
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ravindra Kolhe | + 3040 word(s) | 3040 | 2021-08-18 11:20:00 | | | |
| 2 | Beatrix Zheng | + 298 word(s) | 3338 | 2021-08-19 05:38:16 | | |
Video Upload Options
Non-small cell lung cancer (NSCLC) is a major subtype of lung cancer that accounts for almost 85% of lung cancer cases worldwide. Although recent advances in chemotherapy, radiotherapy, and immunotherapy have helped in the clinical management of these patients, the survival rate in advanced stages remains dismal. Furthermore, there is a critical lack of accurate prognostic and stratification markers for emerging immunotherapies. To harness immune response modalities for therapeutic benefits, a detailed understanding of the immune cells in the complex tumor microenvironment (TME) is required. Among the diverse immune cells, natural killer (NK cells) and dendritic cells (DCs) have generated tremendous interest in the scientific community. NK cells play a critical role in tumor immunosurveillance by directly killing malignant cells. DCs link innate and adaptive immune systems by cross-presenting the antigens to T cells. The presence of an immunosuppressive milieu in tumors can lead to inactivation and poor functioning of NK cells and DCs, which results in an adverse outcome for many cancer patients, including those with NSCLC. Recently, clinical intervention using modified NK cells and DCs have shown encouraging response in advanced NSCLC patients. Herein, we will discuss prognostic and predictive aspects of NK cells and DC cells with an emphasis on NSCLC. Additionally, the discussion will extend to potential strategies that seek to enhance the anti-tumor functionality of NK cells and DCs.
1. Introduction
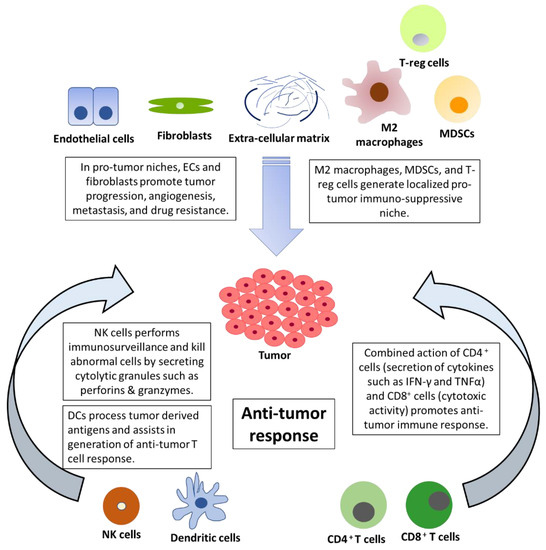
The tumor microenvironment is composed of malignant cells along with fibroblasts, extracellular matrix (ECM), endothelial cells, adipocytes, and immune cells [1] ( Figure 1 ). Tumors can be divided into two major immunological subtypes: hot (high T cell activity) and cold (lack of T cell priming/activation) based on inflammatory cytokine profile [2]. In the cancer progression cycle, excessive secretion of PD-L1 in the tumor and enhanced PD-1 signaling inactivates T cells leading to tumor growth and metastasis [3]. The use of novel immunotherapeutic agents, such as nivolumab (anti-PD-1), pembrolizumab (anti-PD-1), and atezolizumab (anti PD-L1), has led to the improved overall survival of NSCLC patients compared to treatment with docetaxel [4][5][6]. Further, the combination of cytotoxic therapy and immunotherapy has shown synergistic improvements in the anti-tumor response. The combination of pemetrexed (folate antimetabolites), carboplatin (platinum-based antineoplastic drug), and pembrolizumab (anti-PD-1) led to the improvement of the objective response rate and progression-free survival [7][8]. This striking difference was exhibited in the benefits of immunotherapy, as hot tumors show a higher response rate to immunotherapy. The immune checkpoint blockade (ICB) is effective in patients with expression of PD-L1 or high TMB (tumor mutation burden), but there are different approaches required to target cold tumors. TMB is the number of nonsynonymous coding mutations per million bases (Mb) and is a predictive biomarker for ICB therapy efficacy. It is associated with increased secretion of neoantigens and cytotoxic T cell activity in several cancers [9][10]. There is increased interest in exploring strategies to convert cold tumors to hot tumors in an attempt to increase immunotherapy benefits [11]. Recent breakthroughs in the form of personalized RNA mutanome vaccine based on individuals’ genomic information activates lymph-node-DCs and can generate potent T cell response [12]. The ICBs have remarkably improved survival but their benefits are achieved only in a minority of patients. Furthermore, the defects in antigen presentation in the form of reduced expression of HLA class I or loss of B2M can cause resistance to checkpoint inhibitors [13]. Additionally, IFN-γ signaling and corresponding higher production of IDO1 (Indoleamine 2, 3-dioxygenase 1) can also lead to poor response to checkpoint inhibitors [13][14][15]. The IDO expression is promoted by pro-inflammatory stimuli generated by IFN-γ and might result in a suboptimal anti-tumor immune response in cancer patients. Furthermore, APCs with IDO production can activate and promote the production of immunosuppressive regulatory T cells [15]. Despite several therapeutic challenges at the tumor level, recent advancements in the form of adoptive T-cell therapy have shown a durable response in cancer patients. There are several difficulties in its clinical application due to tumor heterogeneity, fewer neoantigens, systemic cytokine toxicities, and challenges associated with the production of cells in compliance with GMP (Good Manufacturing Practice) [16][17]. Recently, interest in NK cells has emerged as a promising alternative due to their killing abilities and as a safer alternative to adoptive T cell therapy because of its lower immune-related adverse events [18]. In this review article, we have discussed the therapeutic and prognostic benefits of NK cells and DCs with a focus on NSCLC.
2. The Emerging Role of NK Cells in the Tumor Microenvironment and Immunotherapy
The process of development and maturation of NK cells makes them competent to identify and kill host cells with aberrant expression of MHC class I molecules (called human leukocyte antigen—HLA) in humans [19]. During NK cell maturation, the interaction between inhibitory killer cell immunoglobulin-like receptors (KIRs) and HLA provides functional competency to NK cells. This process termed ‘licensing’ suppresses NK cell function in the presence of intact MHC and minimizes the destruction of healthy cells [20]. This suppression is eliminated in the presence of downregulated or altered MHC expression as in tumor cells [21]. Aberrant cells can also be killed by antibody-dependent cell cytotoxicity (ADCC), whereby NK cells bind to the Fc region of a tumor cell-bound antibody [22]. The mechanism of killing by NK cells lies in part in their appearance as ‘Large granular lymphocytes’ [23]. When a susceptible cell is identified by the NK cells, specific lytic granules converge toward the immunological synapse through microtubules [24]. There are two major components in these lytic granules—perforins and granzymes. Perforins are cytolytic proteins that are inserted into the plasma membrane and leads to osmotic lysis in a Ca 2+ -dependent manner [21][25]. Perforins are found to play a critical role in controlling tumor metastasis [26][27]. Granzymes are serine proteases that activate caspase signalling and leads to apoptosis of the target cell [28]. Natural killer cells can kill more than a single cell through their degranulation process. NK cells form multiple contacts with target cells and can sequentially kill several abnormal cells in a time-dependent manner [29]. NK cells express a high mRNA pool of granzymes and perforins that are rapidly translated to protein when required [30]. Interestingly, upon a single encounter, an NK cell releases only one-tenth of its cytotoxic lytic granules but it has been determined that even a single granule is sufficient to induce target cell death [25]. NK cells have been shown to shift from fast GrzB-mediated cell death to slow death receptor-mediation killing in the later stages and can serially kill up to 30+ tumor cells [31].
NK cells play an important role in tumor immunosurveillance, which is emphasized by the association between NK cell deficiency and cancer [32][33]. In a large prospective study with 11 years of follow-up period, patients with medium or high NK cell cytotoxic activity were found to be associated with a lower risk of cancer. In this study, NK effector cells isolated from peripheral blood were used to measure specific lysis of target cells (K562, leukemia cell line) using the 51Cr-release assay [34]. In a recent meta-analysis, infiltration of NK cells was found to be associated with better overall survival in solid tumors [35]. NK cells express heparinase to invade primary tumors, and therefore, affect tumor progression [36]. Furthermore, NK cells can also impact metastasis by eliminating circulating tumor cells [37]. There are several ways through which the function of NK cells is negatively affected in an immune-suppressive milieu of the immune-evasive tumor. The enriched metabolites of the kynurenine pathway contribute to the immune escape of cancer cells [38]. This escape is fueled by the secretion of kynurenine by cancer cells, which subsequently leads to immune tolerance in the tumor microenvironment, induction of immunosuppressive T-regulatory cells, and blockade of IL-2 [38]. In the presence of TGF-β, the gene expression profile of NK cells shifts toward lower cytotoxicity. Activin-A binds to type I activin receptor ALK4, which is present on NK cells and suppresses their metabolism [39]. Furthermore, at the metabolic level, impaired glycolysis in NK cells due to overexpression of fructose 1,6-bisphosphatase impairs NK cell activity [40]. In this study, NK cells were shown to prevent tumor initiation in lung cancer but failed to prevent tumor progression due to the metabolic dysfunctional state of NK cells [40]. Further, low levels of nutrients such as glucose and a hypoxic environment suppress the anti-tumor activity of NK cells [41]. Additionally, in the TME, a high concentration of lactate and low pH can also impair NK cells [41][42].
3. The Emerging Role of Dendritic Cells in the Tumor Microenvironment and Immunotherapy
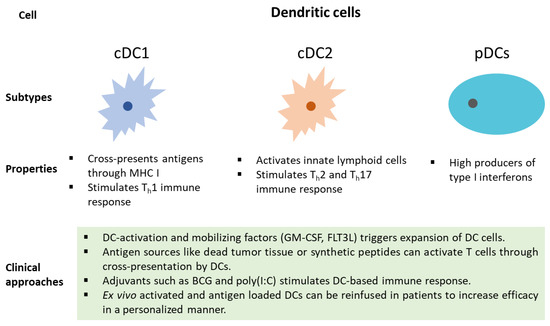
DCs (Dendric cells) play an important role in the initiation, development, and maintenance of the anti-tumor immune response. Dying cells release damage-associated molecular patterns (DAMPs), which are predominantly intracellular proteins and induce the production of cytokines and activation of T cells [43]. Upon uptake of antigens, DCs undergo maturation and migrate to lymph nodes, where they present the antigens to CD8+ T cells [44]. DCs are broadly divided into two classes, conventional type 1 DCs (cDC1s) and conventional type 2 DCs (cDC2s). cDC1s functions primarily by cross-presenting antigens to CD8+ T cells. In contrast, cDC2s function by priming CD4+ T cell response [45]. Furthermore, based on transcriptional and chromatin variations, cDC2s have been divided into anti-inflammatory cDC2A (T-bet + ) and pro-inflammatory cDC2B (T-bet - ) [46]. cDC1s are essential for mounting an anti-tumor immune response. cDC1s infiltration has been found to positively correlate with T cell infiltration and increased survival. Tumor evasi strategies include the prevention of cDC1 infiltration into the tumor microenvironment [47]. cDC1 binds to F-actin exposed necrotic bodies through C-type lectin receptor DNGR-1. This process leads to the uptake and cross-presentation of antigens from dead cell debris to initiate CD8+ T cell response [48]. Recently, tumor-secreted gelsolin (sGSN) has been found to dampen the immune response by impairing the DNGR-1-dependent cross-presentation in cDC1 [49]. The third class of DCs is known as plasmacytoid dendritic cells (pDC), with appearances like plasma cells and characteristic production of high levels of interferon-α [50]. Tumor-promoting features of aberrant pDCs with poor production of type-I IFN and T-reg differentiation were displayed in patients with breast and ovarian cancer [51][52]. The molecular basis of the differentiation of DCs in the tumor microenvironment is an active area of research and may hold promise in the development of future immunotherapies.
Increased infiltration, expansion, and activation of cDC1s play an important role in the immune control of tumors and response to immunotherapies [47][53]. Interestingly, the DC gene signature was found to be associated with improved overall survival in NSCLC patients treated with tezolizumab (PD-L1 blockade) [54]. There are two pre-dominant receptors for PDL-1: PD-1 and B7.1. In an interesting study, the expression of PDL-1 was found to be significantly higher in DCs present in TME and circulation of cancer patients. The blockage of PD-L1 relieved B7.1 that in turn interacted with CD28 to enhance the priming of T cells [54]. Additionally, dendritic cells are also essential in the reactivation of circulating memory T cells [55]. In another study, anti-PD-1 immunotherapy was found to depend on IL-12-secreting DCs in the presence of IFN-γ-secreting T cells [56]. Several factors prevent the anti-tumor effect of DCs in the complex microenvironment of the tumor. The recruitment of cDCs is sparse in the tumor microenvironment in early-stage tumors compared to adjacent normal tissue. The presence of NK cells was found to be significantly reduced in lung adenocarcinoma patients. NK cells in these tumors showed poor cytolytic capacity due to the lower expression of granzyme B, CD57, and IFN-γ [57]. This mechanism could in part be responsible for preventing the anti-tumor immune response [57]. Furthermore, the WNT/β-catenin pathway in tumors can partly prevent the infiltration of cDCs and T cells. Activation of this pathway in the tumor impedes the expression of CCL4, which reduces infiltration of DCs in the tumor. The resulting reduced CXCL10 limits CD8+ T cells and leads to faulty cross-priming [58]. Moreover, the presence of prostanoid lipids leads to the expansion of tumor growth, migration, invasion, and immunosuppression [59]. Necrosis in the tumor releases prostaglandin E2 (PGE 2) and it has been shown to prevent the immunostimulatory activity of DCs [60]. The overexpression of COX1 and 2 Cyclooxygenase (COX) and production of PGE 2 in the hypoxic microenvironment prevent the accumulation and activation of cDCs and assists in immune evasion [61][62]. The presence of Vascular endothelial growth factor (VEGF) in the tumor microenvironment is also a suppressing factor of DCs, as it adversely affects functionality [63][64]. Recently, a new subset of DCs, ‘mature DCs, enriched in immunoregulatory molecules’ (mregDCs), with an immunoregulatory gene signature ( Cd274 , Cd200 , and Pdcd1lg2 ), has been identified [65]. These cDCs continue with the uptake of antigens, but do not stimulate T cell activation in lymph nodes, blocking the trajectory of inflammation. DCs with similar regulatory gene signatures have been identified in normal tissue, which hints at its role in the maintenance of homeostasis. Furthermore, the process of cross-presentation can itself be impaired in the tumor tissue due to the lack of tumor-infiltrating DCs with activating potential [65][66]. Increased production of oxidized lipids in DC adversely affects the cross-presentation process [67][68][69]. One of the reasons for this could be the increased uptake of lipids due to higher expression of scavenging receptor MSR1 in DCs [67]. In another study, infiltrated DCs exhibited ER stress and expression of ER stress response factor XBP1 promoted primary and metastatic ovarian cancer [68]. It has also been reported that DCs with increased lipid content failed to effectively present antigens or stimulate T cells. It was found that oxidized lipids sequester chaperone HSP70; thus, preventing the MHC-peptide complex from reaching the cell surface [69].
Despite these challenges, there are promising approaches that could assist in the expansion of DCs as a central player in future therapeutic strategies. In pre-clinical models, poly I:C (TLR-3, MDA5, and RIG-I. agonist) treatment led to increased IFNα/β-related transcriptomic profile, and increased infiltration of dendritic cells and T cell in the melanoma mouse model [70]. The intra-tumoral activation of STING pathway was found to initiate an immune response and led to regression of established tumors. It was found that STING agonists led to the maturation of DCs and the production of cytokines and chemokines [71]. Modified amidobenzimidazole (ABZI)-based compounds have also been developed to enhance the STING pathway [72]. Also, VEGF blockade therapy through anti-VEGF antibody has been shown to stimulate DCs and T cells to enhance tumor immunity [73]. Infiltration of DCs led to an improved response to checkpoint inhibitor immunotherapy and the administration of tumor-stroma-directed CCL4 administration through the intravenous method led to increased infiltration of DCs and CD8+ T cells even in poor responders to checkpoint inhibitor (CPI) immunotherapy [74]. cDC1 abundance has been reported to be associated with checkpoint blockade immunotherapy [75][76]. Overall, the composition of the TME and infiltrating immune cells play a critical role in determining the efficacy of Checkpoint inhibitors [77]. There is significant interest in the identification of the immune cells and their associated variables that determine the responsiveness to immunotherapy.
Many clinical approaches can be utilized to harness the potential of DCs in cancer patients ( Figure 2 ). Dendritic cell mobilizing factors, such as GM-CSF and FLT3L, can lead to the expansion of DC cells [78]. Additionally, DC-mediated T cell activation through the presentation of antigens and synthetic peptides can lead to an anti-tumor immune response [79]. Furthermore, DC response can be stimulated using adjuvants such as BCG and poly(I:C) [80]. In a personalized approach, ex vivo activated and antigen-loaded DCs can be reinfused in cancer patients [79]. Recently, DCs pulsed with survivin and MUC1 showed promise in resected NSCLC [81]. Advances in the prognostic roles of DCs in NSCLC have hinted at its critical role in the TME ( Table 1 ). DCs are positively correlated with the progression-free survival of NSCLC patients [82]. The expression of individual genes, such as TOP2A and TLR3, and multiple gene signatures have been associated with DC infiltration in NSCLC [83][84][85]. These recent findings related to gene expression signatures and infiltration of DCs in NSCLC will assist in the design of effective strategies for patients with refractory cancer.
| S. No. | The Theme of the Study | Clinical Significance | Reference |
|---|---|---|---|
| 1 | Autologous modified DC vaccine | DC vaccine pulsed with MUC1, survivin, and flagellin was well tolerated and induced anti-tumor activity. | [81] |
| 2 | Prognostic significance of pDCs and mDCs | There was a significant reduction of plasmacytoid dendritic cells (pDCs) and monocytic dendritic cells (mDCs) in NSCLC patients. The overall survival was negatively correlated to mDCs but positively correlated with pDCs. | [86] |
| 3 | Prognostic association of DCs | DCs showed significant association with PFS and disease stage in NSCLC patients. | [82] |
| 4 | MYEOV gene expression was found to be prognostically significant | The expression of the myeloma overexpressed gene (MYEOV) was found to be associated with poorer overall survival and increased infiltration of immune cells, including DCs. | [87] |
| 5 | TP53 mutations were associated with poor survival | There was higher infiltration of immune cells, including CD8+ T cells and DCs in TP53-mutated lung cancer tissues. | [88] |
| 6 | TLR3 expression was prognostically significant in NSCLC patients | The presence of the TLR3-CD1-3+ Dendritic cell axis and corresponding activation of CD8+ T cells was found to be associated with improved overall survival. | [83] |
| 7 | 5-gene prognostic signature | There was higher infiltration of DCs in the lower risk group compared to a higher risk group. | [85] |
| 8 | Identification of differentially expressed genes | TOP2A expression was found to be significantly associated with the infiltration of DCs. | [84] |
| 9 | Association of DCs with anti-tumor immunity | Patients with the non-metastatic disease had higher infiltration of dendritic cells. | [89] |
4. NK Cells and DCs: Prospects in NSCLC
In NSCLC, treatment strategies involved in the management of cancer patients range from conventional chemotherapy regimens to newly approved immunotherapeutic agents [90]. Despite its recent success, several challenges associated with immunotherapy include lack of consistent response, lack of predictive biomarkers, risk of immune-related adverse effects, and resistance to immunotherapy [91]. NK cells and DCs have started to emerge as critical players in the development of a newer generation of therapeutic strategies. For example, monoclonal antibodies targeting NK inhibitory receptors and IL-15 can activate killer properties of NK cells [92]. In a recent clinical trial, patients administered with Pembrolizumab plus NK cell therapy showed improved survival in advanced NSCLC cases. In these patients, infusion of NK cells led to increased circulation of NK cells in the blood and enhancement of cellular immune functions. Interestingly, this combinatorial therapy also led to a reduction in circulating tumor cells [93]. The role of NK cells as a promising therapeutic modality is actively being investigated. Another strategy explored the clinical potential of DC cells as a vaccine in NSCLC patients. In this trial, DCs were silenced for SOCS1 expression (to prevent negative regulation of DCs), pulsed with survivin and MUC1 (heavily expressed proteins in NSCLC tumor), and flagellin (immune stimulant) [81][94]. It was shown that the administration of DCs vaccine led to a reduction in tumor markers and improved the quality of life in cancer patients [81]. In another trial, the efficacy of the adenoviral vector with the CCL21 gene (Ad-CCL21-DC) was explored in stage III/IV NSCLC patients [95]. Higher expression of CCL21 attracts T cells and DCs through interaction with CXCR3 CCR7 receptors [95][96]. In mouse models, CCL21 treatment led to higher infiltration of DCs CD4+ and CD8+ in tumor [97]. Administration of intra-tumoral vaccination led to enhanced infiltration of CD8+ T cell, increased expression of PD-L1 in tumors, and induction of immune response against tumor antigen [95]. In lung tumors, higher infiltration of mature dendritic cells was correlated with the influx of effector T cells [98]. This study identified the presence of tertiary lymphoid structures enriched in DCs, T h 1 subtype and cytotoxic properties with improved prognostic outcomes in the tumor microenvironment [98]. In addition to the therapeutic benefits of NK and DCs, gene signatures associated with the infiltration of these cells can provide vital prognostic and predictive biomarkers [99][100][82][85][89]. Encouragingly, the preliminary results of clinical trials involving NKs and DCs are most likely to play a key role in the design and application of future immunotherapies.
References
- Ribeiro Franco, P.I.; Rodrigues, A.P.; de Menezes, L.B.; Pacheco Miguel, M. Tumor microenvironment components: Allies of cancer progression. Pathol. Res. Pract. 2020, 216, 152729.
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168.
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10.
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639.
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Perez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550.
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265.
- Langer, C.J.; Gadgeel, S.M.; Borghaei, H.; Papadimitrakopoulou, V.A.; Patnaik, A.; Powell, S.F.; Gentzler, R.D.; Martins, R.G.; Stevenson, J.P.; Jalal, S.I.; et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016, 17, 1497–1508.
- Hagner, N.; Joerger, M. Cancer chemotherapy: Targeting folic acid synthesis. Cancer Manag. Res. 2010, 2, 293–301.
- Halbert, B.; Einstein, D.J. Hot or Not: Tumor Mutational Burden (TMB) as a Biomarker of Immunotherapy Response in Genitourinary Cancers. Urology 2021, 147, 119–126.
- Hamilton, G.; Rath, B. Immunotherapy for small cell lung cancer: Mechanisms of resistance. Expert Opin. Biol. Ther. 2019, 19, 423–432.
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618.
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.P.; Simon, P.; Lower, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrors, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226.
- Gettinger, S.; Choi, J.; Hastings, K.; Truini, A.; Datar, I.; Sowell, R.; Wurtz, A.; Dong, W.; Cai, G.; Melnick, M.A.; et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov. 2017, 7, 1420–1435.
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829.
- Holmgaard, R.B.; Zamarin, D.; Munn, D.H.; Wolchok, J.D.; Allison, J.P. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J. Exp. Med. 2013, 210, 1389–1402.
- Morotti, M.; Albukhari, A.; Alsaadi, A.; Artibani, M.; Brenton, J.D.; Curbishley, S.M.; Dong, T.; Dustin, M.L.; Hu, Z.; McGranahan, N.; et al. Promises and challenges of adoptive T-cell therapies for solid tumours. Br. J. Cancer 2021, 124, 1759–1776.
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020, 17, 147–167.
- Gun, S.Y.; Lee, S.W.L.; Sieow, J.L.; Wong, S.C. Targeting immune cells for cancer therapy. Redox Biol. 2019, 25, 101174.
- Karre, K.; Ljunggren, H.G.; Piontek, G.; Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 1986, 319, 675–678.
- Elliott, J.M.; Yokoyama, W.M. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol. 2011, 32, 364–372.
- Chiossone, L.; Dumas, P.Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688.
- Giles, A.J.; Hao, S.; Padget, M.; Song, H.; Zhang, W.; Lynes, J.; Sanchez, V.; Liu, Y.; Jung, J.; Cao, X.; et al. Efficient ADCC killing of meningioma by avelumab and a high-affinity natural killer cell line, haNK. JCI Insight 2019, 4.
- Timonen, T.; Ortaldo, J.R.; Herberman, R.B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J. Exp. Med. 1981, 153, 569–582.
- Orange, J.S. Formation and function of the lytic NK-cell immunological synapse. Nat. Rev. Immunol. 2008, 8, 713–725.
- Gwalani, L.A.; Orange, J.S. Single Degranulations in NK Cells Can Mediate Target Cell Killing. J. Immunol. 2018, 200, 3231–3243.
- Smyth, M.J.; Thia, K.Y.; Cretney, E.; Kelly, J.M.; Snook, M.B.; Forbes, C.A.; Scalzo, A.A. Perforin is a major contributor to NK cell control of tumor metastasis. J. Immunol. 1999, 162, 6658–6662.
- Kodama, T.; Takeda, K.; Shimozato, O.; Hayakawa, Y.; Atsuta, M.; Kobayashi, K.; Ito, M.; Yagita, H.; Okumura, K. Perforin-dependent NK cell cytotoxicity is sufficient for anti-metastatic effect of IL-12. Eur. J. Immunol. 1999, 29, 1390–1396.
- Bots, M.; Medema, J.P. Granzymes at a glance. J. Cell Sci. 2006, 119, 5011–5014.
- Bhat, R.; Watzl, C. Serial killing of tumor cells by human natural killer cells--enhancement by therapeutic antibodies. PLoS ONE 2007, 2, e000326.
- Fehniger, T.A.; Cai, S.F.; Cao, X.; Bredemeyer, A.J.; Presti, R.M.; French, A.R.; Ley, T.J. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity 2007, 26, 798–811.
- Prager, I.; Liesche, C.; van Ooijen, H.; Urlaub, D.; Verron, Q.; Sandstrom, N.; Fasbender, F.; Claus, M.; Eils, R.; Beaudouin, J.; et al. NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J. Exp. Med. 2019, 216, 2113–2127.
- Orange, J.S. Natural killer cell deficiency. J. Allergy Clin. Immunol. 2013, 132, 515–525.
- Morvan, M.G.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19.
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799.
- Nersesian, S.; Schwartz, S.L.; Grantham, S.R.; MacLean, L.K.; Lee, S.N.; Pugh-Toole, M.; Boudreau, J.E. NK cell infiltration is associated with improved overall survival in solid cancers: A systematic review and meta-analysis. Transl. Oncol. 2021, 14, 100930.
- Putz, E.M.; Mayfosh, A.J.; Kos, K.; Barkauskas, D.S.; Nakamura, K.; Town, L.; Goodall, K.J.; Yee, D.Y.; Poon, I.K.; Baschuk, N.; et al. NK cell heparanase controls tumor invasion and immune surveillance. J. Clin. Investig. 2017, 127, 2777–2788.
- Lopez-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154.
- Routy, J.P.; Routy, B.; Graziani, G.M.; Mehraj, V. The Kynurenine Pathway Is a Double-Edged Sword in Immune-Privileged Sites and in Cancer: Implications for Immunotherapy. Int. J. Tryptophan Res. 2016, 9, 67–77.
- Rautela, J.; Dagley, L.F.; de Oliveira, C.C.; Schuster, I.S.; Hediyeh-Zadeh, S.; Delconte, R.B.; Cursons, J.; Hennessy, R.; Hutchinson, D.S.; Harrison, C.; et al. Therapeutic blockade of activin-A improves NK cell function and antitumor immunity. Sci. Signal. 2019, 12, eaat7527.
- Cong, J.; Wang, X.; Zheng, X.; Wang, D.; Fu, B.; Sun, R.; Tian, Z.; Wei, H. Dysfunction of Natural Killer Cells by FBP1-Induced Inhibition of Glycolysis during Lung Cancer Progression. Cell Metab. 2018, 28, 243–255.e5.
- O’Brien, K.L.; Finlay, D.K. Immunometabolism and natural killer cell responses. Nat. Rev. Immunol. 2019, 19, 282–290.
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671.
- Jang, G.Y.; Lee, J.W.; Kim, Y.S.; Lee, S.E.; Han, H.D.; Hong, K.J.; Kang, T.H.; Park, Y.M. Interactions between tumor-derived proteins and Toll-like receptors. Exp. Mol. Med. 2020, 52, 1926–1935.
- Roberts, E.W.; Broz, M.L.; Binnewies, M.; Headley, M.B.; Nelson, A.E.; Wolf, D.M.; Kaisho, T.; Bogunovic, D.; Bhardwaj, N.; Krummel, M.F. Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 2016, 30, 324–336.
- Dudziak, D.; Kamphorst, A.O.; Heidkamp, G.F.; Buchholz, V.R.; Trumpfheller, C.; Yamazaki, S.; Cheong, C.; Liu, K.; Lee, H.W.; Park, C.G.; et al. Differential antigen processing by dendritic cell subsets in vivo. Science 2007, 315, 107–111.
- Brown, C.C.; Gudjonson, H.; Pritykin, Y.; Deep, D.; Lavallee, V.P.; Mendoza, A.; Fromme, R.; Mazutis, L.; Ariyan, C.; Leslie, C.; et al. Transcriptional Basis of Mouse and Human Dendritic Cell Heterogeneity. Cell 2019, 179, 846–863.e24.
- Bottcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e14.
- Canton, J.; Blees, H.; Henry, C.M.; Buck, M.D.; Schulz, O.; Rogers, N.C.; Childs, E.; Zelenay, S.; Rhys, H.; Domart, M.C.; et al. The receptor DNGR-1 signals for phagosomal rupture to promote cross-presentation of dead-cell-associated antigens. Nat. Immunol. 2021, 22, 140–153.
- Giampazolias, E.; Schulz, O.; Lim, K.H.J.; Rogers, N.C.; Chakravarty, P.; Srinivasan, N.; Gordon, O.; Cardoso, A.; Buck, M.D.; Poirier, E.Z.; et al. Secreted gelsolin inhibits DNGR-1-dependent cross-presentation and cancer immunity. Cell 2021, 184, 4016–4031.e22.
- Reizis, B. Plasmacytoid Dendritic Cells: Development, Regulation, and Function. Immunity 2019, 50, 37–50.
- Sisirak, V.; Faget, J.; Gobert, M.; Goutagny, N.; Vey, N.; Treilleux, I.; Renaudineau, S.; Poyet, G.; Labidi-Galy, S.I.; Goddard-Leon, S.; et al. Impaired IFN-alpha production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 2012, 72, 5188–5197.
- Labidi-Galy, S.I.; Sisirak, V.; Meeus, P.; Gobert, M.; Treilleux, I.; Bajard, A.; Combes, J.D.; Faget, J.; Mithieux, F.; Cassignol, A.; et al. Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Res. 2011, 71, 5423–5434.
- Sanchez-Paulete, A.R.; Teijeira, A.; Quetglas, J.I.; Rodriguez-Ruiz, M.E.; Sanchez-Arraez, A.; Labiano, S.; Etxeberria, I.; Azpilikueta, A.; Bolanos, E.; Ballesteros-Briones, M.C.; et al. Intratumoral Immunotherapy with XCL1 and sFlt3L Encoded in Recombinant Semliki Forest Virus-Derived Vectors Fosters Dendritic Cell-Mediated T-cell Cross-Priming. Cancer Res. 2018, 78, 6643–6654.
- Mayoux, M.; Roller, A.; Pulko, V.; Sammicheli, S.; Chen, S.; Sum, E.; Jost, C.; Fransen, M.F.; Buser, R.B.; Kowanetz, M.; et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaav7431.
- Enamorado, M.; Iborra, S.; Priego, E.; Cueto, F.J.; Quintana, J.A.; Martinez-Cano, S.; Mejias-Perez, E.; Esteban, M.; Melero, I.; Hidalgo, A.; et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8(+) T cells. Nat. Commun. 2017, 8, 16073.
- Garris, C.S.; Arlauckas, S.P.; Kohler, R.H.; Trefny, M.P.; Garren, S.; Piot, C.; Engblom, C.; Pfirschke, C.; Siwicki, M.; Gungabeesoon, J.; et al. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-gamma and IL-12. Immunity 2018, 49, 1148–1161.e7.
- Lavin, Y.; Kobayashi, S.; Leader, A.; Amir, E.D.; Elefant, N.; Bigenwald, C.; Remark, R.; Sweeney, R.; Becker, C.D.; Levine, J.H.; et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 2017, 169, 750–765.e17.
- Li, X.; Xiang, Y.; Li, F.; Yin, C.; Li, B.; Ke, X. WNT/beta-Catenin Signaling Pathway Regulating T Cell-Inflammation in the Tumor Microenvironment. Front. Immunol. 2019, 10, 2293.
- Wang, D.; Dubois, R.N. Eicosanoids and cancer. Nat. Rev. Cancer 2010, 10, 181–193.
- Hangai, S.; Ao, T.; Kimura, Y.; Matsuki, K.; Kawamura, T.; Negishi, H.; Nishio, J.; Kodama, T.; Taniguchi, T.; Yanai, H. PGE2 induced in and released by dying cells functions as an inhibitory DAMP. Proc. Natl. Acad. Sci. USA 2016, 113, 3844–3849.
- Zelenay, S.; van der Veen, A.G.; Bottcher, J.P.; Snelgrove, K.J.; Rogers, N.; Acton, S.E.; Chakravarty, P.; Girotti, M.R.; Marais, R.; Quezada, S.A.; et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell 2015, 162, 1257–1270.
- Bonavita, E.; Bromley, C.P.; Jonsson, G.; Pelly, V.S.; Sahoo, S.; Walwyn-Brown, K.; Mensurado, S.; Moeini, A.; Flanagan, E.; Bell, C.R.; et al. Antagonistic Inflammatory Phenotypes Dictate Tumor Fate and Response to Immune Checkpoint Blockade. Immunity 2020, 53, 1215–1229.e8.
- Gabrilovich, D.I.; Chen, H.L.; Girgis, K.R.; Cunningham, H.T.; Meny, G.M.; Nadaf, S.; Kavanaugh, D.; Carbone, D.P. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996, 2, 1096–1103.
- Ohm, J.E.; Carbone, D.P. VEGF as a mediator of tumor-associated immunodeficiency. Immunol. Res. 2001, 23, 263–272.
- Maier, B.; Leader, A.M.; Chen, S.T.; Tung, N.; Chang, C.; LeBerichel, J.; Chudnovskiy, A.; Maskey, S.; Walker, L.; Finnigan, J.P.; et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature 2020, 580, 257–262.
- McDonnell, A.M.; Joost Lesterhuis, W.; Khong, A.; Nowak, A.K.; Lake, R.A.; Currie, A.J.; Robinson, B.W. Restoration of defective cross-presentation in tumors by gemcitabine. Oncoimmunology 2015, 4, e1005501.
- Herber, D.L.; Cao, W.; Nefedova, Y.; Novitskiy, S.V.; Nagaraj, S.; Tyurin, V.A.; Corzo, A.; Cho, H.I.; Celis, E.; Lennox, B.; et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat. Med. 2010, 16, 880–886.
- Cubillos-Ruiz, J.R.; Silberman, P.C.; Rutkowski, M.R.; Chopra, S.; Perales-Puchalt, A.; Song, M.; Zhang, S.; Bettigole, S.E.; Gupta, D.; Holcomb, K.; et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell 2015, 161, 1527–1538.
- Veglia, F.; Tyurin, V.A.; Mohammadyani, D.; Blasi, M.; Duperret, E.K.; Donthireddy, L.; Hashimoto, A.; Kapralov, A.; Amoscato, A.; Angelini, R.; et al. Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer. Nat. Commun. 2017, 8, 2122.
- Aznar, M.A.; Planelles, L.; Perez-Olivares, M.; Molina, C.; Garasa, S.; Etxeberria, I.; Perez, G.; Rodriguez, I.; Bolanos, E.; Lopez-Casas, P.; et al. Immunotherapeutic effects of intratumoral nanoplexed poly I:C. J. Immunother. Cancer 2019, 7, 116.
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.R.; Lemmens, E.; Banda, T.; Leong, J.J.; et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015, 11, 1018–1030.
- Ramanjulu, J.M.; Pesiridis, G.S.; Yang, J.; Concha, N.; Singhaus, R.; Zhang, S.Y.; Tran, J.L.; Moore, P.; Lehmann, S.; Eberl, H.C.; et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature 2018, 564, 439–443.
- Osada, T.; Chong, G.; Tansik, R.; Hong, T.; Spector, N.; Kumar, R.; Hurwitz, H.I.; Dev, I.; Nixon, A.B.; Lyerly, H.K.; et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol. Immunother. 2008, 57, 1115–1124.
- Williford, J.M.; Ishihara, J.; Ishihara, A.; Mansurov, A.; Hosseinchi, P.; Marchell, T.M.; Potin, L.; Swartz, M.A.; Hubbell, J.A. Recruitment of CD103(+) dendritic cells via tumor-targeted chemokine delivery enhances efficacy of checkpoint inhibitor immunotherapy. Sci. Adv. 2019, 5, eaay1357.
- Barry, K.C.; Hsu, J.; Broz, M.L.; Cueto, F.J.; Binnewies, M.; Combes, A.J.; Nelson, A.E.; Loo, K.; Kumar, R.; Rosenblum, M.D.; et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 2018, 24, 1178–1191.
- Michea, P.; Noel, F.; Zakine, E.; Czerwinska, U.; Sirven, P.; Abouzid, O.; Goudot, C.; Scholer-Dahirel, A.; Vincent-Salomon, A.; Reyal, F.; et al. Adjustment of dendritic cells to the breast-cancer microenvironment is subset specific. Nat. Immunol. 2018, 19, 885–897.
- Li, J.; Byrne, K.T.; Yan, F.; Yamazoe, T.; Chen, Z.; Baslan, T.; Richman, L.P.; Lin, J.H.; Sun, Y.H.; Rech, A.J.; et al. Tumor Cell-Intrinsic Factors Underlie Heterogeneity of Immune Cell Infiltration and Response to Immunotherapy. Immunity 2018, 49, 178–193.e7.
- Saxena, M.; Balan, S.; Roudko, V.; Bhardwaj, N. Towards superior dendritic-cell vaccines for cancer therapy. Nat. Biomed. Eng. 2018, 2, 341–346.
- Sabado, R.L.; Balan, S.; Bhardwaj, N. Dendritic cell-based immunotherapy. Cell Res. 2017, 27, 74–95.
- O’Keeffe, M.; Mok, W.H.; Radford, K.J. Human dendritic cell subsets and function in health and disease. Cell Mol. Life Sci. 2015, 72, 4309–4325.
- Ge, C.; Li, R.; Song, H.; Geng, T.; Yang, J.; Tan, Q.; Song, L.; Wang, Y.; Xue, Y.; Li, Z.; et al. Phase I clinical trial of a novel autologous modified-DC vaccine in patients with resected NSCLC. BMC Cancer 2017, 17, 884.
- Wang, Y.; Zhao, N.; Wu, Z.; Pan, N.; Shen, X.; Liu, T.; Wei, F.; You, J.; Xu, W.; Ren, X. New insight on the correlation of metabolic status on (18)F-FDG PET/CT with immune marker expression in patients with non-small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1127–1136.
- Bianchi, F.; Alexiadis, S.; Camisaschi, C.; Truini, M.; Centonze, G.; Milione, M.; Balsari, A.; Tagliabue, E.; Sfondrini, L. TLR3 Expression Induces Apoptosis in Human Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2020, 21, 1440.
- Wang, K.; Chen, R.; Feng, Z.; Zhu, Y.M.; Sun, X.X.; Huang, W.; Chen, Z.N. Identification of differentially expressed genes in non-small cell lung cancer. Aging 2019, 11, 11170–11185.
- Li, J.; Wang, H.; Li, Z.; Zhang, C.; Zhang, C.; Li, C.; Yu, H.; Wang, H. A 5-Gene Signature Is Closely Related to Tumor Immune Microenvironment and Predicts the Prognosis of Patients with Non-Small Cell Lung Cancer. BioMed. Res. Int. 2020, 2020, 2147397.
- Zahran, A.M.; Hetta, H.F.; Mansour, S.; Saad, E.S.; Rayan, A. Reviving up dendritic cells can run cancer immune wheel in non-small cell lung cancer: A prospective two-arm study. Cancer Immunol. Immunother. 2021, 70, 733–742.
- Zhang, R.; Ma, A. High expression of MYEOV reflects poor prognosis in non-small cell lung cancer. Gene 2021, 770, 145337.
- Zhao, L.; Qu, X.; Wu, Z.; Li, Y.; Zhang, X.; Guo, W. TP53 somatic mutations are associated with poor survival in non-small cell lung cancer patients who undergo immunotherapy. Aging 2020, 12, 14556–14568.
- Minkov, P.; Gulubova, M.; Ivanova, K.; Obretenov, E.; Ananiev, J. CD11c- and CD123-positive dendritic cells in development of antitumour immunity in non-small cell lung cancer patients. Pol. J. Pathol. 2019, 70, 109–114.
- Suresh, K.; Naidoo, J.; Lin, C.T.; Danoff, S. Immune Checkpoint Immunotherapy for Non-Small Cell Lung Cancer: Benefits and Pulmonary Toxicities. Chest 2018, 154, 1416–1423.
- Qu, J.; Mei, Q.; Liu, L.; Cheng, T.; Wang, P.; Chen, L.; Zhou, J. The progress and challenge of anti-PD-1/PD-L1 immunotherapy in treating non-small cell lung cancer. Ther. Adv. Med. Oncol. 2021, 13.
- Carrega, P.; Ferlazzo, G. Natural Killers Are Made Not Born: How to Exploit NK Cells in Lung Malignancies. Front. Immunol. 2017, 8, 277.
- Lin, M.; Luo, H.; Liang, S.; Chen, J.; Liu, A.; Niu, L.; Jiang, Y. Pembrolizumab plus allogeneic NK cells in advanced non-small cell lung cancer patients. J. Clin. Investig. 2020, 130, 2560–2569.
- Jiang, T.; Chen, X.; Zhou, W.; Fan, G.; Zhao, P.; Ren, S.; Zhou, C.; Zhang, J. Immunotherapy with Dendritic Cells Modified with Tumor-Associated Antigen Gene Demonstrates Enhanced Antitumor Effect Against Lung Cancer. Transl. Oncol. 2017, 10, 132–141.
- Lee, J.M.; Lee, M.H.; Garon, E.; Goldman, J.W.; Salehi-Rad, R.; Baratelli, F.E.; Schaue, D.; Wang, G.; Rosen, F.; Yanagawa, J.; et al. Phase I Trial of Intratumoral Injection of CCL21 Gene-Modified Dendritic Cells in Lung Cancer Elicits Tumor-Specific Immune Responses and CD8(+) T-cell Infiltration. Clin. Cancer Res. 2017, 23, 4556–4568.
- Chan, V.W.; Kothakota, S.; Rohan, M.C.; Panganiban-Lustan, L.; Gardner, J.P.; Wachowicz, M.S.; Winter, J.A.; Williams, L.T. Secondary lymphoid-tissue chemokine (SLC) is chemotactic for mature dendritic cells. Blood 1999, 93, 3610–3616.
- Yang, S.C.; Batra, R.K.; Hillinger, S.; Reckamp, K.L.; Strieter, R.M.; Dubinett, S.M.; Sharma, S. Intrapulmonary administration of CCL21 gene-modified dendritic cells reduces tumor burden in spontaneous murine bronchoalveolar cell carcinoma. Cancer Res. 2006, 66, 3205–3213.
- Goc, J.; Germain, C.; Vo-Bourgais, T.K.; Lupo, A.; Klein, C.; Knockaert, S.; de Chaisemartin, L.; Ouakrim, H.; Becht, E.; Alifano, M.; et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014, 74, 705–715.
- Picard, E.; Godet, Y.; Laheurte, C.; Dosset, M.; Galaine, J.; Beziaud, L.; Loyon, R.; Boullerot, L.; Lauret Marie Joseph, E.; Spehner, L.; et al. Circulating NKp46(+) Natural Killer cells have a potential regulatory property and predict distinct survival in Non-Small Cell Lung Cancer. Oncoimmunology 2019, 8, e1527498.
- Russick, J.; Joubert, P.E.; Gillard-Bocquet, M.; Torset, C.; Meylan, M.; Petitprez, F.; Dragon-Durey, M.A.; Marmier, S.; Varthaman, A.; Josseaume, N.; et al. Natural killer cells in the human lung tumor microenvironment display immune inhibitory functions. J. Immunother. Cancer 2020, 8, 1–15.




