Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Matteo Sambucci | + 1988 word(s) | 1988 | 2021-08-10 05:05:34 | | | |
| 2 | Peter Tang | Meta information modification | 1988 | 2021-08-19 02:39:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sambucci, M. Geopolymers vs. Cement Matrix Materials. Encyclopedia. Available online: https://encyclopedia.pub/entry/13318 (accessed on 08 February 2026).
Sambucci M. Geopolymers vs. Cement Matrix Materials. Encyclopedia. Available at: https://encyclopedia.pub/entry/13318. Accessed February 08, 2026.
Sambucci, Matteo. "Geopolymers vs. Cement Matrix Materials" Encyclopedia, https://encyclopedia.pub/entry/13318 (accessed February 08, 2026).
Sambucci, M. (2021, August 18). Geopolymers vs. Cement Matrix Materials. In Encyclopedia. https://encyclopedia.pub/entry/13318
Sambucci, Matteo. "Geopolymers vs. Cement Matrix Materials." Encyclopedia. Web. 18 August, 2021.
Copy Citation
Geopolymers are spreading more and more in the cementitious materials field, exhibiting technological properties that are highly competitive to conventional Portland concrete mixes.
CO2 emissions
energy efficiency
Portland replacement
geopolymer concrete mixes
mechanical properties
microstructure
geopolymer nanocomposites
durability
high-temperature behavior
LCA analysis
1. Introduction
After water, concrete is the world’s second most important substance needed for humans, but its hidden effects on the environment and health make it one of the most polluting materials on the planet. Carbon dioxide (CO2) emissions straightforwardly correspond to the cement content used in the concrete mix. Portland cement (PC) is the most common type of cementitious binder in general use around the world as a basic ingredient of concrete or mortars. Typical raw materials used to fabricate PC include limestone, marl, clay, slag, silica sand, and iron ores [1]. The manufacturing process of PC entails the following major steps [1][2]:
-
Raw materials preparation. After quarrying, principal raw materials (such as limestone and clay) are crushed to a maximum of about 7.5 cm in diameter by a two-step grinding treatment. In a “dry” production method, crushed aggregates are fed directly into the kiln. Conversely, the “wet” method consists of the mixing of ground material with water to form a slurry, a suspension of creamy consistency composed of water (35–50%) and fine particles. Next, the slurry is inserted into the kiln. “Wet” preparation ensures an easier control of the chemistry process and is suitable in the presence of moist raw aggregates. However, it has higher energy requirements due to the need for slurry water evaporation. On the other hand, the “dry” process is faster and less energy expensive [3].
-
Clinker production. Ground ingredients are burned in the kiln at a temperature of about 1350 °C to 1500 °C. Various materials can be used as kiln fuels. Traditional sub-stances are fossil fuels, oil, coal, and gas. Secondary raw materials, including waste oils, plastics, waste tires, and sewage sludge, are considered alternative fuels for the cement industry [4]. Thermal treatment involves the partial fusion of raw materials, the breaking of their chemical bonds, and the recombination into new compounds. The result is a nodular-shaped clinker product.
-
Final grinding process. After cooling, clinker nodules are crushed into a superfine powder by steel ball milling treatment. During this process, the clinker is mixed with a small amount of gypsum (3–5%) to produce PC. Gypsum prevents the flash setting of the cement and regulates its hardening.
Specifically, PC results from the calcination of limestone (calcium carbonate or CaCO3) and silico-aluminous materials in burnt lime (CaO)-based products, according to the following reaction [5]:
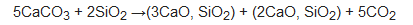
Production of 1 ton of PC is estimated to release an average of 0.86 ton of CO2. Calcination is the major way of CO2 emission. Approximately 540 kg of CO2 per ton of clinker is released from this process. The rest of greenhouse emission mainly results from fuel combustion to provide the thermal energy for maintaining the high temperatures into the kiln. However, there are other secondary sources of polluting emission: CO2 emission from electricity consumption (from 1% to 10% of global CO2 releases, depending on the local energy efficiency source) and contribution of mining and transportation of the mineral raw materials (~5%) [6]. As highlighted in the De Lena et al. analysis [7], cement manufacturing is responsible for about 8% of overall anthropogenic CO2 emissions. This chemical compound is the most abundant component among all greenhouse gasses, having the highest impact on the global warming phenomenon. Benhelal et al. [8] report that the increase in the global mean temperature could led to catastrophic environmental consequences, such as extinction of many animal and plant species, risks for biodiversity hotspots and ecosystems, extremely adverse weather events, and damage associated with excessive sea level rise.
In this framework, how to minimize the dangerous effect of the cement industry in terms of atmospheric pollution and energy saving, has become an urgent question for re-searchers. Several low-carbon strategies were proposed [9]:
-
Use of alternative fuels or raw materials to mitigate CO2 emission for Portland cement manufacturing.
-
Adoption of CO2-capturing technologies in the cement plants.
-
Development of Portland-free alternative binders.
2. CO2 Emission Mitigation Strategies in the Cement Industry: An Overview
2.1. Alternative Fuels in PC Manufacturing
Adoption of biomass or industrial wastes (tires, sludge, waste oil, plastics, fabrics, etc.) in cement manufacturing, as a replacement of conventional fuels, is in progress in most countries [9][10]. These materials, with a high Carbon-to-Hydrogen (C-H) ratio, can remarkably reduce CO2 emission compared to conventional fossil fuels. Other benefits are related to the preservation of non-renewable sources, reduction of landfill sites, and maximization of energy recovery from wastage [9][11]. To demonstrate the eco-efficiency performance of alternative fuels in the cement industry, Rahman et al. [12] proposed a numerical comparative analysis on the employment of some types of waste products (specifically tire, plastics, wastes of animal origin, and municipal solid wastes) as substitutes for traditional fossil fuels in the PC production. The net CO2 release (kg/ton of clinker) is illustrated in Figure 1 for different feed content of alternative material in the fuel mix, where 0% of alternative fuel indicates the carbon emission in the case of traditional coal fuel.
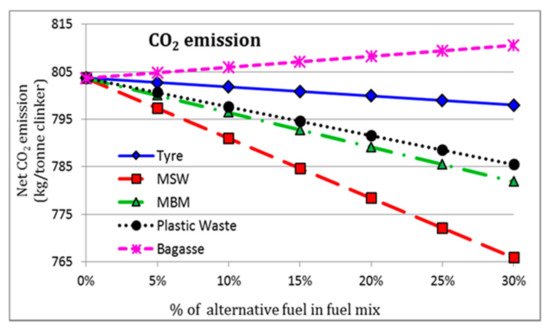
Figure 1. Net CO2 emission for a different feed rate of waste-deriving alternative fuels: used tyres, municipal solid waste (MSW), meat and bone meal (MBM), plastic waste, and bagasse [12].
The overall decrease in CO2 emissions justifies the high efficiency of waste-deriving alternative fuels in terms of low environmental impact. In fact, the results indicated that up to a 4.4% reduction in CO2 emissions and up to a 6.4% reduction in thermal energy requirement could be achieved using these alternative fuels with 20% mix in coal without significantly altering the clinker quality [12]. However, careful controls are required for the integration of these resources during the clinkering process. Inhomogeneous thermal distribution in the kiln, higher emissions of harmful compounds/elements (Carbon monoxide, Sulphur dioxide, Nitrogen oxides, Chloride, heavy metals), and possible accumulation of dust in the apparatus are some of the principal limitations of this approach. Besides, switching from conventional fuel to alternative ones requires extensive economic investments related to the technical modification of the plant and the implementation of new fuels storage and distribution systems [10].
2.2. CO2-Capturing Technologies
Generally, capture and storage technologies (CSTs) refer to a set of methodologies designed to separate the CO2 produced by combustion and then to compress, transport, and inject it into geological formations. In highly efficient cement plants where the fuel consumption is significantly reduced and the potential for improving energy performance is very limited, the integration of CSTs can produce a drastic reduction of CO2 emission coming from the calcination reaction [13].
2.3. Alternative Cementitious Binders
Calcium aluminates cements (CACs), super sulfated slag cements (SSCs), microbial cements (MCs), and geopolymer cements (GCs) are some types of sustainable binders as an alternative to conventional PC. Promising environmental performances are demonstrated by the production and adoption of these alternative materials: 5–90% reduction in CO2 release could lead up to 7% decrease in global CO2 emissions compared to PC manufacturing [14]. Coupled with the interest in finding solutions with low-CO2 emissions and low-energy consumption, the development of “green” binders aims to enhance the recycling and reuse of waste materials. In PC technology, some types of industrial waste (fly ash, silica fume, ground granulated blast furnace slag) are only partially used as supplementary cementing materials (10–50%). Nowadays, the research pushes beyond this evidence to create alternative cementitious compounds made entirely or almost entirely from waste products [15]. Besides, further motivation for exploring novel materials to supplement and/or replace PC concrete in the building architectural field derives from their technological performance. Specifically, many of these alternative and novel binder systems generally demonstrate increased durability when subjected to harsh conditions (such as lower shrinkage, higher acid resistance, fire inertia) compared to PC concrete [15]. However, one of the most relevant obstacles to the application of non-conventional cements concerns the lack of official standards or regulations unanimously accepted by the technical committees [15][16].
3. Geopolymer Cements: Basic Features and Novel Advances
Geopolymer cements result from a chemical reaction between aluminosilicate materials and alkali solution. “Cement” does not mean the chemical characteristics of the material but rather its functionality as a matrix applicable in the field of concrete materials. Specifically, the precursors are rich in SiO2 and Al2O3. When activated by alkali solution, these materials lead the formation of an aluminosilicate gel that develops high strength at early age and after moderate curing temperature condition [17]. Based on the nature and type of the aluminosilicate precursors, geopolymer binders can be mainly classified in [18]: granulated blast furnace slag (GBFS)-based geopolymer cement, fly ash (FA)-based geopolymer cement, rock-based geopolymer cement, and Ferro-sialate-based geopolymer cement. These binders comprised of a repeating unit of silico-oxide (–Si–O–Si-O–), silico-aluminate (–Si–O–Al–O–), Ferro-silico-aluminate (–Fe–O–Si–O–Al–O–) or alumino-phosphate (–Al–O–P–O–), developed through an alkali-activated geopolymerization process [19]. Alkaline compounds, such as Sodium hydroxide (NaOH), Potassium hydroxide (KOH), Sodium silicate (Na2SiO3), and Potassium silicate (K2SiO3), are used to activate the aluminosilicate precursors. The reaction produces SiO4 and AlO4, tetrahedral frameworks linked by shared oxygen as poly(sialates) or poly(sialate–siloxo) or poly(sialate–disiloxo), depending on the SiO2/Al2O3 ratio in the system. The tetrahedral units are alternatively linked to polymeric precursors by sharing oxygen atoms, thus forming polymeric Si–O–Al–O bonds [20]. A comprehensive geopolymerization mechanism was proposed by Zhang et al. [21] and reported in Figure 3. Synthetically, the mechanism involves the following steps: (a) dissolution of aluminosilicate particles; (b) initial polymerization of dissolved alumina and silicate species into Al–O–Si oligomers; (c) further polymerization into large amorphous gels (zeolitic precursors) or direct growth into crystalline structures (zeolitic phase). After the gel is formed, the system will continue to rearrange and the number of bonds between the molecules will also increase, creating a 3D network that is associated with the geopolymer structure.
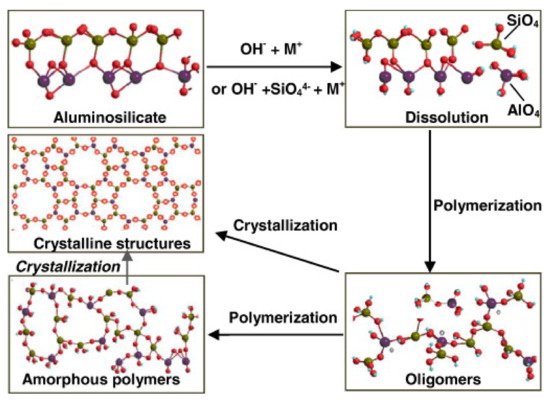
Figure 3. Zhang model of the geopolymerization process. Reprinted with permission from ref. [21]. Copyright 2016 Elsevier.
The geopolymer synthesis and structure are very similar to zeolites. The main difference is related to the crystallinity degree. Zeolite is usually crystalline in nature. It crystallizes from dilute aqueous solution by hydrothermal process, where aluminosilicate precursors have enough mobility and time to orient and align before bonding into a crystal lattice. Conversely, geopolymers are amorphous to semi-crystalline. During the alkali activation of precursors, there is not sufficient time and space for the gel to arrange into a crystalline framework. The result is an amorphous or semi-amorphous C–S–H microstructure [17].
The preparation of geopolymer materials, including concrete and mortars, is summarized in the following steps [22]:
-
Preparation of the activator solution. The alkali solution is prepared at least one day prior to its use, employing distilled water to avoid contamination by unknown substances. Common molarity values investigated are in the range of 8 to 16 M.
-
Raw material mixing. One or more precursor types are mixed with the mineral aggregates (fine sand or coarse fraction), in dry conditions, for 2–3 min. The aggregate part occupies about 75–80% by mass of geopolymer concrete/mortar.
-
Activator addition. The alkaline solution is added to the solid fractions and mixed for 3–5 min. To improve the rheology of the compound, in terms of workability, a part of water-reducing admixture could be added to the mixture. Firstly, the admixture is mixed with the alkali solution and then added to the solid dry material.
-
Casting. The fresh material is cast in specific molds (cylinder, cubes, or beams) kept under vibration for 10 s.
-
Curing. The specimen curing can be performed under various temperature and time regimes. The material hardening can be completed entirely at room temperature or provide oven treatments between 30 °C to 90 °C, accelerating the curing. Typical treatment times are selected in the range from 6 h to 96 h.
As clearly discussed above, the production of geopolymer cements involves a considerable number of process variables. The microstructure and the physical, mechanical, chemical, and thermal properties vary greatly depending on the nature of raw material from which they derive, the curing conditions (time and temperature), and the type and concentration of the activating alkaline solution [23].
References
- Datis Export Group. Available online: https://datis-inc.com/ (accessed on 24 December 2020).
- Hanle, L.J.; Jayaraman, K.R.; Smith, J.S. CO2 Emissions Profile of the US Cement Industry; EPA: Washington, DC, USA, 2004.
- Barker, D.J.; Turner, S.A.; Napier-Moore, P.A.; Clark, M.; Davison, J.E. CO2 capture in the cement industry. Energy Procedia 2009, 1, 87–94.
- Kääntee, U.; Zevenhoven, R.; Backman, R.; Hupa, M. Cement manufacturing using alternative fuels and the advantages of process modelling. Fuel Process. Technol. 2004, 85, 293–301.
- Geopolymer Institute. Available online: https://www.geopolymer.org/applications/global-warming (accessed on 24 December 2020).
- Hasanbeigi, A.; Price, L.; Lin, E. Emerging energy-efficiency and CO2 emission-reduction technologies for cement and concrete production: A technical review. Renew. Sustain. Energy Rev. 2012, 16, 6220–6238.
- de Lena, E.; Spinelli, M.; Gatti, M.; Scaccabarozzi, R.; Campanari, S.; Consonni, S.; Cinti, G.; Romano, M.C. Techno-economic analysis of calcium looping processes for low CO2 emission cement plants. Int. J. Greenh. Gas Control. 2019, 82, 244–260.
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2013, 51, 142–161.
- Gartner, E.; Hirao, H. A review of alternative approaches to the reduction of CO2 emissions associated with the manufacture of the binder phase in concrete. Cem. Concr. Res. 2015, 78, 126–142.
- Rahman, A.; Rasul, M.G.; Khan, M.M.K.; Sharma, S. Recent development on the uses of alternative fuels in cement manufacturing process. Fuel 2015, 145, 84–99.
- Chatziaras, N.; Psomopoulos, C.S.; Themelis, N.J. Use of waste derived fuels in cement industry: A review. Manag. Environ. Qual. Int. J. 2016, 27, 178–193.
- Rahman, A.; Rasul, M.G.; Khan, M.; Sharma, S.C. Assessment of energy performance and emission control using alternative fuels in cement industry through a process model. Energies 2017, 10, 1996.
- Fernández, J.R.; Abanades, J.C. CO2 capture from the calcination of CaCO3 using iron oxide as heat carrier. J. Clean. Prod. 2016, 112, 1211–1217.
- Gevaudan, J.P.; Osio-Norgaard, J.; Srubar, W.V., III. Alternative cements: Recent developments and future directions. In Proceedings of the AEI 2019: Integrated Building Solutions—The National Agenda, Tysons, VA, USA, 3–6 April 2019.
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243.
- Maddalena, R.; Roberts, J.J.; Hamilton, A. Can Portland cement be replaced by low-carbon alternative materials? A study on the thermal properties and carbon emissions of innovative cements. J. Clean. Prod. 2018, 186, 933–942.
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746.
- Davidovits, J. Geopolymer cement. A review. Geopolymer Inst. Tech. Pap. 2013, 21, 1–11.
- Longos, A., Jr.; Tigue, A.A.; Dollente, I.J.; Malenab, R.A.; Bernardo-Arugay, I.; Hinode, H.; Kurniawan, W.; Promentilla, M.A. Optimization of the mix formulation of geopolymer using nickel-laterite mine waste and coal fly ash. Minerals 2020, 10, 1144.
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90.
- Zhang, Z.H.; Zhu, H.J.; Zhou, C.H.; Wang, H. Geopolymer from kaolin in China: An overview. Appl. Clay Sci. 2016, 119, 31–41.
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.; Rangan, B.V. On the development of fly ash-based geopolymer concrete. Mater. J. 2004, 101, 467–472.
- Sambucci, M.; Sibai, A.; Valente, M. Recent advances in geopolymer technology. A potential eco-friendly solution in the construction materials industry: A review. J. Compos. Sci. 2021, 5, 109.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
24 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




