| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yong Chool Boo | + 2035 word(s) | 2035 | 2021-07-26 08:29:16 | | | |
| 2 | Dean Liu | Meta information modification | 2035 | 2021-08-18 04:25:10 | | |
Video Upload Options
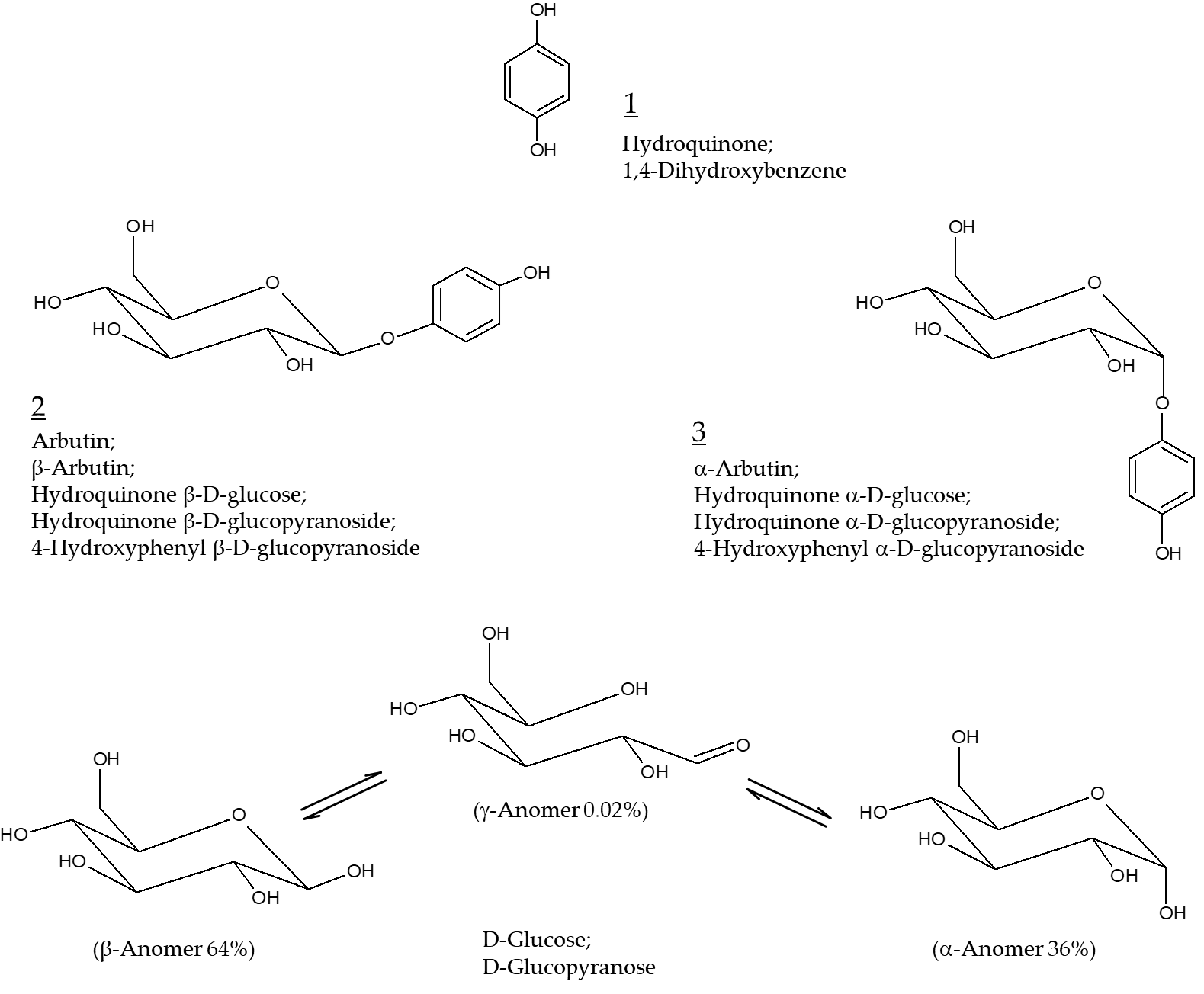
Arbutin is a compound with a structure in which one molecule of D-glucose is bound to hydroquinone.
1. Anti-Melanogenic Effect of Arbutin
Arbutin (Chemical structure 2, Figure 1) is a compound with a structure in which one molecule of D-glucose is bound to hydroquinone. β-Arbutin (this stereoisomer is called arbutin) in which the β-anomer of D-glucose is bound to hydroquinone is mainly found in plants, such as wheat, pear, and bearberry[1]. α-Arbutin (Chemical structure 3, Figure 1) is a compound of hydroquinone and the α-anomer of D-glucose[2]. Arbutin and α-arbutin have been used as hydroquinone alternatives for skin lightening purposes[3].
Figure 1. Chemical structures of hydroquinone, arbutin (β-arbutin), and α-arbutin. Anomeric structures of D-glucose are also shown for comparative purposes.
Arbutin has the effect of reducing melanin content at a concentration that has little effect on the viability of cultured human melanocytes. Maeda et al. showed that arbutin dose-dependently reduced TYR activity in human melanocytes at concentrations between 0.1 and 1.0 mM without significantly decreasing cell viability, and its inhibitory effect against cellular melanin synthesis was more potent than that of kojic acid or L-ascorbic acid when compared at a fixed concentration (0.5 mM)[4]. Akiu et al. reported that the melanin content of cultured murine melanoma B16 cells was reduced by arbutin, and the effect was explained by the decrease in intracellular TYR activity[3]. Arbutin was shown to inhibit melanin production in B16 cells stimulated by α-MSH and abrogate the hyperpigmentation effects of α-MSH in brownish guinea pig and human skin explants in organ culture experiments [5].
The decrease in TYR activity in human melanocytes by arbutin does not appear to be due to the decrease in the expression level of this enzyme. Maeda et al. reported that arbutin at 0.5 mM reduced the activity of intracellular TYR by 50% but did not affect the mRNA expression level of TYR[6]. Chakraborty et al. showed that arbutin (0.37 mM) lowered cellular melanin content but did not reduce the protein levels of TYR, TYRP-1, and TYRP-2 [7]. If so, arbutin may inhibit post-translational modification or maturation of newly synthesized TYR or may induce irreversible inactivation of already synthesized mature TYR. In an experiment in which lysates obtained from human melanocytes treated with 1.0 mM arbutin or not were analyzed by zymography, the TYR activity was reduced to 87% in the former [6]. Therefore, it is reasonable to consider arbutin as an inactivator of cellular TYR, rather than a suppressor of TYR gene expression.
In an in vitro assay using crude protein extracts derived from murine melanoma B16 cells, arbutin was shown to be able to directly inhibit the catalytic activity of TYR[3]. In the experiment with mushroom TYR, when L-DOPA was used as a substrate, arbutin showed a lower inhibitory effect than kojic acid and L-ascorbic acid: Their 50% inhibitory (IC50) values were 10 mM, 0.12 mM, and 0.2 mM, respectively[6]. In the experiment using human TYR (derived from melanocytes of Asian neonatal foreskins), when either L-tyrosine (for monophenolase activity) or L-DOPA (for diphenolase activity) were used as substrates, the IC50 values of arbutin were 5.7 mM and 18.9 mM, respectively: Arbutin appeared to be an inhibitor in a competitive relationship with L-tyrosine[6]. It is presumed that arbutin competes with a structurally similar substrate to bind to the active site of the TYR enzyme. More importantly, the concentration at which arbutin inhibits the catalytic activity of TYR in vitro is higher than the concentration that reduces cellular melanin, so there is no conviction as to whether this mechanism works in the cell.
Inoue et al. compared the effects of hydroquinone and arbutin on the differentiation of melanocytes[8]. The results showed that hydroquinone downregulated the early stage of differentiation of mouse embryonic stem cells to neural crest cells, and the late stage of differentiation to melanocytes with melanogenic capability. On the other hand, arbutin did not affect the early and late stages of differentiation of melanocytes and only suppressed elevations in TYR expression in the late stage of differentiation.
2. Pro-Melanogenic Effect of Arbutin
There is another report that conflicts with many other studies that have reported the melanin-lowering action of arbutin. Nakajima et al. observed that in cultured normal human melanocytes (from neonatal Caucasian foreskins) treated with increasing concentrations of arbutin, the pigmentation became darker (effective concentrations, 2–8 mM), whereas the viability (2–8 mM) and the TYR activity (0.5–4 mM) of the cells decreased[9]. One possible explanation for the particular observation is that arbutin increases the synthesis of intracellular melanin by acting as a substrate of TYR as discussed above. Another possibility is that although arbutin inhibits the synthesis of new melanin, it blocks the release of synthesized melanin out of the cell, thereby causing the accumulation of melanin inside cells. Melanosome transfer is a unique biological process that delivers a package of organelles from melanocytes to keratinocytes[10], and various mechanisms, such as cytophagocytosis, membrane fusion, shedding-phagocytosis, and exocytosis-endocytosis, have been proposed to explain this process[11].
3. Anti-Melanogenic Effect of α-Arbutin
Many studies have compared the inhibitory effects of arbutin (β-arbutin) and α-arbutin on TYR catalytic activity in an in vitro experiment, with inconsistent results (Table 1). Kiato et al. reported that α-arbutin inhibited monophenolase activity of mushroom TYR with potency slightly lower than arbutin or hydroquinone[12]. Funayama et al. reported that α-arbutin inhibited diphenolase activity of TYR derived from murine melanoma 10 times more potently than β-arbutin, and their IC50 values were 0.48 mM and 4.8 mM, respectively[13]. On the other hand, the inhibitory effect against mushroom TYR diphenolase was not observed in the case of α-arbutin, unlike β-arbutin (IC50, 8.4 mM). Later, Qin et al. reported that α-arbutin inhibited monophenolase activity (IC50, 4.5 mM), whereas it activated diphenolase activity of mushroom TYR[14].
Table 1. Tyrosinase inhibitory effects of arbutin and α-arbutin.
|
Literature |
Compounds |
Tyrosinase Inhibitory Effects |
Enzymes and Substrates Used |
|
|
Monophenolase Activity |
Diphenolase Activity |
|||
|
[12] |
Hydroquinone |
97.2% inhibition at 3 mM |
Mushroom tyrosinase; 0.3 mM L-tyrosine |
|
|
Arbutin |
82.0% inhibition at 3 mM |
|||
|
α-Arbutin |
72.8% inhibition at 3 mM |
|||
|
[13] |
α-Arbutin |
IC50 = 0.48 mM |
B16 mouse tyrosinase; 3.3 mM L-DOPA |
|
|
Arbutin |
IC50 = 4.8 mM |
|||
|
α-Arbutin |
No inhibition |
Mushroom tyrosinase; 0.83 mM L-DOPA |
||
|
Arbutin |
IC50 = 8.4 mM |
|||
|
[15] |
α-Arbutin |
IC50 = 8 mM |
IC50 = 8.87 mM |
Mushroom tyrosinase; 0.25 mM L-tyrosine plus 0.01 mM L-DOPA for monophenolase activity; 0.5 mM L-DOPA for diphenolase activity |
|
Arbutin |
IC50 = 0.9 mM |
IC50 = 0.7 mM |
||
Garcia-Jimenez et al. demonstrated that both α and β-arbutin are apparent competitive inhibitors against both the monophenolase and diphenolase activities of TYR [15]. In their study, IC50 values of β-arbutin for TYR monophenolase and diphenolase activities were 0.9 and 0.7 mM, respectively, which were much lower than those for α-arbutin (IC50, 8.0 and 8.87 mM for TYR monophenolase and diphenolase activities, respectively). They also kinetically characterized α and β-arbutin as substrates of TYR and obtained their Michaelis constant values of 6.5 mM and 3.0 mM, respectively, supporting that the TYR enzyme has a higher affinity for β-arbutin than α-arbutin.
As such, contradictory results have been reported regarding which arbutin or α-arbutin is a more potent TYR inhibitor. The causes for this extreme inconsistency between studies are not clear and it is only assumed that the inconsistent results may be due to differences in the origin and purity of the enzyme, the conformational state of the enzyme, the type and concentration of substrates, oxygen concentration, pH, temperature, the purity of arbutin and α-arbutin, and the possibility of hydroquinone contamination or production. A conclusion could be reached if several institutions conduct studies comparing the activity of the same substances under standardized experimental conditions.
The antimelanogenic effects of α-arbutin were reported by Sugimoto et al. [16]. They showed that α-arbutin decreased melanin content and TYR activity in cultured human melanoma cells, at a concentration below 1.0 mM, without significant effects on cell growth and TYR mRNA level. They further showed that treatment of the human skin model with α-arbutin (250 g per tissue) reduced melanin content to a 40% level of the control, without causing cell death.
4. Human Skin Depigmenting Efficacy of Arbutin
Choi et al. evaluated the depigmenting efficacy of aloesin and arbutin in a human study[17]. After irradiating the skin area of the forearm with UVR, a 10% solution of each substance was treated alone or together 4 times daily for 15 days. As a result, aleosin, arbutin, and their co-treatment reduced the UVR-induced hyperpigmentation by 34%, 43.5%, and 63.3%, respectively compared to the vehicle treatment.
A randomized, prospective, open-label study of female patients aged 26-50 years with epidermal or mixed melasma evaluated the skin-lightening effects of arbutin and ellagic acid[18]. A gel formulation containing arbutin (1%), ellagic acid (1%), or ellagic acid plus plant extract (each 1%) was applied to the face twice a day for 6 months, and the skin melanin index was measured before and after using the product. The above three gel formulations reduced the melanin index to 71% (p = 0.05), 79% (p = 0.38) and 76% (p < 0.05) levels of baseline, respectively. No evaluation of control products without an active ingredient was performed and this is a limitation of this study.
A randomized, placebo-controlled, double-blind trial involving 102 women, aged 26– 55, with melasma and solar lentigines, evaluated depigmenting efficacy of arbutin derived from Serratulae quinquefoliae[19]. The study group (n = 54) applied the cream containing the plant extract (final concentration of arbutin 2.51%) twice a day on the discolored side for 8 weeks. The results showed that the cream with the plant extract decreased melanin level in the skin pigmentation spot, compared to the control group (n = 48) applied with a placebo cream without the active ingredient. During 8 weeks of application, the melanin level of the test group decreased from 182.60 39.41 to 168.76 36.30 (p < 0.000001), and there was no significant change from 158.9 34.41 to 166.84 39.72 in the control group. Clinical improvement was observed in 75.86% of the female patients with melasma and 56.00% of the female patients with solar lentigines.
5. Antioxidant Properties of Arbutin and α-Arbutin.
Takebayashi et al. examined the antioxidant activity of arbutin compared to that of hydroquinone[20]. Arbutin was a weaker scavenger against 1,1-diphenyl-2-picrylhydrazyl radical and a more potent scavenger against 2,2′-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) cation radical compared to hydroquinone. Arbutin exerted slower but long-lasting scavenging activity against 2,2′-azobis (2-methylpropionamidine) dihydrochloride (AAPH)-derived peroxyl radical compared to hydroquinone. At 50 μM arbutin prevented AAPH-induced hemolysis of erythrocytes more effectively than hydroquinone. Arbutin (125–1000 μM) rescued skin fibroblasts exposed to AAPH, whereas hydroquinone (125 μM) exerted cytotoxicity.
Tada et al. detected hydroxyl radical generation from the TYR-catalyzed oxidations of L-tyrosine and L-DOPA, using an electron spin resonance-spin trapping technique[21]. They also observed that arbutin attenuated the hydroxyl radical generation in both reactions, suggesting that arbutin can reduce the levels of ROS derived from the melanogenic pathway. Arbutin at 500 μM was also shown to reduce intracellular hydroxyl radical production and prevent mitochondrial membrane potential loss and played an anti-apoptotic role in human lymphoma U937 cells irradiated with X-ray[22]. Arbutin attenuated the tert-butyl hydroperoxide-induced oxidative stress in human liver cancer HepG2 cell line (effective concentration, 150 μM)[23], human prostate cancer LNCaP cell line, and human fibroblasts (250 and 1000 µM)[24].
Polouliakh et al. conducted in silico comparative genomics analysis in human dermal fibroblasts treated with α-arbutin, and identified transcription factors with a potential role in tumor suppression, toxicity response, and wound healing[25]. α-Arbutin upregulated Nrf2 transcription factor which consequently activates target genes involved in antioxidant defense. Arbutin attenuated lipopolysaccharide-induced acute kidney injury in rats, by inhibiting inflammation and apoptosis via the phosphoinositide 3-kinase/protein kinase B/Nrf2 pathway[26]. Arbutin also decreased the levels of pro-inflammatory cytokines and enhanced myocardial antioxidant status, attenuating isoproterenol-induced cardiac hypertrophy in mice[27].
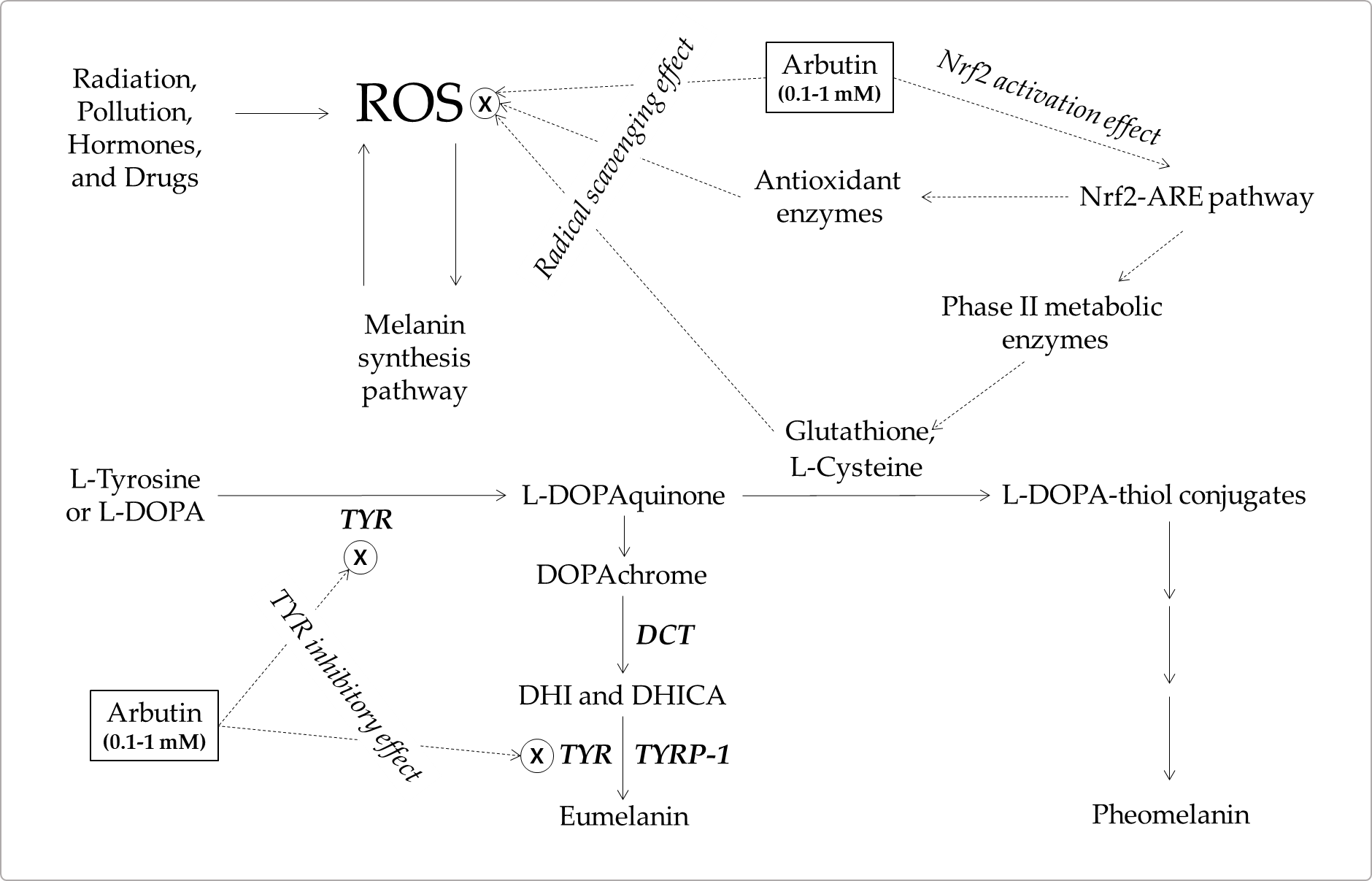
Thus, arbutin and α-arbutin may reduce ROS levels by directly scavenging free radicals or indirectly enhancing the antioxidant capacity of cells through the activation of the Nrf2-ARE pathway. These antioxidant properties may contribute to the inhibitory action of arbutin and α-arbutin on melanin synthesis in cells. The antioxidant action-based mechanism and the mechanism based on the TYR inhibitory action are not mutually exclusive and are assumed to work together for the inhibition of eumelanin synthesis (Figure 2).
Figure 2. A hypothetical mechanism for the inhibition of eumelanin synthesis by arbutin involving its tyrosinase (TYR) inhibitory and antioxidant activities. Arbutin inhibits the catalytic activity of TYR. It also scavenges reactive oxygen species (ROS) from various sources that can induce melanin synthesis, apoptosis, or tumorigenesis. It can activate the erythroid 2-associated factor 2 (Nrf2)-antioxidant responsive elements (ARE) pathway to enhance the antioxidant capacity of cells. Increased thiol compounds, such as L-cysteine and glutathione, can react with L-DOPAquinon to form DOPA-thiol conjugates, which enter the pheomelanin synthesis pathway. As a result, eumelanin synthesis via DOPAchrome can be selectively downregulated by arbutin. Inhibitory targets of arbutin are indicated with ⓧ.
References
- Xu, W.H.; Liang, Q.; Zhang, Y.J.; Zhao, P. Naturally occurring arbutin derivatives and their bioactivities. Chem Biodivers 2015, 12, 54-81.
- Saeedi, M.; Khezri, K.; Seyed Zakaryaei, A.; Mohammadamini, H. A comprehensive review of the therapeutic potential of alpha-arbutin. Phytother Res 2021, doi: 10.1002/ptr.7076.
- Akiu, S.; Suzuki, Y.; Asahara, T.; Fujinuma, Y.; Fukuda, M. Inhibitory effect of arbutin on melanogenesis--biochemical study using cultured B16 melanoma cells. Nippon Hifuka Gakkai Zasshi 1991, 101, 609-613.
- Maeda, K.; Fukuda, M. In vitro effectiveness of several whitening cosmetic components in human melanocytes. Journal of the society of cosmetic chemists 1991, 42, 361-368.
- Lim, Y.J.; Lee, E.H.; Kang, T.H.; Ha, S.K.; Oh, M.S.; Kim, S.M.; Yoon, T.J.; Kang, C.; Park, J.H.; Kim, S.Y. Inhibitory effects of arbutin on melanin biosynthesis of alpha-melanocyte stimulating hormone-induced hyperpigmentation in cultured brownish guinea pig skin tissues. Arch Pharm Res 2009, 32, 367-373.
- Maeda, K.; Fukuda, M. Arbutin: mechanism of its depigmenting action in human melanocyte culture. J Pharmacol Exp Ther 1996, 276, 765-769.
- Chakraborty, A.K.; Funasaka, Y.; Komoto, M.; Ichihashi, M. Effect of arbutin on melanogenic proteins in human melanocytes. Pigment Cell Res 1998, 11, 206-212.
- Inoue, Y.; Hasegawa, S.; Yamada, T.; Date, Y.; Mizutani, H.; Nakata, S.; Matsunaga, K.; Akamatsu, H. Analysis of the effects of hydroquinone and arbutin on the differentiation of melanocytes. Biol Pharm Bull 2013, 36, 1722-1730.
- Nakajima, M.; Shinoda, I.; Fukuwatari, Y.; Hayasawa, H. Arbutin increases the pigmentation of cultured human melanocytes through mechanisms other than the induction of tyrosinase activity. Pigment Cell Res 1998, 11, 12-17.
- Rok, J.O., M.;Buszman, E.; Wrześniok, D. Melanin – from melanocyte to keratinocyte, that is how melanin is transported within the skin. Ann Acad Med Sil 2012, 66, 60-66.
- Wu, X.F.; Hammer, J.A. Melanosome transfer: it is best to give and receive. Current Opinion in Cell Biology 2014, 29, 1-7.
- Kitao, S.; Sekine, H. alpha-D-Glucosyl Transfer to Phenolic Compounds by Sucrose Phosphorylase from Leuconostoc mesenteroides and Production of alpha-Arbutin. Biosci Biotechnol Biochem 1994, 58, 38-42.
- Funayama, M.; Arakawa, H.; Yamamoto, R.; Nishino, T.; Shin, T.; Murao, S. Effects of alpha- and beta-arbutin on activity of tyrosinases from mushroom and mouse melanoma. Biosci Biotechnol Biochem 1995, 59, 143-144.
- Qin, L.; Wu, Y.; Liu, Y.; Chen, Y.; Zhang, P. Dual effects of alpha-arbutin on monophenolase and diphenolase activities of mushroom tyrosinase. PLoS One 2014, 9, e109398.
- Garcia-Jimenez, A.; Teruel-Puche, J.A.; Berna, J.; Rodriguez-Lopez, J.N.; Tudela, J.; Garcia-Canovas, F. Action of tyrosinase on alpha and beta-arbutin: A kinetic study. PLoS One 2017, 12, e0177330.
- Sugimoto, K.; Nishimura, T.; Nomura, K.; Sugimoto, K.; Kuriki, T. Inhibitory effects of alpha-arbutin on melanin synthesis in cultured human melanoma cells and a three-dimensional human skin model. Biol Pharm Bull 2004, 27, 510-514.
- Choi, S.; Park, Y.I.; Lee, S.K.; Kim, J.E.; Chung, M.H. Aloesin inhibits hyperpigmentation induced by UV radiation. Clinical and Experimental Dermatology 2002, 27, 513-515.
- Ertam, I.; Mutlu, B.; Unal, I.; Alper, S.; Kivcak, B.; Ozer, O. Efficiency of ellagic acid and arbutin in melasma: a randomized, prospective, open-label study. J Dermatol 2008, 35, 570-574.
- Morag, M.; Nawrot, J.; Siatkowski, I.; Adamski, Z.; Fedorowicz, T.; Dawid-Pac, R.; Urbanska, M.; Nowak, G. A double-blind, placebo-controlled randomized trial of Serratulae quinquefoliae folium, a new source of beta-arbutin, in selected skin hyperpigmentations. J Cosmet Dermatol 2015, 14, 185-190.
- Takebayashi, J.; Ishii, R.; Chen, J.B.; Matsumoto, T.; Ishimi, Y.; Tai, A. Reassessment of antioxidant activity of arbutin: Multifaceted evaluation using five antioxidant assay systems. Free Radical Research 2010, 44, 473-478.
- Tada, M.; Kohno, M.; Niwano, Y. Alleviation effect of arbutin on oxidative stress generated through tyrosinase reaction with L-tyrosine and L-DOPA. BMC Biochem 2014, 15, 23.
- Wu, L.H.; Li, P.; Zhao, Q.L.; Piao, J.L.; Jiao, Y.F.; Kadowaki, M.; Kondo, T. Arbutin, an intracellular hydroxyl radical scavenger, protects radiation-induced apoptosis in human lymphoma U937 cells. Apoptosis 2014, 19, 1654-1663.
- Seyfizadeh, N.; Tazehkand, M.Q.; Palideh, A.; Maroufi, N.F.; Hassanzadeh, D.; Rahmati-Yamchi, M.; Elahimanesh, F.; Borzoueisileh, S. Is arbutin an effective antioxidant for the discount of oxidative and nitrosative stress in Hep-G2 cells exposed to tert-butyl hydroperoxide? Bratislava Medical Journal-Bratislavske Lekarske Listy 2019, 120, 569-575.
- Ebadollahi, S.H.; Pouramir, M.; Zabihi, E.; Golpour, M.; Aghajanpour-Mir, M. The Effect of Arbutin on The Expression of Tumor Suppressor P53, BAX/BCL-2 Ratio and Oxidative Stress Induced by Tert-Butyl Hydroperoxide in Fibroblast and LNcap Cell Lines. Cell J 2021, 22, 532-541.
- Polouliakh, N.; Ludwig, V.; Meguro, A.; Kawagoe, T.; Heeb, O.; Mizuki, N. Alpha-Arbutin Promotes Wound Healing by Lowering ROS and Upregulating Insulin/IGF-1 Pathway in Human Dermal Fibroblast. Front Physiol 2020, 11, 586843.
- Zhang, B.; Zeng, M.; Li, B.; Kan, Y.; Wang, S.; Cao, B.; Huang, Y.; Zheng, X.; Feng, W. Arbutin attenuates LPS-induced acute kidney injury by inhibiting inflammation and apoptosis via the PI3K/Akt/Nrf2 pathway. Phytomedicine 2021, 82, 153466.
- Nalban, N.; Sangaraju, R.; Alavala, S.; Mir, S.M.; Jerald, M.K.; Sistla, R. Arbutin Attenuates Isoproterenol-Induced Cardiac Hypertrophy by Inhibiting TLR-4/NF-kappaB Pathway in Mice. Cardiovasc Toxicol 2020, 20, 235-248.