
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexander Zarbock | + 1815 word(s) | 1815 | 2021-08-03 12:31:38 |
Video Upload Options
"Neutrophil extracellular traps" (NETs) are released by neutrophils. Neutrophils act as the first line of defense during infection and inflammation. Once activated, they are able to fulfil numerous tasks to fight inflammatory insults while keeping a balanced immune response. Besides well-known functions, such as phagocytosis and degranulation, neutrophils are also able to release “neutrophil extracellular traps” (NETs).. In response to most stimuli, the neutrophils release decondensed chromatin in a NADPH oxidase-dependent manner decorated with histones and granule proteins, such as neutrophil elastase, myeloperoxidase, and cathelicidins. Although primarily supposed to prevent microbial dissemination and fight infections, there is increasing evidence that an overwhelming NET response correlates with poor outcome in many diseases. Lung-related diseases especially, such as bacterial pneumonia, cystic fibrosis, chronic obstructive pulmonary disease, aspergillosis, influenza, and COVID-19, are often affected by massive NET formation. Highly vascularized areas as in the lung are susceptible to immunothrombotic events promoted by chromatin fibers. Keeping this fragile equilibrium seems to be the key for an appropriate immune response. Therapies targeting dysregulated NET formation might positively influence many disease progressions.
1. Mechanisms of NET formation
The underlying mechanisms leading to NET formation show variable characteristics, and studies demonstrated that the signaling pathway vary depending on the respective stimulus. NET formation can be stimulated via G protein-coupled receptors (GPCRs), chemokine and cytokine receptors, Toll-like receptors (TLR), and Fc receptors (FcR). The subsequent downstream signaling comprises mostly the activation of the NADPH oxidase (NOX) complex, but exceptions were also described [1]. Upstream of oxidant production, the molecules Raf-MEK-ERK have been shown to be involved [2]. Cytoskeletal rearrangement [3] and glycolytic ATP production [4] are both required for NET formation and are dependent on ROS produced in the context of mitochondrial dysfunction and NOX activation [5]. ROS initiates the dissociation of NE from a membrane-associated complex into the cytosol and activates its proteolytic activity in an MPO-dependent manner. Subsequently, NE degrades F-actin to arrest actin dynamics followed by translocation into the nucleus, where NE and MPO drive chromatin decondensation and histone cleavage [6][7], which can be supported by PAD4-dependent histone citrullination [8] ( Figure 1 ).
Nevertheless, NE- and PAD4-independent pathways have been described, too [9][10]. Van Avondt and colleagues demonstrated that the inhibition of the signal inhibitory receptor on leukocytes-1 (SIRL-1) could prevent NET production without affecting oxidant production [11]. Cell cycle proteins [12] support nuclear envelope breakdown followed by the release of chromatin into the cytosol, where nuclear and cytosolic proteins are mixed [13]. The final cell lysis and NET release involves Gasdermin D (GSDMD), which is able to form pores in granule and plasma membranes [14][15]. This kind of NET formation ends up with cell death and is often described as lytic NET release or NETosis and occurs within a rather long time frame of three to eight hours. In contrast, the non-lytic NET release can be observed rapidly after 5–60 min of stimulation. Here, neutrophils do not undergo cell death, which was observed for neutrophils in close contact with activated platelets [16][17] or in response to Staphylococcus aureus infections [18]. Similar to NETosis, the non-lytic NET formation also involves the translocation of NE to the nucleus, histone citrullination, and chromatin decondensation [19]. Conversely, the membrane does not disintegrate, and the protein-decorated chromatin is released via vesicles [20] ( Figure 1 ). Even the remnants of non-lytic NET formation, cytoplasts, are able to keep their mobility and fulfill important functions, such as phagocytosis, the activation of DCs, and the release of cytotoxic molecules [20][21][22].
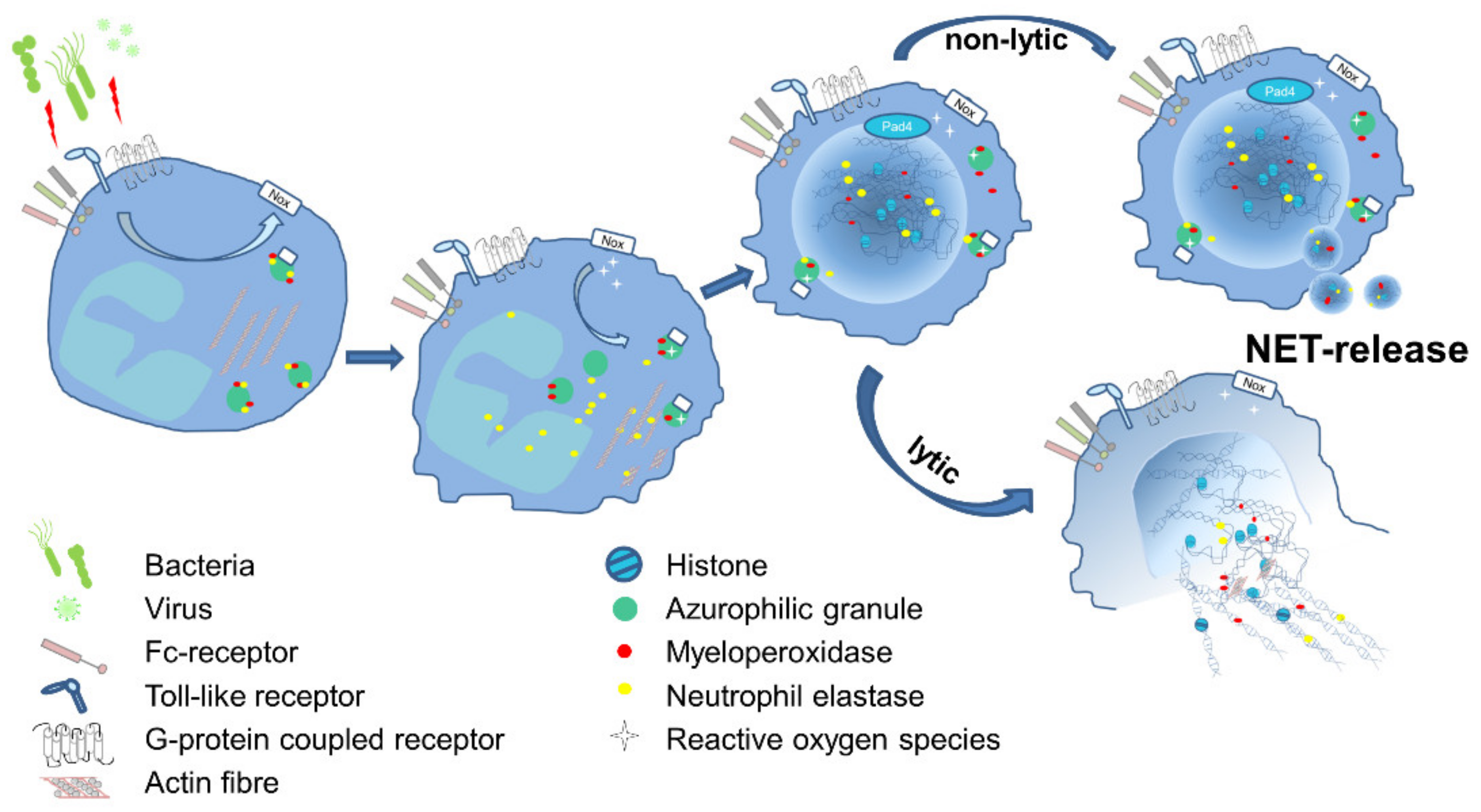 Figure 1. Schematic illustration of the essential steps of NET formation. Several pathogens are capable of inducing NET formation directly or via release of peptides or damage associated molecular patterns (DAMPs). Receptors like TLR, FcR or GPCR transmit signals into the cell and activate predominantly the NADPH-oxidase complex (NOX), which subsequently catalyzes production of reactive oxygen species (ROS). In azurophilic granules, NE gets released from the membranes in a ROS-dependent manner and translocates into the nucleus and in parallel degrades actin. NE activity results in the decondensation of chromatin, which is further supported by the PAD4-dependent citrullination of histones. The chromatin, decorated with microbicidal molecules like histones, MPO and NE, is released in the environment. This occurs either in a non-lytic procedure, where DNA fibers are suggested to be released via vesicles, or in a lytic process, followed by the breakdown of the nuclear envelope and the cell membrane, ending with cell death.
Figure 1. Schematic illustration of the essential steps of NET formation. Several pathogens are capable of inducing NET formation directly or via release of peptides or damage associated molecular patterns (DAMPs). Receptors like TLR, FcR or GPCR transmit signals into the cell and activate predominantly the NADPH-oxidase complex (NOX), which subsequently catalyzes production of reactive oxygen species (ROS). In azurophilic granules, NE gets released from the membranes in a ROS-dependent manner and translocates into the nucleus and in parallel degrades actin. NE activity results in the decondensation of chromatin, which is further supported by the PAD4-dependent citrullination of histones. The chromatin, decorated with microbicidal molecules like histones, MPO and NE, is released in the environment. This occurs either in a non-lytic procedure, where DNA fibers are suggested to be released via vesicles, or in a lytic process, followed by the breakdown of the nuclear envelope and the cell membrane, ending with cell death.
2. NET-Targeting Therapies
2.1. DNase1
2.2. Histones
2.3. Neutrophil Elastase
2.4. Other Treatments
| Compound | Target | Application | Reference |
|---|---|---|---|
| Dornase Alfa/DNase | DNA | Bronchiolitis Cystic fibrosis |
[23][24][25][26][27] |
| Clinical Phase 2 study: COVID19 | NCT04359654 | ||
| C1 esterase inhibitor | Histones | Sepsis patients | [31][32][33][34] |
| tACPA α-H3-cit |
Citrullinated Histones | Inflammatory murine disease models |
[30][35] |
| Sivelestat | Neutrophil elastase | ARDS patients ALI patients |
[37][38][39][40][41] |
| Clinical phase 4 study: ARDS | NCT00036062 | ||
| Aspirin αCLEC Glucocorticoids NET-inhibiting factors |
Inhibition of NET formation | Critically ill patients Inflammatory murine disease models |
[42][43][44][48] |
| Metformin | HMGB/NET clearance | Diabetes patients | [45][46] |
| CXCR2 antagonist | Neutrophil recruitment | LPS-challenged humans | [49] |
| CD40L-M7 | Mac1 | Inflammatory murine disease models |
[50] |
3. Summary
References
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822.
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011, 7, 75–77.
- Stojkov, D.; Amini, P.; Oberson, K.; Sokollik, C.; Duppenthaler, A.; Simon, H.U.; Yousefi, S. ROS and glutathionylation balance cytoskeletal dynamics in neutrophil extracellular trap formation. J. Cell Biol. 2017, 216, 4073–4090.
- Amini, P.; Stojkov, D.; Felser, A.; Jackson, C.B.; Courage, C.; Schaller, A.; Gelman, L.; Soriano, M.E.; Nuoffer, J.M.; Scorrano, L.; et al. Neutrophil extracellular trap formation requires OPA1-dependent glycolytic ATP production. Nat. Commun. 2018, 9, 2958.
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153.
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014, 8, 883–896.
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691.
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010, 207, 1853–1862.
- Diaz-Godinez, C.; Fonseca, Z.; Nequiz, M.; Laclette, J.P.; Rosales, C.; Carrero, J.C. Entamoeba histolytica Trophozoites Induce a Rapid Non-classical NETosis Mechanism Independent of NOX2-Derived Reactive Oxygen Species and PAD4 Activity. Front. Cell Infect. Microbiol. 2018, 8, 184.
- Martinod, K.; Witsch, T.; Farley, K.; Gallant, M.; Remold-O’Donnell, E.; Wagner, D.D. Neutrophil elastase-deficient mice form neutrophil extracellular traps in an experimental model of deep vein thrombosis. J. Thromb. Haemost. 2016, 14, 551–558.
- Van Avondt, K.; van der Linden, M.; Naccache, P.H.; Egan, D.A.; Meyaard, L. Signal Inhibitory Receptor on Leukocytes-1 Limits the Formation of Neutrophil Extracellular Traps, but Preserves Intracellular Bacterial Killing. J. Immunol. 2016, 196, 3686–3694.
- Amulic, B.; Knackstedt, S.L.; Abu Abed, U.; Deigendesch, N.; Harbort, C.J.; Caffrey, B.E.; Brinkmann, V.; Heppner, F.L.; Hinds, P.W.; Zychlinsky, A. Cell-Cycle Proteins Control Production of Neutrophil Extracellular Traps. Dev. Cell 2017, 43, 449–462.
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241.
- Chen, K.W.; Monteleone, M.; Boucher, D.; Sollberger, G.; Ramnath, D.; Condon, N.D.; von Pein, J.B.; Broz, P.; Sweet, M.J.; Schroder, K. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci. Immunol. 2018, 3.
- Sollberger, G.; Choidas, A.; Burn, G.L.; Habenberger, P.; Di Lucrezia, R.; Kordes, S.; Menninger, S.; Eickhoff, J.; Nussbaumer, P.; Klebl, B.; et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol. 2018, 3.
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469.
- McDonald, B.; Urrutia, R.; Yipp, B.G.; Jenne, C.N.; Kubes, P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 2012, 12, 324–333.
- Pilsczek, F.H.; Salina, D.; Poon, K.K.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.; Surette, M.G.; Sugai, M.; et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425.
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287.
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393.
- Roos, D.; Voetman, A.A.; Meerhof, L.J. Functional activity of enucleated human polymorphonuclear leukocytes. J. Cell Biol. 1983, 97, 368–377.
- Krishnamoorthy, N.; Douda, D.N.; Bruggemann, T.R.; Ricklefs, I.; Duvall, M.G.; Abdulnour, R.E.; Martinod, K.; Tavares, L.; Wang, X.; Cernadas, M.; et al. Neutrophil cytoplasts induce TH17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci. Immunol. 2018, 3.
- Nasr, S.Z.; Strouse, P.J.; Soskolne, E.; Maxvold, N.J.; Garver, K.A.; Rubin, B.K.; Moler, F.W. Efficacy of recombinant human deoxyribonuclease I in the hospital management of respiratory syncytial virus bronchiolitis. Chest 2001, 120, 203–208.
- Hodson, M.E.; McKenzie, S.; Harms, H.K.; Koch, C.; Mastella, G.; Navarro, J.; Strandvik, B.; Investigators of the Epidemiologic Registry of Cystic Fibrosis. Dornase alfa in the treatment of cystic fibrosis in Europe: A report from the Epidemiologic Registry of Cystic Fibrosis. Pediatr. Pulmonol. 2003, 36, 427–432.
- Frederiksen, B.; Pressler, T.; Hansen, A.; Koch, C.; Hoiby, N. Effect of aerosolized rhDNase (Pulmozyme) on pulmonary colonization in patients with cystic fibrosis. Acta Paediatr. 2006, 95, 1070–1074.
- Weber, A.G.; Chau, A.S.; Egeblad, M.; Barnes, B.J.; Janowitz, T. Nebulized in-line endotracheal dornase alfa and albuterol administered to mechanically ventilated COVID-19 patients: A case series. Mol. Med. 2020, 26, 91.
- Desilles, J.P.; Gregoire, C.; Le Cossec, C.; Lambert, J.; Mophawe, O.; Losser, M.R.; Lambiotte, F.; Le Tacon, S.; Cantier, M.; Engrand, N.; et al. Efficacy and safety of aerosolized intra-tracheal dornase alfa administration in patients with SARS-CoV-2-induced acute respiratory distress syndrome (ARDS): A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 548.
- Tsourouktsoglou, T.D.; Warnatsch, A.; Ioannou, M.; Hoving, D.; Wang, Q.; Papayannopoulos, V. Histones, DNA, and Citrullination Promote Neutrophil Extracellular Trap Inflammation by Regulating the Localization and Activation of TLR4. Cell Rep. 2020, 31, 107602.
- Wygrecka, M.; Kosanovic, D.; Wujak, L.; Reppe, K.; Henneke, I.; Frey, H.; Didiasova, M.; Kwapiszewska, G.; Marsh, L.M.; Baal, N.; et al. Antihistone Properties of C1 Esterase Inhibitor Protect against Lung Injury. Am. J. Respir. Crit. Care Med. 2017, 196, 186–199.
- Chirivi, R.G.S.; van Rosmalen, J.W.G.; van der Linden, M.; Euler, M.; Schmets, G.; Bogatkevich, G.; Kambas, K.; Hahn, J.; Braster, Q.; Soehnlein, O.; et al. Therapeutic ACPA inhibits NET formation: A potential therapy for neutrophil-mediated inflammatory diseases. Cell. Mol. Immunol. 2021, 18, 1528–1544.
- Caliezi, C.; Wuillemin, W.A.; Zeerleder, S.; Redondo, M.; Eisele, B.; Hack, C.E. C1-Esterase inhibitor: An anti-inflammatory agent and its potential use in the treatment of diseases other than hereditary angioedema. Pharm. Rev. 2000, 52, 91–112.
- Liu, D.; Cai, S.; Gu, X.; Scafidi, J.; Wu, X.; Davis, A.E., 3rd. C1 inhibitor prevents endotoxin shock via a direct interaction with lipopolysaccharide. J. Immunol. 2003, 171, 2594–2601.
- Zeerleder, S.; Caliezi, C.; van Mierlo, G.; Eerenberg-Belmer, A.; Sulzer, I.; Hack, C.E.; Wuillemin, W.A. Administration of C1 inhibitor reduces neutrophil activation in patients with sepsis. Clin. Diagn. Lab. Immunol. 2003, 10, 529–535.
- Igonin, A.A.; Protsenko, D.N.; Galstyan, G.M.; Vlasenko, A.V.; Khachatryan, N.N.; Nekhaev, I.V.; Shlyapnikov, S.A.; Lazareva, N.B.; Herscu, P. C1-esterase inhibitor infusion increases survival rates for patients with sepsis*. Crit. Care Med. 2012, 40, 770–777.
- Deng, Q.; Pan, B.; Alam, H.B.; Liang, Y.; Wu, Z.; Liu, B.; Mor-Vaknin, N.; Duan, X.; Williams, A.M.; Tian, Y.; et al. Citrullinated Histone H3 as a Therapeutic Target for Endotoxic Shock in Mice. Front. Immunol. 2019, 10, 2957.
- Hagio, T.; Matsumoto, S.; Nakao, S.; Matsuoka, S.; Kawabata, K. Sivelestat, a specific neutrophil elastase inhibitor, prevented phorbol myristate acetate-induced acute lung injury in conscious rabbits. Pulm. Pharm. 2005, 18, 285–290.
- Okayama, N.; Kakihana, Y.; Setoguchi, D.; Imabayashi, T.; Omae, T.; Matsunaga, A.; Kanmura, Y. Clinical effects of a neutrophil elastase inhibitor, sivelestat, in patients with acute respiratory distress syndrome. J. Anesth. 2006, 20, 6–10.
- Tamakuma, S.; Ogawa, M.; Aikawa, N.; Kubota, T.; Hirasawa, H.; Ishizaka, A.; Taenaka, N.; Hamada, C.; Matsuoka, S.; Abiru, T. Relationship between neutrophil elastase and acute lung injury in humans. Pulm. Pharm. 2004, 17, 271–279.
- Hashimoto, S.; Okayama, Y.; Shime, N.; Kimura, A.; Funakoshi, Y.; Kawabata, K.; Ishizaka, A.; Amaya, F. Neutrophil elastase activity in acute lung injury and respiratory distress syndrome. Respirology 2008, 13, 581–584.
- Tagami, T.; Tosa, R.; Omura, M.; Fukushima, H.; Kaneko, T.; Endo, T.; Rinka, H.; Murai, A.; Yamaguchi, J.; Yoshikawa, K.; et al. Effect of a selective neutrophil elastase inhibitor on mortality and ventilator-free days in patients with increased extravascular lung water: A post hoc analysis of the PiCCO Pulmonary Edema Study. J. Intensive Care 2014, 2, 67.
- Miyoshi, S.; Hamada, H.; Ito, R.; Katayama, H.; Irifune, K.; Suwaki, T.; Nakanishi, N.; Kanematsu, T.; Dote, K.; Aibiki, M.; et al. Usefulness of a selective neutrophil elastase inhibitor, sivelestat, in acute lung injury patients with sepsis. Drug Des. Devel. 2013, 7, 305–316.
- Lapponi, M.J.; Carestia, A.; Landoni, V.I.; Rivadeneyra, L.; Etulain, J.; Negrotto, S.; Pozner, R.G.; Schattner, M. Regulation of neutrophil extracellular trap formation by anti-inflammatory drugs. J. Pharm. Exp. 2013, 345, 430–437.
- Hirose, T.; Hamaguchi, S.; Matsumoto, N.; Irisawa, T.; Seki, M.; Tasaki, O.; Hosotsubo, H.; Yamamoto, N.; Yamamoto, K.; Akeda, Y.; et al. Presence of neutrophil extracellular traps and citrullinated histone H3 in the bloodstream of critically ill patients. PLoS ONE 2014, 9, e111755.
- Lamphier, M.; Zheng, W.; Latz, E.; Spyvee, M.; Hansen, H.; Rose, J.; Genest, M.; Yang, H.; Shaffer, C.; Zhao, Y.; et al. Novel small molecule inhibitors of TLR7 and TLR9: Mechanism of action and efficacy in vivo. Mol. Pharm. 2014, 85, 429–440.
- Menegazzo, L.; Scattolini, V.; Cappellari, R.; Bonora, B.M.; Albiero, M.; Bortolozzi, M.; Romanato, F.; Ceolotto, G.; Vigili de Kreutzeberg, S.; Avogaro, A.; et al. The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo. Acta Diabetol. 2018, 55, 593–601.
- Gregoire, M.; Uhel, F.; Lesouhaitier, M.; Gacouin, A.; Guirriec, M.; Mourcin, F.; Dumontet, E.; Chalin, A.; Samson, M.; Berthelot, L.L.; et al. Impaired efferocytosis and neutrophil extracellular trap clearance by macrophages in ARDS. Eur. Respir. J. 2018, 52.
- Vargas, A.; Boivin, R.; Cano, P.; Murcia, Y.; Bazin, I.; Lavoie, J.P. Neutrophil extracellular traps are downregulated by glucocorticosteroids in lungs in an equine model of asthma. Respir. Res. 2017, 18, 207.
- Yost, C.C.; Schwertz, H.; Cody, M.J.; Wallace, J.A.; Campbell, R.A.; Vieira-de-Abreu, A.; Araujo, C.V.; Schubert, S.; Harris, E.S.; Rowley, J.W.; et al. Neonatal NET-inhibitory factor and related peptides inhibit neutrophil extracellular trap formation. J. Clin. Invest. 2016, 126, 3783–3798.
- Leaker, B.R.; Barnes, P.J.; O’Connor, B. Inhibition of LPS-induced airway neutrophilic inflammation in healthy volunteers with an oral CXCR2 antagonist. Respir. Res. 2013, 14, 137.
- Wolf, D.; Anto-Michel, N.; Blankenbach, H.; Wiedemann, A.; Buscher, K.; Hohmann, J.D.; Lim, B.; Bauml, M.; Marki, A.; Mauler, M.; et al. A ligand-specific blockade of the integrin Mac-1 selectively targets pathologic inflammation while maintaining protective host-defense. Nat. Commun. 2018, 9, 525.
- Boeltz, S.; Amini, P.; Anders, H.J.; Andrade, F.; Bilyy, R.; Chatfield, S.; Cichon, I.; Clancy, D.M.; Desai, J.; Dumych, T.; et al. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019, 26, 395–408.




