
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Maria Bassi | + 885 word(s) | 885 | 2021-08-04 08:50:12 | | | |
| 2 | Ron Wang | + 99 word(s) | 984 | 2021-08-23 04:39:08 | | | | |
| 3 | Lindsay Dong | Meta information modification | 984 | 2021-10-09 11:42:42 | | |
Video Upload Options
Retinal ganglion cells (RGCs) are a population of neurons of the central nervous system (CNS) extending with their soma to the inner retina and with their axons to the optic nerve. Glaucoma represents a group of neurodegenerative diseases where the slow progressive death of RGCs results in a permanent loss of vision. To date, although Intra Ocular Pressure (IOP) is considered the main therapeutic target, the precise mechanisms by which RGCs die in glaucoma have not yet been clarified.
1. Introduction
Glaucoma represents a group of neurodegenerative diseases characterized by optic nerve damage and the slow progressive death of retinal ganglion cells (RGCs). Indeed, glaucoma is regarded as the second cause of irreversible blindness worldwide and it is estimated that its incidence will increase to more than 112 million cases in the future [1][2][3].
In connection with an increased susceptibility to glaucoma and the progression of such a disease, several risk factors, including age, Intra Ocular Pressure (IOP), race, severe myopia, genetic background, vascular dysregulation and central corneal thickness, have been identified [4][5]. Such a multitude of risk factors can be explained by glaucoma etiological complexity, as well as by the several glaucoma forms in which pressure does not increase [6]. Indeed, although IOP has been recognized as one of the main glaucoma risk factors due to it being responsible for both mechanical axonal damage and nutrient interruption [5][7] , there is a particular glaucoma form, corresponding to 20–25% of glaucomatous optic neuropathy, characterized by IOP within a normal range, i.e., Normal Tension Glaucoma (NTG), which, however, leads to progressive blindness. Moreover, not all people who have elevated IOP suffer from glaucoma [8][9][10].
From an anatomical point of view all glaucoma forms are classified in two types, i.e., the open angle and closed-angle glaucoma, according to the geometry of iridocorneal angle, the point where the iris and the cornea meet [6]. However, Primary Open Angle Glaucoma (POAG) is the most common glaucoma type, accounting for over 70% of cases.
Currently, clinical treatments for all glaucoma types aim for lowering IOP through topical hypotensive drugs or surgery. However, these approaches are not sufficiently successful for many patients who continue to lose their vision [11]. Therefore, it would seem evident that RGC death is also driven by different converging molecular pathways, engaged in additional damage more or less closely connected with IOP elevation, which are able to trigger or exacerbate the glaucomatous cascade. The purpose of this present review is to summarize the most recent evidence about some of the possible upstream causes which are responsible for RGC death, as well as neuroprotective strategies to prevent or at least to slow down progression of the retinal distress.
2. Retinal Ganglion Cells
Although the precise mechanisms by which RGCs die in glaucoma have not yet been clarified, three main causes of severe and extensive damage have been proposed: (i) axonal transport blockade; (ii) Glutamate excitotoxicity; and (iii) changes in pro-inflammatory cytokine along the RGC projection ( Figure 1 ).
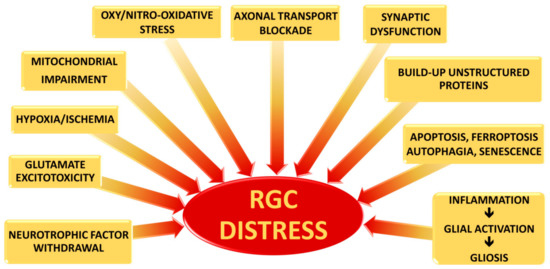
As known, neurotrophic factors (NTFs) are critical for the differentiation and maintenance of the nervous system, as well as for the support of neuronal cell survival. NTFs generally include: the neutrophin family, the glial cell-line derived neurotrophic factor (GDNF) family and the family represented by Ciliary Neurotrophic Factor (CNTF) [12][13].
Although the direct role of glutamate excitotoxicity in glaucoma pathogenesis remains circumstantial [14][15][16][17], its role has been demonstrated during both ischemic and hypoxia injuries [18][19]. Therefore, since both conditions have been found in glaucoma, it is conceivable that glutamate excitotoxicity could be involved in RGC death as a possible outcome of extensive damage rather than being the main cause [20].
Under prolonged hypoxic-ischemic conditions, the neural protective mechanisms are lost, leading to cell death and tissue damage. Therefore, under such conditions, neuronal degeneration can result from different detrimental mechanisms triggered by oxygen and substrate deprivation, including glutamate excitotoxicity, free oxygen radicals, and pro-inflammatory cytokines, causing a loss in the function of the blood retinal barrier (BRB) [20].
3. Neuroprotection
Even though several pre-clinical studies have demonstrated that NTF supplementation could attenuate the RGC loss, it is necessary to better understand the NTF-signaling pathways, as well as improving the delivery strategies with which NTFs are administered.
Therefore, SBSs should also be considered as neuroprotective agents for their involvement in anti-inflammatory actions, acting as inhibitors of NF-κB-signaling in glial cells and, at the same time, as promoters of the TGFβ-cascade pathway [21][22].
It is important to underline that several other innovative neuroprotective approaches are currently being assessed ( Figure 2 ). However, since almost all studies are based on animal models, attempts to perform clinical trials has resulted in many difficulties due to the results not necessarily reproducing those obtained in animal glaucoma models, which in themselves differ from one species to another. Moreover, some of these potential drugs have shown not only instability and difficulty in their delivery to the retina, but also several adverse outcomes [23][24].
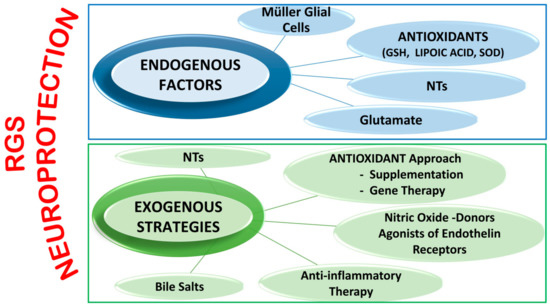
Endogenous factors include Müller Glial Cells functions, antioxidant systems, NTsand glutamate.
4. Conclusions
Although the first-line treatment for patients with glaucoma is lowering the IOP, it should be taken into consideration that glaucoma manifests various and important underlying molecular changes, which, in a certain way, lead to RGC death.
Therefore, it is crucial to increasingly broaden our knowledge of the initial conditions that bring about glaucoma, such as those reported in this present review, to develop new therapeutic approaches.
References
- You, M.; Rong, R.; Zeng, Z.; Xia, X.; Ji, D. Transneuronal Degeneration in the Brain During Glaucoma. Front. Aging Neurosci. 2021, 13, 643685.
- Quigley, H.A.; Broman, A.T. The Number of People with Glaucoma Worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267.
- Kingman, S. Glaucoma Is Second Leading Cause of Blindness Globally. Bull. World Health Organ. 2004, 82, 887–888.
- Calkins, D.J. Adaptive Responses to Neurodegenerative Stress in Glaucoma. Prog. Retin. Eye Res. 2021, 100953.
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The Molecular Basis of Retinal Ganglion Cell Death in Glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181.
- King, A.; Azuara-Blanco, A.; Tuulonen, A. Glaucoma. BMJ 2013, 346, f3518.
- Quaranta, L.; Bruttini, C.; Micheletti, E.; Konstas, A.G.P.; Michelessi, M.; Oddone, F.; Katsanos, A.; Sbardella, D.; De Angelis, G.; Riva, I. Glaucoma and Neuroinflammation: An Overview. Surv. Ophthalmol. 2021, 66, 693–713.
- Gordon, M.O. The Ocular Hypertension Treatment Study: Baseline Factors That Predict the Onset of Primary Open-Angle Glaucoma. Arch. Ophthalmol. 2002, 120, 714.
- Friedman, D.S.; Wilson, M.R.; Liebmann, J.M.; Fechtner, R.D.; Weinreb, R.N. An Evidence-Based Assessment of Risk Factors for the Progression of Ocular Hypertension and Glaucoma. Am. J. Ophthalmol. 2004, 138, 19–31.
- Fahmy, I.A.; Amer, A.K.; EL-Ghaffar, N.A. The Role of T-Cell Subsets and Natural Killer Lymphocytes in the Pathogenesis of Primary Open Angle Glaucoma. Maced. J. Med. Sci. 2010, 3, 307–313.
- Susanna, R.; Moraes, C.G.D.; Cioffi, G.A.; Ritch, R. Why Do People (Still) Go Blind from Glaucoma? Transl. Vis. Sci. Technol. 2015, 4.
- Ip, N.Y.; Yancopoulos, G.D. The Neurotrophins and CNTF: Two Families of Collaborative Neurotrophic Factors. Annu. Rev. Neurosci. 1996, 19, 491–515.
- Kimura, A.; Namekata, K.; Guo, X.; Harada, C.; Harada, T. Neuroprotection, Growth Factors and BDNF-TrkB Signalling in Retinal Degeneration. Int. J. Mol. Sci. 2016, 17, 1584.
- Dreyer, E.B. Elevated Glutamate Levels in the Vitreous Body of Humans and Monkeys with Glaucoma. Arch. Ophthalmol. 1996, 114, 299.
- Dreyer, E.B. A Proposed Role for Excitotoxicity in Glaucoma. J. Glaucoma 1998, 7, 62–67.
- Honkanen, R.A. Vitreous Amino Acid Concentrations in Patients with Glaucoma Undergoing Vitrectomy. Arch. Ophthalmol. 2003, 121, 183.
- Wamsley, S. Vitreous Glutamate Concentration and Axon Loss in Monkeys with Experimental Glaucoma. Arch. Ophthalmol. 2005, 123, 64.
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity from the Perspective of Glial Cells. Front. Cell. Neurosci. 2020, 14, 51.
- Dallas, M.; Boycott, H.E.; Atkinson, L.; Miller, A.; Boyle, J.P.; Pearson, H.A.; Peers, C. Hypoxia Suppresses Glutamate Transport in Astrocytes. J. Neurosci. 2007, 27, 3946–3955.
- Kaur, C. Hypoxia-Ischemia and Retinal Ganglion Cell Damage. Clin. Ophthalmol. 2008, 879.
- Noailles, A.; Fernández-Sánchez, L.; Lax, P.; Cuenca, N. Microglia Activation in a Model of Retinal Degeneration and TUDCA Neuroprotective Effects. J. Neuroinflam. 2014, 11.
- Romero-Ramírez, L.; Nieto-Sampedro, M.; Barreda-Manso, M.A. Integrated Stress Response as a Therapeutic Target for CNS Injuries. BioMed Res. Int. 2017, 2017, 6953156.
- Naik, S.; Pandey, A.; Lewis, S.A.; Rao, B.S.S.; Mutalik, S. Neuroprotection: A Versatile Approach to Combat Glaucoma. Eur. J. Pharmacol. 2020, 881, 173208.
- Sharif, N.A. IDrugs and IDevices Discovery Research: Preclinical Assays, Techniques, and Animal Model Studies for Ocular Hypotensives and Neuroprotectants. J. Ocul. Pharmacol. Ther. 2018, 34, 7–39.




