
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Milena Matuszczak | + 2596 word(s) | 2596 | 2020-05-13 10:46:23 | | | |
| 2 | Rita Xu | Meta information modification | 2596 | 2021-08-13 03:40:34 | | |
Video Upload Options
The high occurrence of bladder cancer and its tendency to recur combined with lifelong surveillance make the treatment of superficial bladder cancer one of the most expensive and time-consuming. Moreover, carcinoma in situ often leads to muscle invasion with an unfavourable prognosis. Currently, invasive methods including cystoscopy and cytology remain a gold standard. The aim is to find biomarkers with the best specificity and sensitivity, allowing the treatment plan to optimise and have potential applications in clinical practice. Such non-invasive methods can be measure in human body fluids, for example, urine or serum: Cytokeratin fragments (CYFRA 21.1), Excision Repair Cross-Complementation 1 (ERCC1), Tumour Protein p53 (Tp53), Fibroblast Growth Factor Receptor 3 (FGFR3), Tumor-Associated Trypsin Inhibitor (TATI).
1. Introduction
Bladder cancer is the most common urinary site of malignancy and the second most common reason of cancer deaths from the genitourinary tract after prostate cancer in the United States, with 81,400 new cases and 17,980 deaths in the year 2020[1]. Globally there are about 430,000 new cases diagnosed each year [2].
Favourably, non-invasive lesions constitute approximately 75–80% of newly diagnosed urothelial bladder cancers (UBC). More than 50% of UBCs are caused by smoking. Other important factors include occupational exposure to aromatic amines and polycyclic hydrocarbons. Less evident is the impact of diet and environmental pollution. Increasing data indicate that genetic predisposition plays a role in UBC pathogenesis [2][3][4]
There are two major groups of patients with distinct prognoses and molecular features.
Carcinoma in situ (CIS) and tumours staged as Ta, T1 (Figure 1.) are grouped as non-muscle-invasive bladder cancers (NMIBC) [5]. NMIBC patients generally have a significant risk of recurrence and potential clinical course for progression [6]For NMIBC, the major problem is that after the initial transurethral resection of the bladder (TURB), they characteristically recur in 50–70% of cases, with only approximately 10–20% of patients progressing to muscle-invasive bladder cancer (MIBC) [7]. Still, their life expectancy is long, and cancer rarely progresses to muscle invasion.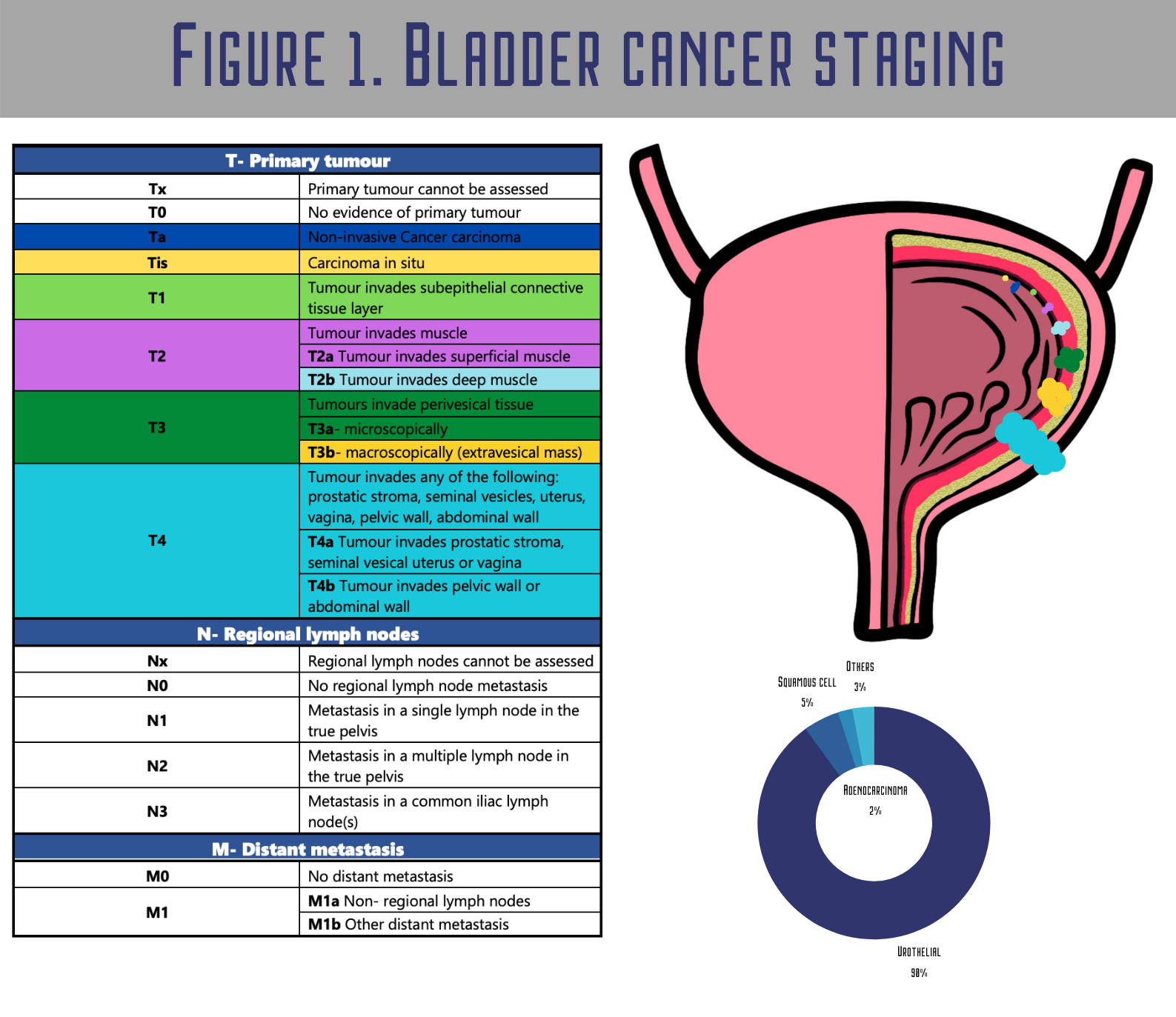
Muscle-invasive tumours often metastasize and are usually diagnosed de novo, the prognosis is unfavourable, and for decades, no major innovation has been made in therapy. Papillary non-invasive cancers (pTa) grow up from carcinoma in situ (CIS) of the urothelium (frequently TP53-mutated, a high-grade lesion) and often metastasize and evolve into muscle invasion [8]. Robertson et al. demonstrated that MIBC shows high overall mutation rates. Still, fortunately, most of them seem to be passenger variations without any functional meaning or repeated genetic alterations, including the TP53, FGFR3, PIK3CA and RB1 genes’ mutations [9].
Muscle-invasive bladder cancer (MIBC) is a high risk but potentially curable disease. Unfortunately, nearly half of patients die from MIBC despite getting the appropriate treatment [10][11]. The major problem in the management of superficial bladder cancer is its tendency to recur. Lifelong surveillance with a relatively long-life expectancy (5-year survival rate > 90%) makes it the most expensive and time-consuming malignancy to treat.
In recent years, a great effort has been put in the search for new potential biomarkers such as protein 53 (p53), ERCC1, CYFRA 21.1, FGFR3 and TATI in the prognosis and prediction of bladder cancer. The FGFR3 mutations could be a marker of low-grade and early-stage tumours, while the changes in p53 appear better in detecting high-grade or advanced cancers.
2. Diagnostic and Prognostic Potential of Bladder Cancer Biomarkers
2.1. Cytokeratin Fragment 21.1 (CYFRA 21.1)
Cytokeratin fragments (CYFRA 21.1) is an ELISA-based assay that detects the concentration of a soluble fragment of cytokeratin 19 by using two monoclonal antibodies[12]. The studies have shown that the differentiation between liquid biopsies of healthy (non-cancer) individuals and BC patients may be done using this biomarker.
Authors[13] concluded that serum and urine CYFRA21.1 (Figure 2.) can be used as diagnostic biomarkers and distinguish between local and metastatic bladder cancer. All healthy individuals had a lower CYFRA21.1 level than patients with bladder cancer. The locally invasive disease also showed lower CYFRA 21.1 levels than the subgroup with metastatic bladder cancer. Notwithstanding, between patients with bladder cancer stage I and stage II, and among the group of patients with local stage II and III were no significant differences in the CYFRA21.1 level. Therefore, CYFRA 21.1 cannot help differentiate grades I–III of local bladder cancer but may be used as a diagnostic biomarker to detect metastases. Notably, the abnormal serum level of CYFRA 21.1 [14] corresponds with a worse response.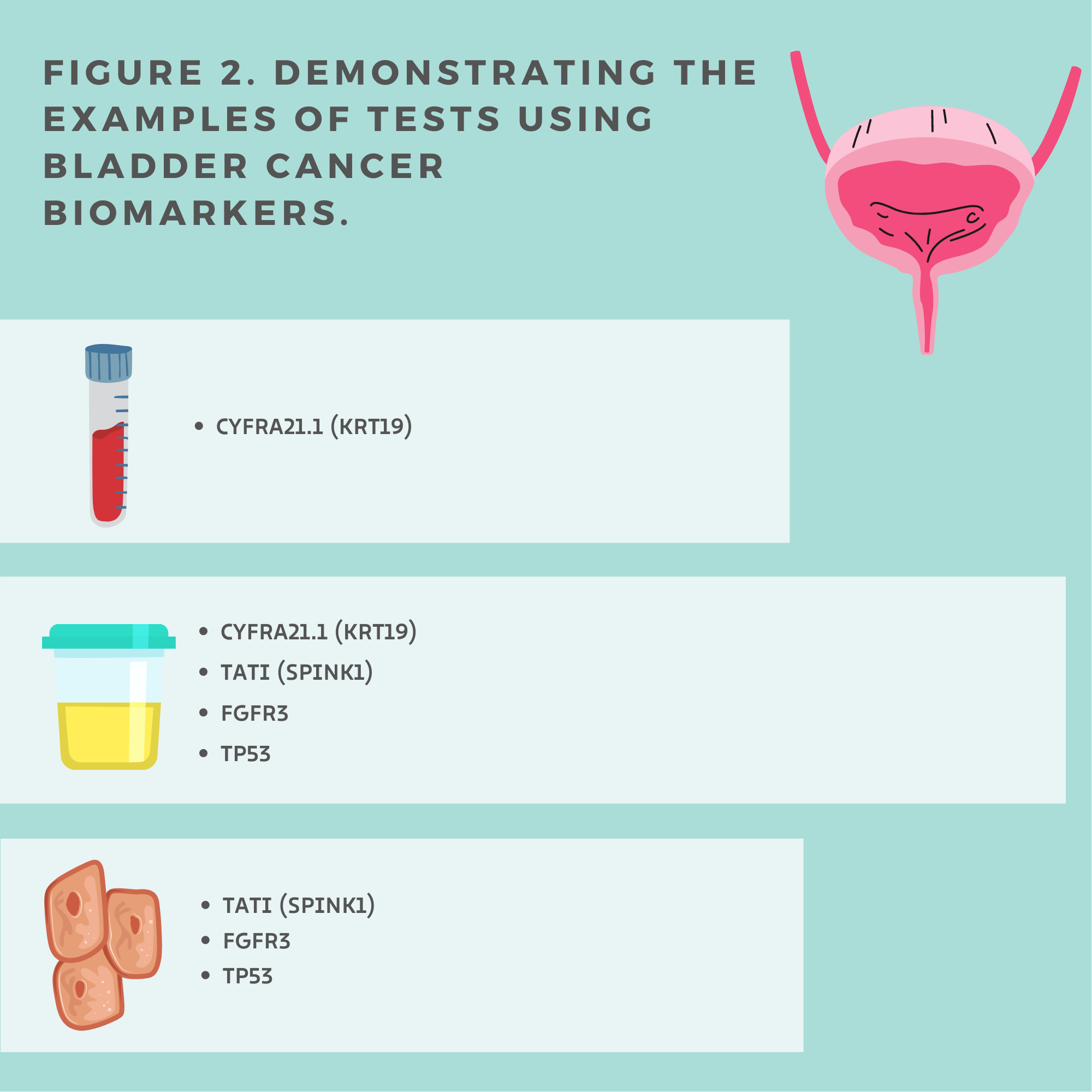
Unfortunately, CYFRA 21.1 is a false positive in the group of patients with urinary tract infections, stones, history of pelvic radiotherapy, urethral catheterization or BCG intravesical instillation within the three previous months. Even years following intravesical immunotherapy with the BCG level of urinary CYFRA 21.1 may be elevated. There is a disadvantage being that the concentrations marker is strongly influenced by benign urological diseases, intravesical installations and also a disappointing performance in low-stage bladder cancer.
2.2. Excision Repair Cross-Complementation 1 (ERCC1)
The nucleotide excision repair (NER) pathway is essential for protecting genomic stability and for the removal of platinum-induced DNA adducts and cisplatin resistance [15][16]. The key molecules in this pathway belong to the excision repair cross-complementing group 1 (ERCC1) [17].
The ERCC1 role is to detect, repairing and rate-limiting the interstrand cross-links in DNA [18]. Therefore, this enzyme may represent the crucial DNA damage repairability of the cell [19][20]. In a group of patients treated with surgical resection, ERCC1 as the DNA repair protein may also be engaged in weakening tumour malignancy by reducing the number of mutations. Moreover, genetic testing of ERCC1 expression levels could personalize the chemotherapy by selecting the patients who would benefit from platinum-based chemotherapy. A variety of tumours, including the bladder, show that the ERCC1 level is strongly associated with cisplatin resistance [21].
Klatte and colleagues [22] presented the work assessing ERCC1 as a prognostic and predictive biomarker of bladder cancer after cystectomy. In a group of 432 patients, a positive expression was found in 71% of patients. Patients with an ERCC1-positive expression had a significantly better five-year disease-free survival (DFS) than those with an ERCC1-negative expression, 62% to 49%, and cancer-specific survival (CSS), 70% to 59%, respectively. In the ERCC1-positive group, the risk of bladder cancer (BC) recurrence and death due to BC was 30% lower. Patients undergoing radical cystectomy with an ERCC1-positive expression had better survival values than those with a negative expression. Therefore, ERCC1 may be an independent prognostic marker for bladder cancer.
Similar conclusions were made in Hemdan's report [23]. They evaluated a group of 244 patients who underwent radical cystectomy or neoadjuvant chemotherapy and radical cystectomy. Negative ERCC1 correlated with worse overall survival in the group with only surgical treatment. It was noted that neoadjuvant chemotherapy would benefit mainly patients with an ERCC1-negative expression, while for those who were ERCC1-positive, the influence was minimal.
The role of ERCC1 as a prognostic factor of survival was assessed in patients with advanced bladder cancer treated with platinum-based chemotherapy. Another meta-analysis was published by Urun [24] performed on 1425 patients from 13 studies, and patients with an ERCC1-positive expression constituted 24–76% of the examined populations. The conclusions were that a positive ERCC1 expression is not significantly related to overall survival but has a significant impact on worse progression-free survival, and maybe an indicator of worse survival in patients with advanced bladder cancer, but large prospective studies are needed to consider ERCC1 as a prognostic marker in patients with advanced bladder cancer.
In 2018, Eldehna[21] conducted a descriptive study on 80 patients with muscle-invasive bladder cancer (stages T2–T4a) who received platinum-based chemotherapy. They were analyzing the previous studies gives controversial information about predicting the prognostic role of ERCC1 in the treatment of advanced bladder cancer. Their research showed a significant relationship between platinum-based treatment response and the ERCC1 expression in bladder cancer tissue samples (p = 0.013). It was an indicative association between a negative immuno-expression and a more favourable outcome. Still, no difference between the ERCC1 expression and mean overall survival or progression-free survival in different immune-expression levels in patients was apparent. Therefore, ERCC1 may be a potential predictive but not prognostic marker. For this reason, genetic testing could personalize chemotherapy by selecting the patients who would benefit from a platinum-based treatment in bladder cancer.
In summary, ERCC1-positive tumours were associated with a better prognosis in cases without chemotherapies. However, in patients with chemotherapies, ERCC1-negative tumours were associated with a better outcome.
The possible explanation for the above scenario seems related to the function of this enzyme, which appears crucial in the DNA damage repairability of the cell. However, the above DNA repair, related to the ERCC1 activity, is non-beneficial for patients treated with chemotherapy, potentially leading to an “antichemotherapeutic” activity.
2.3. Tumour Protein p53 (TP53)
The common oncosuppressor gene mutated in all human cancers and the most frequently mutated gene in MIBC is the tumour protein p53 (TP53)[25]. Genomic integrity and stability by TP53 via triggering a cell-cycle arrest, apoptosis, autophagy and DNA repair. Mutant p53 proteins silence the autophagy-related gene (ATG), which affects the autophagic flow, and therefore suppresses regulation to the autophagic vesicles formation and their fusion with lysosomes [26]. Additionally, p53 preferentially binds to the AMPKα subunit and inhibits the AMPK activation. Mutp53s become oncogenic via the activation of AMPK [27].
Bladder carcinogenesis is closely associated with tumour suppressor dysfunction and the inactivation of TP53 [28]. Therefore, p53 has been studied as a marker of urothelial cell carcinoma recurrence and progression. A cheap and simple method to detect the abnormal function of p53 is immunohistochemistry staining (IHC). The short half-life of wild-type p53 prevents its intra-nuclear accumulation [29].
Increased p53 accumulation in the cell nucleus is a result of TP53 mutations. Immunohistochemical patterns of TP53 mutations are strongly associated with the progression of urothelial cell carcinoma. Plenty of data illustrate that from non-missense mutations (i.e., nonsense, insertion and deletion) to wild-type TP53, the expression of p53’s IHC increases. That promotes the growing up of an invasive phenotype of bladder cancer [30]. The high expression of p53 has been associated with features of tumour aggressiveness and correlated with poor oncological outcomes [31]. Therefore, this protein level was higher in more advanced bladder cancer [32][33]. Plenty of studies have indicated that p53 can be helpful to assess the level of progress and prognoses urothelial cell carcinoma [31].
However, Ciccasese and colleagues published a study with a contradictory opinion. In their opinion, the single p53 marker is not good enough as a prognostic marker of MIBC [34].
Moreover, the most aggressive T1 high-grade cancers appear to be also associated with the expression of this protein. The progression from T1 NMIBC to T1HG can be predicted by a p53 overexpression [35].
Authors [33] collected data from 70 patients and showed that 16% of patients with low-grade and 91% of patients with high-grade lesions were p53-positive. There was 33% positivity in Tis, 55% in T1, 72% in T2 and 100% in T3a and T3b. These results indicated a strong intensification of p53 staining—94.6% of high-grade and 5.4% of low-grade tumours. Moreover, the p53 accumulation in the nucleus, in a group treated with radical cystectomy and in other MIBCs, has a prognostic value [36].
Another study [25] showed that an aggressive tumour phenotype is strongly associated with the overexpression of p53. MIBC and CIS correlated with a high level of TP53 deletion and mutation [37]. According to the TCGA cohort data [4], 89% of MIBCs have an inactivated TP53 cell-cycle pathway, with TP53 mutations in 48%. Bladder epithelial cells become malignant by the TP53/RB1 pathway or the FGFR3/RAS pathway [37].
2.4. Fibroblast Growth Factor Receptor 3 (FGFR3)
Fibroblast growth factor receptor 3 (FGFR3) alternations are associated with urothelial cell carcinoma pathogenesis [38][39]. FGFR3 is activated by the mutation or overexpression in many bladder tumours at any stage but is predominantly active in low-grade NMIBCs [40][41][43]. Higher levels of FGFR3 expression were observed in low-grade, non-invasive tumours and recurrent non-invasive tumours than in invasive and non-invasive high-grade carcinoma[39].
This marker is associated with a lower chance of progression to muscle-invasive disease, and it is like a hallmark of the low-grade pathway. FGFR3 alternations occur mainly in non-invasive tumours [41][42] specifically in the luminal-papillary subtype (35%), which has the best overall survival and is characterized by a papillary morphology [41]Moreover, many studies indicated that FGFR3 mutation and the risk of progression are an inverse interaction. Therefore, patients with MIBC and the FGFR3 mutation have better survival rates [44]. Another study suggests that the progression in pT1 tumours is negatively correlated with the FGFR3 mutation [45]. Many studies confirm that FGFR3 mutations correlate with an overall benign effect [45][46]. The presence of the FGFR3 mutation in urine is observed not only in low-grade tumours but also seems to be associated with future recurrence [47][48]
FGFR3 is involved in tumorigenesis in ∼40% of invasive bladder cancer and the majority (∼80%) of low-grade non-invasive (stage Ta) bladder cancers [15]. Tomlinson et al [49]. observed an FGFR3 overexpression in nearly 40% of MIBC, whereas mutations occurred in 21% of MIBC Sung [38] observed that an FGFR3 overexpression results in the worst overall survival and disease-free survival in a group of patients with adjuvant chemotherapy. In a group without this treatment, no prognostic significance was observed.
High levels of FGFR3- and PIK3CA-mutated DNA in urine can be useful in predicting later metastasis and progression in NMIBC [50]. Choi et al. indicated that FGFR3 mutations are characteristic for the luminal type of MIBC [51]. In conclusion, FGFR3 may be used as a urine-based assay to detect primary tumours, recurrences for prognosis and targeted therapies. in both non-invasive and invasive BC
2.5. Tumor-Associated Trypsin Inhibitor (TATI)
TATI is a peptide produced at lower concentrations in many healthy tissues, especially in the gastrointestinal and urogenital tracts. In cancers, an increased TATI concentration is associated not only with tumour production but also acute phase reactions caused by tissue destruction during cancer invasion [52]
Serum values of TATI have also been used in patients with muscle-invasive and metastatic transitional cell carcinoma to monitor the response to therapy. In 1996, Pectasides [53] suggested that TATI might be potentially useful in monitoring treatment efficacy in transitional cell carcinoma of the bladder. Significantly modified values of TATI were observed in metastatic diseases, in patients with complete or partial remission and non-responders. A significant increase in TATI in T2-T4-N0M0 tumours were in the non-responders.
Kelloniemi et al. showed that TATI might be an independent prognostic factor for the identification group of patients with adverse prognosis in transitional cell carcinoma serum [54].
Gkialas [55] showed that TATI was significantly more sensitive in stage Ta (80%) than was CYFRA 21-1 (32%), UBC (12%) and cytology (20%). TATI was different also between stages and was more sensitive compared with other tumour markers for stage T1.
Patschan and colleagues [56] confirmed that the TATI level positively correlates with low-stage tumours and the favourable differentiation of bladder cancer. They also showed in univariate analyses that a decreased level of TATI was associated with high recurrences and cancer-specific mortality.
Liu [57]made a similar conclusion that a decrease in the TATI expression correlated with more advanced disease. Moreover, in the progression of bladder cancer, the prognostic value of a p53 overexpression can be enhanced by TATI.
Bladder cancer management is one of the most complex and expensive in uro-oncology. An ideal biomarker of the future should be potentially able to detect the disease before its clinical manifestation. The BC mortality rate is another major reason to obtain a similar screening method to that available in other cancers, i.e., prostate and colon.
Currently, flexible cystoscopy remains a mainstay in BC diagnosis, and it appears unlikely that available biomarkers would quickly rule out this standard approach in clinical practice. On the other hand, developing markers showing a correlation with cancer aggressiveness and distinguishing between aggressive and non-aggressive tumours appears of utmost clinical importance. Hopefully, one of the discussed markers might become helpful in patients’ selection for an appropriate treatment plan and personalized cancer medicine. The prospective studies on a larger group of individuals are still needed to obtain additional prognostic information that will improve results, reduce adverse effects and in future, allow us to individualize bladder cancer treatments.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30.
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur. Urol. 2017, 71, 96–108.
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Primers 2017, 3, 1–19.
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 2017, 171, 540–556.
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO classification of tumours of the urinary system and male genital organs—Part B: Prostate and bladder tumours. Eur. Urol. 2016, 70, 106–119.
- Czerniak, B.; Dinney, C.; McConkey, D. Origins of bladder cancer. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 149–174.
- Babjuk, M.; Burger, M.; Zigeuner, R.; Shariat, S.F.; van Rhijn, B.W.G.; Compérat, E.; Sylvester, R.J.; Kaasinen, E.; Böhle, A.; Redorta, J.P.; et al. EAU Guidelines on Non–Muscle-invasive urothelial carcinoma of the bladder: Update 2013. Eur. Urol. 2013, 64, 639–653.
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2014, 15, 25–41.
- Inamura, K. Bladder cancer: New insights into its molecular pathology. Cancers 2018, 10, 100.
- Burger, M.; Catto, J.W.F.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; Vecchia, C.L.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241.
- Woldu, S.L.; Bagrodia, A.; Lotan, Y. Guideline of guidelines: Non-muscle-invasive bladder cancer. BJU Int. 2017, 119, 371–380.
- Huang, Y.-L.; Chen, J.; Yan, W.; Zang, D.; Qin, Q.; Deng, A.-M. Diagnostic accuracy of cytokeratin-19 fragment (CYFRA 21–1) for bladder cancer: a systematic review and meta-analysis. Tumor Biol. 2015, 36, 3137–3145.
- Kuang, L.I.; Song, W.J.; Qing, H.M.; Yan, S.; Song, F.L. CYFRA21-1 levels could be a biomarker for bladder cancer: A meta-analysis. Genet. Mol. Res. 2015, 14, 3921–3931.
- Nisman, B.; Yutkin, V.; Peretz, T.; Shapiro, A.; Barak, V.; Pode, D. The follow-up of patients with non-muscle-invasive bladder cancer by urine cytology, abdominal ultrasound and urine CYFRA 21-1: A pilot study. Anticancer Res. 2009, 29, 4281–4285.
- Rabik, C.A.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23.
- Martin, L.P.; Hamilton, T.C.; Schilder, R.J. Platinum resistance: The role of DNA repair pathways. Clin. Cancer Res. 2008, 14, 1291–1295.
- Metzger, R.; Bollschweiler, E.; Hölscher, A.H.; Warnecke-Eberz, U. ERCC1: Impact in multimodality treatment of upper gastrointestinal cancer. Future Oncol. 2010, 6, 1735–1749.
- Olaussen, K.A.; Dunant, A.; Fouret, P.; Brambilla, E.; André, F.; Haddad, V.; Taranchon, E.; Filipits, M.; Pirker, R.; Popper, H.H.; et al. DNA repair by ERCC1 in Non–Small-Cell Lung Cancer and Cisplatin-Based Adjuvant Chemotherapy. N. Engl. J. Med. 2006, 355, 983–991.
- Simon, G.R.; Sharma, S.; Cantor, A.; Smith, P.; Bepler, G. ERCC1 expression is a predictor of survival in resected patients with non-small cell lung cancer. Chest 2005, 127, 978–983.
- Rosell, R.; Pifarré, A.; Monzó, M.; Astudillo, J.; López-Cabrerizo, M.P.; Calvo, R.; Moreno, I.; Sánchez-Céspedes, M.; Font, A.; Navas-Palacios, J.J. Reduced survival in patients with stage-I non-small-cell lung cancer associated with DNA-replication errors. Int. J. Cancer 1997, 74, 330–334.
- Eldehna, W.M.; Fouda, M.M.; Eteba, S.M.; Abdelrahim, M.; Elashry, M.S. Gene expression of excision repair cross-complementation group 1 enzyme as a novel predictive marker in patients receiving platinum-based chemotherapy in advanced bladder cancer. Benha Med. J. 2018, 35, 42–48.
- Klatte, T.; Seitz, C.; Rink, M.; Rouprêt, M.; Xylinas, E.; Karakiewicz, P.; Susani, M.; Shariat, S.F. ERCC1 as aprognostic and predictive biomarker for urothelial carcinoma of the bladder following radical cystectomy. J. Urol. 2015, 194, 1456–1462.
- Hemdan, T.; Segersten, U.; Malmström, P.-U. 122 ERCC1-negative tumors benefit from neoadjuvant cisplatin-based chemotherapy whereas patients with ERCC1-positive tumors do not—Results from a cystectomy trial database. Eur. Urol. Suppl. 2014, 13, e122.
- Urun, Y.; Leow, J.J.; Fay, A.P.; Albiges, L.; Choueiri, T.K.; Bellmunt, J. ERCC1 as a prognostic factor for survival in patients with advanced urothelial cancer treated with platinum based chemotherapy: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2017, 120, 120–126.
- Chen, L.; Liu, Y.; Zhang, Q.; Zhang, M.; Han, X.; Li, Q.; Xie, T.; Wu, Q.; Sui, X. p53/PCDH17/Beclin-1 proteins as prognostic predictors for urinary bladder cancer. J. Cancer 2019, 10, 6207–6216.
- Choundhury, S.; Kolukula, V.; Preet, A.; Albanese, C.; Maria, A. Dissecting the pathways that destabilize mutant p53: The proteasome or autophagy? Cell Cycle 2013, 12, 1022–1029.
- Zhou, G.; Wang, J.; Zhao, M.; Xie, T.-X.; Tanaka, N.; Sano, D.; Patel, A.A.; Ward, A.M.; Sandulache, V.C.; Jasser, S.A.; et al. Gain-of-function mutant p53 promotes cell growth and cancer cell metabolism via inhibition of AMPK activation. Mol. Cell 2014, 54, 960–974.
- Mitra, A.P. Molecular substratification of bladder cancer: Moving towards individualized patient management. Ther. Adv. Urol. 2016, 8, 215–233.
- Ando, K.; Oki, E.; Saeki, H.; Yan, Z.; Tsuda, Y.; Hidaka, G.; Kasagi, Y.; Otsu, H.; Kawano, H.; Kitao, H.; et al. Discrimination of p53 immunohistochemistry-positive tumors by its staining pattern in gastric cancer. Cancer Medicine 2014, 4, 75–83.
- Puzio-Kuter, A.M.; Castillo-Martin, M.; Kinkade, C.W.; Wang, X.; Shen, T.H.; Matos, T.; Shen, M.M.; Cordon-Cardo, C.; Abate-Shen, C. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009, 23, 675–680.
- Shariat, S.F.; Chade, D.C.; Karakiewicz, P.I.; Ashfaq, R.; Isbarn, H.; Fradet, Y.; Bastian, P.J.; Nielsen, M.E.; Capitanio, U.; Jeldres, C. Combination of multiple molecular markers can improve prognostication in patients with locally advanced and lymph node positive bladder cancer. J. Urol. 2010, 183, 68–75.
- Daizumoto, K.; Yoshimaru, T.; Matsushita, Y.; Fukawa, T.; Uehara, H.; Ono, M.; Komatsu, M.; Kanayama, H.; Katagiri, T. A DDX31/Mutant–p53/EGFR axis promotes multistep progression of muscle-invasive bladder cancer. Cancer Res. 2018, 78, 2233–2247.
- Qamar, S.; Inam, Q.A.; Ashraf, S.; Khan, M.S.; Khokhar, M.A.; Awan, N. Prognostic Value of p53 expression intensity in urothelial cancers. J. Coll. Physicians Surg. Pak. 2017, 27, 232–236.
- Ciccarese, C.; Massari, F.; Blanca, A.; Tortora, G.; Montironi, R.; Cheng, L.; Scarpelli, M.; Raspollini, M.R.; Vau, N.; Fonseca, J.; et al. Tp53 and its potential therapeutic role as a target in bladder cancer. Expert Opin. Ther. Targets 2017, 21, 401–414.
- Du, J.; Wang, S.; Yang, Q.; Chen, Q.; Yao, X. p53 status correlates with the risk of progression in stage T1 bladder cancer: A meta-analysis. World J. Surg. Oncol. 2016, 14, 137.
- Shariat, S.F.; Lotan, Y.; Karakiewicz, P.I.; Ashfaq, R.; Isbarn, H.; Fradet, Y.; Bastian, P.J.; Nielsen, M.E.; Capitanio, U.; Jeldres, C.; et al. p53 predictive value for pT1-2 N0 disease at radical cystectomy. J. Urol. 2009, 182, 907–913.
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO classification of tumours of the urinary system and male genital organs—Part A: Renal, penile, and testicular tumours. Eur. Urol. 2016, 70, 93–105.
- Sung, J.-Y.; Sun, J.-M.; Chang Jeong, B.; Il Seo, S.; Soo Jeon, S.; Moo Lee, H.; Choi, H.Y.; Kang, S.Y.; Choi, Y.-L.; Young Kwon, G. FGFR3 overexpression is prognostic of adverse outcome for muscle-invasive bladder carcinoma treated with adjuvant chemotherapy11This work was supported by Grant CB-2011-04-01 from Korean Foundation for Cancer Research grant and by a Global Frontier Project Grant (NRF-M1AXA002-2010-0029795) of the National Research Foundation funded by the Ministry of Education, Science and Technology of Korea. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 49.e23–49.e31.
- Akanksha, M.; Sandhya, S. Role of FGFR3 in Urothelial Carcinoma. Iran. J. Pathol. 2019, 14, 148–155.
- Williams, S.V.; Hurst, C.D.; Knowles, M.A. Oncogenic FGFR3 gene fusions in bladder cancer. Hum. Mol. Genet. 2012, 22, 795–803.
- Di Martino, E.; Tomlinson, D.C.; Knowles, M.A. A decade of FGF receptor research in bladder cancer: Past, present, and future challenges. Adv. Urol. 2012, 2012, 1–10.
- Beukers, W.; van der Keur, K.A.; Kandimalla, R.; Vergouwe, Y.; Steyerberg, E.W.; Boormans, J.L.; Jensen, J.B.; Lorente, J.A.; Real, F.X.; Segersten, U.; et al. FGFR3, TERT and OTX1 as a urinary biomarker combination for surveillance of patients with bladder cancer in a large prospective multicenter study. J. Urol. 2017, 197, 1410–1418.
- Hurst, C.D.; Knowles, M.A. Multiomic profiling refines the molecular view. Nat. Rev. Clin. Oncol. 2017, 15, 203–204.
- Van Oers, J.M.M.; Zwarthoff, E.C.; Rehman, I.; Azzouzi, A.-R.; Cussenot, O.; Meuth, M.; Hamdy, F.C.; Catto, J.W.F. FGFR3 mutations indicate better survival in invasive upper urinary tract and bladder tumours. Eur. Urol. 2009, 55, 650–658.
- Van Rhijn, B.W.G.; van der Kwast, T.H.; Liu, L.; Fleshner, N.E.; Bostrom, P.J.; Vis, A.N.; Alkhateeb, S.S.; Bangma, C.H.; Jewett, M.A.S.; Zwarthoff, E.C.; et al. The FGFR3 mutation is related to favorable pT1 bladder cancer. J. Urol. 2012, 187, 310–314.
- Hernandez, S.; Lopez-Knowles, E.; Lloreta, J.; Kogevinas, M.; Amorós, A.; Tardón, A.; Carrato, A.; Serra, C.; Malats, N.; Real, F.X. Prospective study of fgfr3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J. Clin. Oncol. 2006, 24, 3664–3671.
- Critelli, R.; Fasanelli, F.; Oderda, M.; Polidoro, S.; Assumma, M.B.; Viberti, C.; Preto, M.; Gontero, P.; Cucchiarale, G.; Lurkin, I.; et al. Detection of multiple mutations in urinary exfoliated cells from male bladder cancer patients at diagnosis and during follow-up. Oncotarget 2016, 7, 67435.
- Frantzi, M.; Makridakis, M.; Vlahou, A. Biomarkers for bladder cancer aggressiveness. Curr. Opin. Urol. 2012, 22, 390–396.
- Tomlinson, D.; Baldo, O.; Harnden, P.; Knowles, M. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J. Pathol. 2007, 213, 91–98.
- Christensen, E.; Birkenkamp-Demtröder, K.; Nordentoft, I.; Høyer, S.; van der Keur, K.; van Kessel, K.; Dyrskjøt, L. Liquid biopsy analysis of FGFR3 and PIK3CA hotspot mutations for disease surveillance in bladder cancer. Eur. Urol. 2017, 71, 961–969.
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; McConkey, D.J. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014, 25, 152–165.
- Tramonti, P.G.; Ferdeghini, M.; Donadio, C.; Annichiarico, C.; Norpoth, M.; Bianchi, R.; Bianchi, C. Serum levels of tumor associated trypsin inhibitor (TATT) and glomerular filtration rate. Ren. Fail. 1998, 20, 295–302.
- Pectasides, D.; Bafaloucos, D.; Antoniou, F.; Gogou, L.; Economides, N.; Varthalitis, J.; Athanassiou, A. TPA, TATI, CEA, AFP, β-HCG, PSA, SCC, and CA 19-9 for monitoring transitional cell carcinoma of the bladder. Am. J. Clin. Oncol. 1996, 19, 271–277
- Kelloniemi, E.; Rintala, E.; Finne, P.; Stenman, U.-H. Tumor-associated trypsin inhibitor as a prognostic factor during follow-up of bladder cancer. Urology 2003, 62, 249–253.
- Gkialas, I.; Papadopoulos, G.; Iordanidou, L.; Stathouros, G.; Tzavara, C.; Gregorakis, A.; Lykourinas, M. Evaluation of urine tumor-associated trypsin inhibitor, CYFRA 21-1, and urinary bladder cancer antigen for detection of high-grade bladder Carcinoma. Urology 2008, 72, 1159–1163.
- Patschan, O.; Shariat, S.F.; Chade, D.C.; Karakiewicz, P.I.; Ashfaq, R.; Lotan, Y.; Hotakainen, K.; Stenman, U.-H.;Bjartell, A. Association of tumor-associated trypsin inhibitor (TATI) expression with molecular markers, pathologic features and clinical outcomes of urothelial carcinoma of the urinary bladder. World J. Urol. 2011, 30, 785–794.
- Liu, A.; Xue, Y.; Liu, F.; Tan, H.; Xiong, Q.; Zeng, S.; Zhang, Z.; Gao, X.; Sun, Y.; Xu, C. Prognostic value of the combined expression of tumor-associated trypsin inhibitor (TATI) and p53 in patients with bladder cancer undergoing radical cystectomy. Cancer Biomark. 2019, 26, 281–289.





