
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sara Posé | + 2464 word(s) | 2464 | 2020-07-01 04:39:04 | | | |
| 2 | Nora Tang | Meta information modification | 2464 | 2020-07-15 12:03:20 | | | | |
| 3 | Nora Tang | Meta information modification | 2464 | 2020-07-16 08:34:09 | | | | |
| 4 | Nora Tang | Meta information modification | 2464 | 2020-07-16 08:40:16 | | |
Video Upload Options
Fleshy fruits are crucial in a healthy human diet but highly perishable due to their delicate texture at full ripe stage. Tomato and strawberry are leading crops which belongs to this fleshy fruit category, as well as models for ripening of climacteric and non-climateric fruits, respectively. In this regard, cell cultures derived from strawberry fruit at different developmental stages have been obtained to evaluate their potential use to study different aspects of strawberry ripening. Callus from leaf and cortical tissue of unripe-green, white, and mature-red strawberry fruits were induced in a medium supplemented with 11.3 M 2,4-dichlorophenoxyacetic acid (2,4-D) under darkness. This 2,4-D chemical reagent is an herbicide which mimics the action of the natural plant hormone auxin, widely used in plant cell culture. The transfer of the established callus from darkness to light induced the production of anthocyanin. The replacement of 2,4-D by abscisic acid (ABA) noticeably increased anthocyanin accumulation in green-fruit callus. Cell walls were isolated from the different fruit cell lines and from fruit receptacles at equivalent developmental stages and sequentially fractionated to obtain fractions enriched in soluble pectins, ester bound pectins, xyloglucans (XG), and matrix glycans tightly associated with cellulose microfibrils. These fractions were analyzed by cell wall carbohydrate microarrays. In fruit receptacle samples, pectins were abundant in all fractions, including those enriched in matrix glycans. The amount of pectin increased from green to white stage, and later these carbohydrates were solubilized in red fruit. Apparently, XG content was similar in white and red fruit, but the proportion of galactosylated XG increased in red fruit. Cell wall fractions from callus cultures were enriched in extensin and displayed a minor amount of pectins. Stronger signals of extensin antibodies were detected in sodium carbonate fraction, suggesting that these proteins could be linked to pectins. Overall, the results obtained suggest that fruit cell lines could be used to analyze hormonal regulation of color development in strawberry but that the cell wall remodelling process associated with fruit softening might be masked by the high presence of extensin in callus cultures.
1. Introduction
Ripening of fleshy fruits is a complex developmental process involving changes in color, flavor, and texture that make the tissue edible to seed-dispersing animals [1]. From an agricultural point of view, textural changes are of major importance since the loss of firm texture is the main determinant of the postharvest shelf life in most commodities [2][3]. Furthermore, fruit texture is one of the main attributes for the acceptance in the market from the consumer’s point of view [2]. Soft fruits such as strawberry acquire an undesirable melting texture very soon after ripening, increasing their susceptibility to pathogen attack and reducing their shelf life to a few days [4][5].
It is generally accepted that the modification of the mechanical properties of the primary cell walls due to cell wall disassembly, the reduction of intercellular adhesion as a result of middle lamella dissolution, and the reduction in cell turgor are the major causes of fruit softening [6][7][8][9]. Among these three factors, most studies have focused on the cell wall disassembly process taking place during fruit ripening; however, despite the large amount of information available, a general model of cell wall remodeling leading to fruit softening remains elusive [9]. Functional analyses of genes encoding pectinases such as polygalacturonase and pectate lyase point to the pectin fraction as a key factor involved in strawberry softening [10][11].
An additional area of active research in strawberry is the genetic regulation of fruit color. Anthocyanin is the principal pigment leading to the red color of ripe strawberry fruit [12][13]. Some key genes involved in the biosynthesis and the regulation of flavonoid/phenylpropanoid compounds have been characterized using transgenic approaches [12][14][15][16]. All these studies have been performed with whole strawberry fruit, a complex organ containing different tissues that differ in their metabolism and that undergo ripening at different rates.
In many cases, cell cultures can be used as simple model systems to study developmental processes, such as stem cell regulation, mineral deficiency, or disease and stress responses [17][18][19][20]. In vitro cultures provide a source of uniform plant material that can be easy to handle, avoiding complex interactions among different plant organs and/or tissues. In the case of fruit, calyx and fruit cultures as well as callus derived from fruit tissues have been employed to study different aspects of ripening, such as hormonal regulation [21], flavor and color development [22], or defense responses [23]. In strawberry, callus from immature fruit has been used to analyze phenol metabolism during the in vitro culture period and its relationship with cell growth [24][25]. Strawberry leaf callus has also been employed to characterize anthocyanin synthesis [26][27]. As regards cell wall metabolism, the effect of plant growth regulators in cell wall composition of callus cultures obtained from immature apple fruits has been determined [28]. As far as is known, the remodeling of cell walls associated with fruit softening has not yet been addressed using callus cultures. The aims of this study were firstly to produce cell lines from strawberry fruits at different developmental stages and secondly to characterize these lines to determine if they could be a useful model system to gain insight into the fruit ripening process, particularly into the regulation of anthocyanin synthesis and the cell wall disassembly associated with fruit softening.
2. Development of Callus Cultures from Fruit Receptacle
Callus cultures have been induced in strawberry from leaf [26], petioles [29], apical meristems [30], and immature fruit [25][31] using Murashige and Skoog (MS) [32] or Linsmaier and Skoog (LS) [33] medium supplemented with 2,4-D. Previous studies demonstrated that N30K, a mineral formulation with lower ionic strength than MS, was more suitable for micropropagation and regeneration of strawberry cv. ‘Chandler’ [34][35]. In this research, this medium was used successfully to obtain callus cultures in leaf explants from this genotype (Figure 1). The presence of 2,4-D was needed to induce callus formation, as previously observed in some strawberry genotypes [31]. The addition of BA to the induction medium diminished the amount of callus formed. Asahira and Kano [36] also found that callus was only established on a medium containing 2,4-D but lacking BA. N30K medium supplemented with a low 2,4-D concentration was also suitable for isolation and proliferation of cell cultures from fruit receptacle at different developmental stages. Explants from red fruit showed the lowest capacity to develop callus in this medium. Similarly, the proliferation rate of the cell lines obtained diminished accordingly to the developmental age of the initial explant, being higher in green immature fruits and lower in ripe fruits. As observed in this research, Hong et al. [31] reported that the rate of callus formation from strawberry fruit decreased with the age of the explant, but they were unable to obtain callus from nearly mature fruits collected four weeks after flowering. In other species, immature fruits have also been chosen as the preferred explant to initiate cell lines [37]; to the best of our knowledge, this is the first time that callus cultures have been obtained from highly differentiated mature fruit.
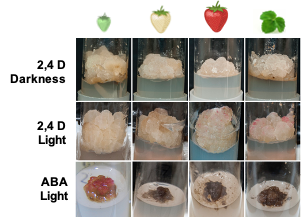
Figure 1. Strawberry callus obtained from leaves and fruit receptacle at different developmental stages (modified from original article published on 10.3390/plants9070805)
3. Anthocyanin Production in Strawberry Fruit Cultures
Light slightly increased the proliferation rate of green and white callus cultures, and it induced the accumulation of red pigmentation, especially in leaf and ripe-fruit lines (Figure 2). A similar observation was made in cv. ‘Brighton’ [31]. In strawberry fruit, anthocyanin biosynthesis is regulated by the transcription factor FaMYB10. This gene is mainly expressed in ripe receptacle and directly controls the expression of early and late-regulated biosynthesis genes involved in the flavonoid/phenylpropanid pathway, including anthocyanin production genes [38]. Kadomura-Ishikawa et al. [39] found that light and ABA independently regulate FaMYB10 expression and therefore anthocyanin production in strawberry fruit, their effect being additive. The replacement of 2,4-D by ABA at low concentrations notably increased anthocyanin production in callus from immature green fruit but did not significantly affect that of the other lines. ABA and auxins play antagonistic effects on strawberry fruit ripening. Decreasing ABA biosynthesis in green fruit by virus induced gene silencing of FaNCDE1, a key gene in ABA biosynthesis, or injection of the ABA inhibitor fluridone impaired fruit development, obtaining a colorless fruit phenotype [40]. A similar phenotype was obtained when down-regulating ABA receptors [41]. Contrary to ABA, auxins produced by achenes reach a maximum level in green receptacle [42], and the removal of the achenes induced the accumulation of anthocyanin [43]. The results obtained in this research suggest large differences in the regulation of the flavonoid pathway among the different fruit cell lines. Auxin/ABA hormonal balance seems to be the main regulator of anthocyanin production in the cell line from green fruits; however, cultures from ripe fruits mainly respond to light, while those from white fruits showed a low capacity for anthocyanin production. The differential behavior of the fruit cell lines could be exploited to decipher the genetic regulation of pigment accumulation in strawberry fruit.
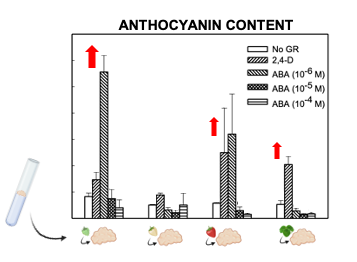
Figure 2. Anthocyanin production in calli obtained from strawberry leaf and fruits at different developmental stages (modified from original article published on 10.3390/plants9070805).
4. Characterization of Cell Walls from Cell Cultures
Carbohydrate profiles from cell cultures were quite different from fruit (Figure 3). In callus cell walls, pectin antibodies showed a very weak signal. By contrast, callus cell wall extracts were enriched in extensin epitopes associated with tightly bound pectins (sodium carbonate fraction) and matrix glycans.
The pectin profile in cell wall fractions isolated from fruits showed that the amounts of HG and RGI epitopes increased from green to white fruit and then declined in red ripe fruit to very low levels. This pattern was observed in all fractions analyzed except in the water fraction from ripe fruit that contained significant amounts of low methyl ester HG recognized by JIM5. These findings support previous observations of pectin solubilization, i.e., a reduction in the amount of pectins tightly bound to the cell wall concomitant to an increase in water-soluble pectins as a typical feature of strawberry remodeling during fruit ripening [4][44][45][46]. It is noteworthy that a large fraction of pectins, especially RGI, was extracted with KOH and cadoxen. Pectin and xyloglucan can be covalently linked [47][48] and recently, Cornuault et al. [49] found that a sub-population of pectin was attached to XG in KOH fractions from different fruits, including strawberry. As regards hemicellulose, it has been reported that its content diminished during strawberry ripening in cultivars with contrasting fruit firmness [50][51]. Contrary to those results, the carbohydrate microarray showed that the signal intensity of extractable XG epitopes changed little during ripening of strawberry cultivar ‘Chandler’; however, the results obtained suggest a change in the chemical structure of XG in ripe fruit, increasing the amount of galactosylated XG recognized by LM25. Significant amounts of glucuronoxylans were also detected in KOH and cadoxen extracts and with an intriguing pattern of relatively lower abundance in white fruit. Heteroxylans are not abundant in primary cell walls of dicotyledonous plants, and the role of glucuronoxylan is unknown. Cornuault et al. [52] found glucuronoxylan associated with RGI-enriched fraction from potato tubers. However, our results indicate a negative correlation between the glucuronoxylan signal and RGI abundance in matrix glycan fractions. Extensins are one of the main classes of hydroxyproline-rich glycoproteins (HRGPs) that contain multiple Ser-(Pro)3–5 repeats and Tyr motifs acting as cross-link sites [53][54]. These proteins are involved in the building and the maintenance of primary cell wall. During the cell plate formation, the self-assembling ability of extensins generates scaffolding networks that may serve as a template for pectin matrix assembly due to the acid-base interactions between extensin and pectin [53]. On the other hand, extensin cross-linking strengthens cell walls and many biotic and abiotic stresses induce extensin biosynthesis, e.g., pathogen attack or wounding [55][56][57]. In grape berries, extensin epitopes detected with LM1 and JIM20 increased at véraison when ripening started; however, its physiological function was unclear [58]. Contrary to grape, the carbohydrate microarray analysis showed that extensin was a minor component in the strawberry cell wall during fruit development. The high extensin content in the strawberry cell cultures may reflect a tightly cross-linked cell wall and could be related to the stressful conditions of in vitro tissue culture (Figure 3); in fact, high amounts of extensin have also been found in suspension cells from other species [59][60]. The stronger extensin epitope signals were detected in sodium carbonate fraction, especially in leaf and green fruit lines, suggesting that these proteins might be linked to pectins, as reported by [61]. This interaction could also explain the low label of monoclonal antibodies against pectins, i.e., pectin epitopes might be masked in pectin-extensin complexes, resulting in a low binding efficiency of HG and RGI antibodies. Indeed, pectin and other cell wall polysaccharides may be more tightly linked into the cell wall structures of callus cells and therefore less extractable to appear in the solubilized fractions. On the other hand, LM23 binding was detected in all cell wall fractions from callus samples except water, indicating the presence of xylogalacturonan (XGA) epitopes. This pectin has not previously been described in strawberry fruit, but it has been found in hairy regions of apple pectin, another member of Rosaceae family [62]. XGA has been related to cell adhesion in carrot-cultured cells [63].
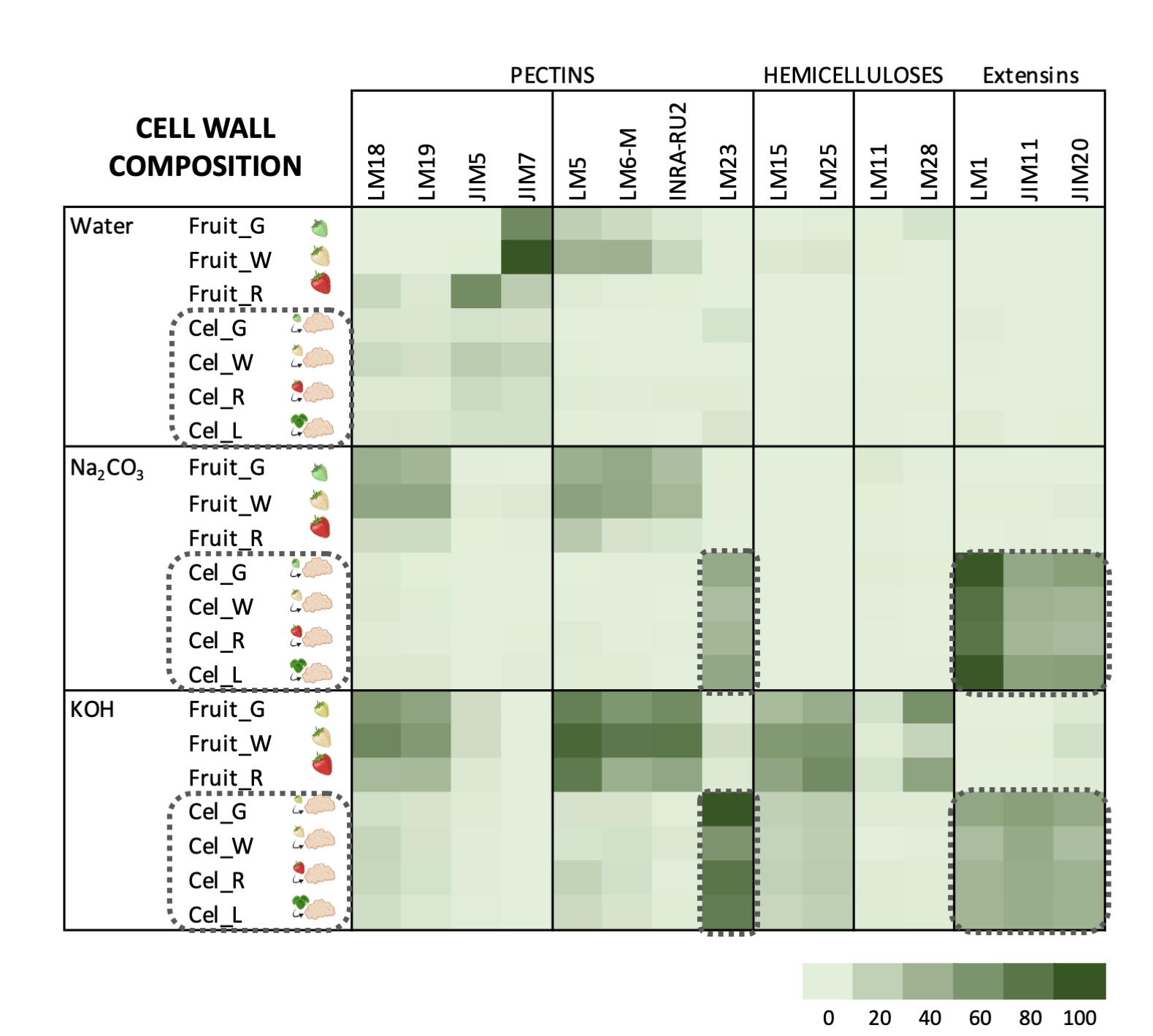
Figure 3. Carbohydrate microarray displayed as heatmap showing the relative abundance of cell wall epitopes recognized by different monoclonal antibodies in cell wall fractions extracted from strawberry fruits and cell cultures obtained from leaf and fruit receptacle at different developmental stages. A value of 100 was assigned to the highest mean spot signal, and all other signals were adjusted accordingly. (modified from original article published on 10.3390/plants9070805)
Carbohydrate microarrays provide information about the relative levels of glycan epitopes but do not allow quantifying absolute levels of cell wall components [64]. In callus cultures, the large amounts of extensin and the apparent lower presence of pectin epitopes make it difficult to compare these cell walls with their corresponding samples from fruits using this approach (Figure 3). As previously indicated, polyuronides are extensively modified during strawberry ripening [4][44]. Other techniques such as carbohydrate structural analyses would be needed to precisely determine whether cell walls at different fruit developmental stages and their corresponding cell lines are equivalent.
5. Conclusions
The results obtained in this research suggest that cell cultures obtained from strawberry receptacle at different developmental stages could be a useful model system to achieve a better understanding of the ripening process in strawberry. Metabolic pathways involved in anthocyanin production or hormonal regulation of ripening could be investigated using this system. Despite the differences observed in cell wall composition in fruits and callus cultures, some aspects of cell wall disassembly during fruit development, e.g., pectin remodeling, could also be addressed using this system. Overall, strawberry fruit cell culture could be a useful tool to study development and regulation of agronomical traits like fruit color, aiming to improve the quality and consumer acceptance of fleshy fruits.
References
- Giovannoni, J.J. Genetic regulation of fruit development and ripening. Plant Cell 2004, 16, 170–180.
- Contador, L.; Shinya, P.; Infante, R. Texture phenotyping in fresh fleshy fruit. Sci. Hortic. 2015, 193, 40–46.
- Goulao, L.; Oliveira, C. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25.
- Moya-León, M.A.; Mattus-Araya, E.; Herrera, R. Molecular events occurring during softening of strawberry fruit. Front. Plant Sci. 2019, 10, 615.
- Perkins-Veazie, P. Growth and ripening of strawberry fruit. Hortic. Rev. 1995, 17, 267–297.
- Brummell, D.A. Cell wall disassembly in ripening fruit. Funct. Plant Biol. 2006, 33, 103–119.
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001, 47, 311–340.
- Posé, S.; Paniagua, C.; Matas, A.J.; Gunning, A.P.; Morris, V.J.; Quesada, M.A.; Mercado, J.A. A nanostructural view of the cell wall disassembly process during fruit ripening and postharvest storage by atomic force microscopy. Trends Food Sci. Technol. 2018, 87, 47–58.
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit softening: Revisiting the role of pectin. Trends Plant Sci. 2018, 23, 302–310.
- Jiménez-Bermúdez, S.; Redondo-Nevado, J.; Muñoz-Blanco, J.; Caballero, J.L.; López-Aranda, J.M.; Valpuesta, V.; Pliego-Alfaro, F.; Quesada, M.A.; Mercado, J.A. Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant Physiol. 2002, 128, 751–759.
- Posé, S.; Paniagua, C.; Cifuentes, M.; Blanco-Portales, R.; Quesada, M.A.; Mercado, J.A. Insights into the effects of polygalacturonase FaPG1 gene silencing on pectin matrix disassembly, enhanced tissue integrity, and firmness in ripe strawberry fruits. J. Exp. Bot. 2013, 64, 3803–3815.
- Schaart, J.G.; Dubos, C.; Romero De La Fuente, I.; van Houwelingen, A.M.M.L.; de Vos, R.C.H.; Jonker, H.H.; Xu, W.; Routaboul, J.-M.; Lepiniec, L.; Bovy, A.G. Identification and characterization of MYB-bHLH-WD40 regulatory complexes controlling proanthocyanidin biosynthesis in strawberry (Fragaria × ananassa) fruits. New Phytol. 2013, 197, 454–467.
- Silva, F.L.D.; Escribano-Bailón, M.T.; Alonso, J.J.P.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. LWT Food Sci. Technol. 2007, 40, 374–382.
- Gao, Q.; Luo, H.; Li, Y.; Liu, Z.; Kang, C. Genetic modulation of RAP alters fruit coloration in both wild and cultivated strawberry. Plant Biotechnol. J. 2020, 18, 1550–1561.
- Palomo-Ríos, E.; Quesada, M.A.; Matas, A.J.; Pliego-Alfaro, F.; Mercado, J.A. The history and current status of genetic transformation in berry crops. In The Genomes of Rosaceous Berries and Their Wild Relatives; Hytönen, T., Graham, J., Harrison, R., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 139–160.
- Wang, H.; Zhang, H.; Yang, Y.; Li, M.; Zhang, Y.; Liu, J.; Dong, J.; Li, J.; Butelli, E.; Xue, Z.; et al. The control of red colour by a family of MYB transcription factors in octoploid strawberry (Fragaria × ananassa) fruits. Plant Biotechnol. J. 2019, 18, 1169–1184.
- Fenning, T.M. The use of tissue culture and in-vitro approaches for the study of tree diseases. Plant Cell Tissue Organ Cult. 2019, 136, 415–430.
- Fernandes, J.C.; García-Angulo, P.; Goulao, L.F.; Acebes, J.L.; Amâncio, S. Mineral stress affects the cell wall composition of grapevine (Vitis vinifera L.) callus. Plant Sci. 2013, 205–206, 111–120.
- Liu, J.H.; Nada, K.; Honda, C.; Kitashiba, H.; Wen, X.P.; Pang, X.M.; Moriguchi, T. Polyamine biosynthesis of apple callus under salt stress: Importance of the arginine decarboxylase pathway in stress response. J. Exp. Bot. 2006, 57, 2589–2599.
- Perez-Garcia, P.; Moreno-Risueno, M.A. Stem cells and plant regeneration. Dev. Biol. 2018, 442, 3–12.
- Cohen, J.D. In vitro tomato fruit cultures demonstrate a role for indole-3-acetic acid in regulating fruit ripening. J. Am. Soc. Hortic. Sci. 1996, 121, 520–524.
- Ishida, B.K.; Baldwin, E.A.; Buttery, R.G.; Chui, S.H.; Ling, L.C. Flavor volatiles, sugars and color development in ripening in vitro-cultured tomato fruit and calyx. Physiol. Plant. 1993, 89, 861–867.
- Belhadj, A.; Telef, N.; Saigne, C.; Cluzet, S.; Barrieu, F.; Hamdi, S.; Mérillon, J.-M. Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol. Biochem. 2008, 46, 493–499.
- Arnaldos, T.L.; Ferrer, M.A.; García, A.A.C.; Muñoz, R. Changes in peroxidase activity and isoperoxidase pattern during strawberry (Fragaria × ananassa) callus development. J. Plant Physiol. 2002, 159, 429–435.
- López Arnaldos, T.; Muñoz, R.; Ferrer, M.A.; Calderón, A.A. Changes in phenol content during strawberry (Fragaria x ananassa, cv. Chandler) callus culture. Physiol. Plant. 2001, 113, 315–322.
- Mori, T.; Sakurai, M.; Seki, M.; Furusaki, S. Use of auxin and cytokinin to regulate anthocyanin production and composition in suspension cultures of strawberry cell. J. Sci. Food Agric. 1994, 65, 271–276.
- Zhang, W.; Furusaki, S. Regulation of anthocyanin synthesis in suspension cultures of strawberry cell by pH. Biotechnol. Lett. 1997, 19, 1057–1061.
- Alayón-Luaces, P.; Ponce, N.M.A.; Mroginski, L.A.; Stortz, C.A.; Sozzi, G.O. Compositional changes in cell wall polysaccharides from apple fruit callus cultures modulated by different plant growth regulators. Plant Sci. 2012, 185–186, 169–175.
- Edahiro, J.; Seki, M. Phenylpropanoid metabolite supports cell aggregate formation in strawberry cell suspension culture. J. Biosci. Bioeng. 2006, 102, 8–13.
- Sato, K.; Nakayama, M.; Shigeta, J. Culturing conditions affecting the production of anthocyanin in suspended cell cultures of strawberry. Plant Sci. 1996, 113, 91–98.
- Hong, Y.C.; Read, P.E.; Harlander, S.K.; Labuza, T.P. Development of a tissue culture system from immature strawberry fruits. J. Food Sci. 1989, 54, 388–392.
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497.
- Linsmaier, E.M.; Skoog, F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant. 1965, 18, 100–127.
- Barceló, M.; El-Mansouri, I.; Mercado, J.A.; Quesada, M.A.; Pliego-Alfaro, F. Regeneration and transformation via Agrobacterium tumefaciens of the strawberry cultivar Chandler. Plant Cell Tissue Organ Cult. 1998, 54, 29–36.
- López-Aranda, J.M.; Pliego-Alfaro, F.; López-Navidad, I.; Barceló-Muñoz, M. Micropropagation of strawberry (Fragaria x ananassa Duch.). Effect of mineral salts, benzyladenine levels and number of subcultures on the in vitro and field behaviour of the obtained microplants and the fruiting capacity of their progeny. J. Hortic. Sci. 1994, 69, 625–637.
- Asahira, T.; Kano, Y. Shoot formation from cultured tissue of strawberry fruits. J. Jpn. Soc. Hortic. Sci. 1977, 46, 317–324.
- Alayón-Luaces, P.; Pagano, E.A.; Mroginski, L.A.; Sozzi, G.O. Four glycoside hydrolases are differentially modulated by auxins, cytokinins, abscisic acid and gibberellic acid in apple fruit callus cultures. Plant Cell Tissue Organ Cult. 2008, 95, 257–263.
- Medina-Puche, L.; Cumplido-Laso, G.; Amil-Ruiz, F.; Hoffmann, T.; Ring, L.; Rodríguez-Franco, A.; Caballero, J.L.; Schwab, W.; Muñoz-Blanco, J.; Blanco-Portales, R. MYB10 plays a major role in the regulation of flavonoid/phenylpropanoid metabolism during ripening of Fragaria × ananassa fruits. J. Exp. Bot. 2014, 65, 401–417.
- Kadomura-Ishikawa, Y.; Miyawaki, K.; Takahashi, A.; Masuda, T.; Noji, S. Light and abscisic acid independently regulated FaMYB10 in Fragaria × ananassa fruit. Planta 2015, 241, 953–965.
- Jia, H.-F.; Chai, Y.-M.; Li, C.-L.; Lu, D.; Luo, J.-J.; Qin, L.; Shen, Y.-Y. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 2011, 157, 188–199.
- Chai, Y.-M.; Jia, H.-F.; Li, C.-L.; Dong, Q.-H.; Shen, Y.-Y. FaPYR1 is involved in strawberry fruit ripening. J. Exp. Bot. 2011, 62, 5079–5089.
- Estrada-Johnson, E.; Csukasi, F.; Pizarro, C.M.; Vallarino, J.G.; Kiryakova, Y.; Vioque, A.; Brumos, J.; Medina-Escobar, N.; Botella, M.A.; Alonso, J.M.; et al. Transcriptomic analysis in strawberry fruits reveals active auxin biosynthesis and signaling in the ripe receptacle. Front. Plant Sci. 2017, 8, 889.
- Given, N.K.; Venis, M.A.; Grierson, D. Hormonal regulation of ripening in the strawberry, a non-climacteric fruit. Planta 1988, 174, 402–406.
- Paniagua, C.; Santiago-Doménech, N.; Kirby, A.R.; Gunning, A.P.; Morris, V.J.; Quesada, M.A.; Matas, A.J.; Mercado, J.A. Structural changes in cell wall pectins during strawberry fruit development. Plant Physiol. Biochem. 2017, 118, 55–63.
- Quesada, M.A.; Blanco-Portales, R.; Pose, S.; Garcia-Gago, J.A.; Jiménez-Bermúdez, S.; Muñoz-Serrano, A.; Caballero, J.L.; Pliego-Alfaro, F.; Mercado, J.A.; Muñoz-Blanco, J. Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiol. 2009, 150, 1022–1032.
- Redgwell, R.J.; Macrae, E.; Hallett, I.; Fischer, M.; Perry, J.; Harker, R. In vivo and in vitro swelling of cell walls during fruit ripening. Planta 1997, 64, 162–173.
- Popper, Z.A.; Fry, S.C. Widespread occurrence of a covalent linkage between xyloglucan and acidic polysaccharides in suspension-cultured angiosperm cells. Ann. Bot. 2005, 96, 91–99.
- Thompson, J.E.; Fry, S.C. Evidence for covalent linkage between xyloglucan and acidic pectins in suspension-cultured rose cells. Planta 2000, 211, 275–286.
- Cornuault, V.; Posé, S.; Knox, J.P. Disentangling pectic homogalacturonan and rhamnogalacturonan-I polysaccharides: Evidence for sub-populations in fruit parenchyma systems. Food Chem. 2018, 246, 275–285. [Google Scholar] [CrossRef]
- Heng Koh, T.; Melton, L.D. Ripening-related changes in cell wall polysaccharides of strawberry cortical and pith tissues. Postharvest Biol. Technol. 2002, 26, 23–33.
- Rosli, H.G.; Civello, P.M.; Martínez, G.A. Changes in cell wall composition of three Fragaria x ananassa cultivars with different softening rate during ripening. Plant Physiol. Biochem. 2004, 42, 823–831.
- Cornuault, V.; Buffetto, F.; Rydahl, M.G.; Marcus, S.E.; Torode, T.A.; Xue, J.; Crépeau, M.-J.; Faria-Blanc, N.; Willats, W.G.T.; Dupree, P.; et al. Monoclonal antibodies indicate low-abundance links between heteroxylan and other glycans of plant cell walls. Planta 2015, 242, 1321–1334.
- Lamport, D.T.A.; Kieliszewski, M.J.; Chen, Y.; Cannon, M.C. Role of the extensin superfamily in primary cell wall architecture. Plant Physiol. 2011, 156, 11–19.
- Marzol, E.; Borassi, C.; Bringas, M.; Sede, A.; Rodríguez Garcia, D.R.; Capece, L.; Estevez, J.M. Filling the gaps to solve the extensin puzzle. Mol. Plant 2018, 11, 645–658.
- Liu, X.; Wolfe, R.; Welch, L.R.; Domozych, D.S.; Popper, Z.A.; Showalter, A.M. Bioinformatic identification and analysis of extensins in the plant kingdom. PLoS ONE 2016, 11, e0150177.
- Merkouropoulos, G.; Shirsat, A.H. The unusual Arabidopsis extensin gene atExt1 is expressed throughout plant development and is induced by a variety of biotic and abiotic stresses. Planta 2003, 217, 356–366.
- Portieles, R.; Canales, E.; Hernández, I.; López, Y.; Rodríguez, M.; Rodríguez, M.; Terauchi, R.; Borroto, C.; Santos, R.; Ayra-Pardo, C.; et al. NmEXT Extensin gene: A positive regulator of resistance response against the oomycete Phytophthora nicotianae. Plant Mol. Biol. Rep. 2018, 36, 484–490.
- Moore, J.P.; Fangel, J.U.; Willats, W.G.T.; Vivier, M.A. Pectic-β(1,4)-galactan, extensin and arabinogalactan-protein epitopes differentiate ripening stages in wine and table grape cell walls. Ann. Bot. 2014, 114, 1279–1294.
- Dey, P.M.; Brownleader, M.D.; Pantelides, A.T.; Trevan, M.; Smith, J.J.; Saddler, G. Extensin from suspension-cultured potato cells: A hydroxyproline-rich glycoprotein, devoid of agglutinin activity. Planta 1997, 202, 179–187.
- Jackson, P.A.P.; Galinha, C.I.R.; Pereira, C.S.; Fortunato, A.; Soares, N.C.; Amâncio, S.B.Q.; Pinto Ricardo, C.P. Rapid deposition of extensin during the elicitation of grapevine callus cultures is specifically catalyzed by a 40-kilodalton peroxidase. Plant Physiol. 2001, 127, 1065–1076.
- Nunez, A.; Fishman, M.L.; Fortis, L.L.; Cooke, P.H.; Hotchkiss, A.T. Identification of extensin protein associated with sugar beet pectin. J. Agric. Food Chem. 2009, 57, 10951–10958.
- Schols, H.A.; Bakx, E.J.; Schipper, D.; Voragen, A.G.J. A xylogalacturonan subunit present in the modified hairy regions of apple pectin. Carbohydr. Res. 1995, 279, 265–279.
- Satoh, S. Functions of the cell wall in the interactions of plant cells: Analysis using carrot cultured cells. Plant Cell Physiol. 1998, 39, 361–368.
- Moller, I.; Sørensen, I.; Bernal, A.J.; Blaukopf, C.; Lee, K.; Øbro, J.; Pettolino, F.; Roberts, A.; Mikkelsen, J.D.; Knox, J.P.; et al. High-throughput mapping of cell-wall polymers within and between plants using novel microarrays. Plant J. 2007, 50, 1118–1128.





