
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hemant Khanna | + 2636 word(s) | 2636 | 2021-08-02 04:52:17 | | | |
| 2 | Dean Liu | Meta information modification | 2636 | 2021-08-13 02:38:14 | | | | |
| 3 | Dean Liu | Meta information modification | 2636 | 2021-08-13 02:38:37 | | |
Video Upload Options
The eye is at the forefront of developing therapies for genetic diseases. With the FDA approval of the first gene-therapy drug for a form of congenital blindness, numerous studies have been initiated to develop gene therapies for other forms of eye diseases. These examinations have revealed new information about the benefits as well as restrictions to using drug-delivery routes to the different parts of the eye. In this entry, authors will discuss the ocular delivery landscape that is currently being investigated and provide insights into their advantages and disadvantages. Efficient delivery routes and vehicle are crucial for an effective, safer, and longer-lasting therapy.
1. Introduction
The complexity of eyes has perplexed scientists of the likes of Charles Darwin. The eye is considered one of the greatest leaps in evolution. Fossil evidence has revealed that eyes appeared ~500 million years ago and became an indispensable tool for survival [1].
The human eye is a camera-type sense organ that allows external visual cues to be transmitted to the brain. The light enters through the anterior chamber of the eye and passes through the cornea, aqueous humor, and the lens before entering the vitreous humor and traversing the inner retina to reach the retina in the posterior compartment. Here, the light signal is converted into an electrical impulse that communicates with the inner retinal neurons and eventually transports to the optic nerve. The signal is then sent to the processing centers in the central nervous system [2].
The vertebrate retina is a light-sensitive tissue containing five major types of neurons (photoreceptors, bipolar cells, amacrine cells, horizontal cells, and retinal ganglion cells) and three types of glial cells (Muller glia, microglia, and astroglia) organized in three distinct layers of cell bodies separated by two synaptic layers. The photoreceptors (rods and cones) account for >70% of the cell types in the retina and are the first responders to light. They contain the photopigment opsin that isomerizes in response to light and generates action potential. The rods are sensitive to lower-intensity light and help us see in starlight (at night). Cones, on the other hand, respond to brighter light and help us see during the day. Commensurately, we depend upon our cones for our day-to-day activities. The importance of cones in maintaining our quality of life is also exemplified by the presence of a cone-rich and rodless central area in the primate retina called the fovea. This structure is part of the macula, which contains the highest density of rods and cones in the central region [2].
Given the importance of visual input for human survival, vision disabilities are one of the top ten disabilities in humans. According to the Centers for Disease Control and Prevention, >3 million people in the United States have vision impairment. By 2050, this number is expected to double to ~6 million people ( https://www.cdc.gov/visionhealth/risk/burden.htm ; accessed on 6 July 2021). Although the most prevalent eye disorders include complex genetic diseases such as age-related macular degeneration, diabetic retinopathy, cataracts, and glaucoma, the rare forms of inherited retinal degenerations (IRDs) have presented unique challenges in management and treatment.
2. Gene Therapy
The subretinal injection is an invasive surgical procedure in which the therapeutics are delivered between the photoreceptors and the RPE [3] (Figure 1). It requires an operating room, is usually performed under general anesthesia, and carries the risk of retinal tears, detachments, and macular holes. Intravitreal injections (IVIs) on the other hand, are relatively safer and can be performed in the doctor’s office [3]. It is currently used clinically for injecting anti-angiogenic agents for age-related macular degeneration and diabetic retinopathy. Thus, IVI is a preferable procedure for ocular injections.
Suprachoroidal injections are a recent breakthrough in the retinal gene-delivery landscape [4] (Figure 1). These are less invasive than subretinal injection and involve accessing the retina by injecting into the space between the choroid (overlaying the RPE) and the sclera [5]. This method has been successful in large-animal models and was demonstrated to be a safer approach in a phase 3 clinical trial to treat uveitis with macular edema [6]. Some disadvantages of this method include the use of specialized needles, inaccessibility in smaller animals and difficulty of the AAV vectors to traverse the choroid layer to reach the RPE and the photoreceptors. Nonetheless, the suprachoroidal delivery route provides a unique opportunity to perform less invasive surgeries for retinal gene delivery.
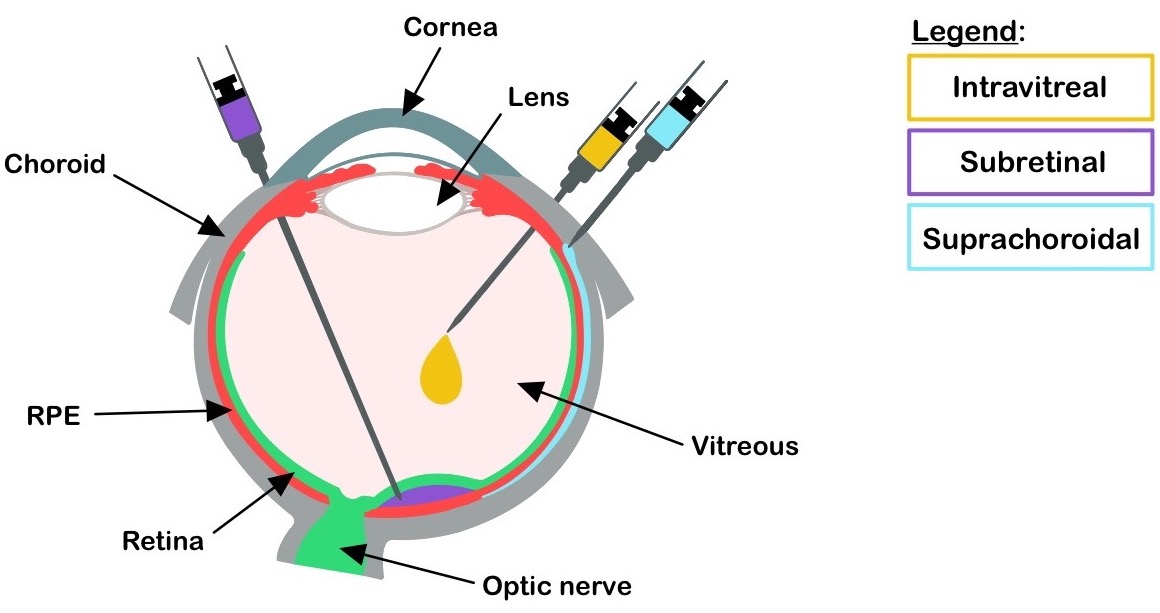 Figure 1. Drug delivery routes. Ocular delivery routes at the indicated locations are depicted. RPE: retinal pigment epithelium.
Figure 1. Drug delivery routes. Ocular delivery routes at the indicated locations are depicted. RPE: retinal pigment epithelium.
2.1. Vectors for Gene Delivery
Vectors for gene therapy are vehicles that carry the gene of interest to the host cells. There are two major subclasses of vectors: nonviral and viral vectors (Figure 2).
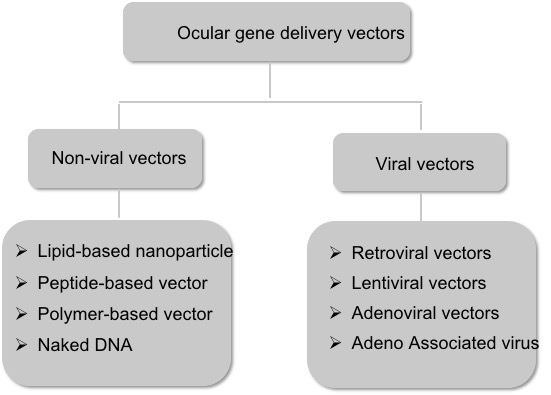 Figure 2. Schematic representation of the major subtypes of the ocular gene delivery vectors.
Figure 2. Schematic representation of the major subtypes of the ocular gene delivery vectors.
2.1.1. Non-Viral Vectors
2.1.2. Viral Vectors
The capsid determines the cell and tissue tropism of the rAAV. Through capsid development, novel rAAV capsids have been discovered or developed that have new and favorable characteristics. Over the years, the AAV virus capsid was modified to infect diverse cell types in the retina. AAV2 and AAV5 were isolated from humans, while AAV4 and AAV8 were isolated from monkeys.
The prevalence of neutralizing antibodies and immunological response in humans against viral capsids is an important consideration when selecting rAAVs for gene delivery. Although rAAVs are non-integrating and efficiently transduce retinal populations, intraocular inflammation and loss of efficacy have been associated with rAAV-gene delivery. This response was observed in both intravitreal and subretinal delivery routes and is linked to the capsid and the dose of the AAV. AAV activates innate immune response, which releases the inflammatory cytokines and type-1 interferons. Neutralizing antibodies against the capsid can also reduce the therapeutic potential of the gene delivery [38]. To evade the immune system, George Church and colleagues recently reported the generation of engineered AAV vectors that are intrinsically less immunogenic. They achieved this by incorporating immunomodulatory noncoding sequences to “cloak” the vector from immune responses [47].
3. Conclusions
Non-viral delivery systems offer distinct advantages, including unlimited payload size, low immunogenicity, and minimal side effects. Although the efficacy of this approach has been reported earlier, such as in Abca4 −/− mice, the non-viral gene delivery strategy has not yet been used in any clinical trials for ocular diseases. Anatomical barriers in the retina and pH sensitivity of the nanoparticles are some of the many environmental challenges for efficient non-viral gene delivery and prolonged gene expression.
Based on the considerable research on the different viral vectors for gene delivery to date, rAAVs seem to be the safest and the most reliable vehicles for gene delivery. Advances in capsid identification, safety, and transduction are needed for their robust, successful, and wider clinical applications. Viral gene therapies are promising tools to transfer genetic material. The insights of precise gene therapy increase the speed for the discovery to restore vision that is destroyed by blinding diseases.
References
- Collin, S.P.; Davies, W.L.; Hart, N.S.; Hunt, D.M. The evolution of early vertebrate photoreceptors. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2925–2940.
- Kandel, E.R. Principles of Neural Science, 5th ed.; McGraw-Hill: New York, NY, USA, 2013; pp. 1227–1246.
- Ross, M.; Ofri, R. The future of retinal gene therapy: Evolving from subretinal to intravitreal vector delivery. Neural Regen. Res. 2021, 16, 1751–1759.
- Raghava, S.; Hammond, M.; Kompella, U.B. Periocular routes for retinal drug delivery. Expert Opin. Drug Deliv. 2004, 1, 99–114.
- Swan, R.; Kim, S.J.; Campbell, J.P.; Paul Chan, R.V.; Sonmez, K.; Taylor, K.D.; Li, X.; Chen, Y.I.; Rotter, J.I.; Simmons, C.; et al. The genetics of retinopathy of prematurity: A model for neovascular retinal disease. Ophthalmol. Retina 2018, 2, 949–962.
- Patel, S.R.; Lin, A.S.; Edelhauser, H.F.; Prausnitz, M.R. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm. Res. 2011, 28, 166–176.
- Chen, J.; Guo, Z.; Tian, H.; Chen, X. Production and clinical development of nanoparticles for gene delivery. Mol. Ther. Methods Clin. Dev. 2016, 3, 16023.
- Fink, T.L.; Klepcyk, P.J.; Oette, S.M.; Gedeon, C.R.; Hyatt, S.L.; Kowalczyk, T.H.; Moen, R.C.; Cooper, M.J. Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using compacted DNA nanoparticles. Gene Ther. 2006, 13, 1048–1051.
- Heth, C.A.; Bernstein, M.H. Mannose-sensitive HRP endocytosis by the retinal pigment epithelium. Exp. Eye Res. 1991, 52, 75–82.
- Farjo, R.; Skaggs, J.; Quiambao, A.B.; Cooper, M.J.; Naash, M.I. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS ONE 2006, 1, e38.
- Young, R.W. The renewal of photoreceptor cell outer segments. J. Cell Biol. 1967, 33, 61–72.
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244.
- Dalby, B.; Cates, S.; Harris, A.; Ohki, E.C.; Tilkins, M.L.; Price, P.J.; Ciccarone, V.C. Advanced transfection with Lipofectamine 2000 reagent: Primary neurons, siRNA, and high-throughput applications. Methods 2004, 33, 95–103.
- Sung, Y.K.; Kim, S.W. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 1–7.
- Balazs, D.A.; Godbey, W. Liposomes for use in gene delivery. J. Drug Deliv. 2011, 2011, 326497.
- Masuda, I.; Matsuo, T.; Yasuda, T.; Matsuo, N. Gene transfer with liposomes to the intraocular tissues by different routes of administration. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1914–1920.
- Wang, Y.; Rajala, A.; Rajala, R.V. Lipid Nanoparticles for Ocular Gene Delivery. J. Funct. Biomater. 2015, 6, 379–394.
- Sun, D.; Sahu, B.; Gao, S.; Schur, R.M.; Vaidya, A.M.; Maeda, A.; Palczewski, K.; Lu, Z.R. Targeted Multifunctional Lipid ECO Plasmid DNA Nanoparticles as Efficient Non-viral Gene Therapy for Leber’s Congenital Amaurosis. Mol. Ther. Nucleic Acids 2017, 7, 42–52.
- Zulliger, R.; Conley, S.M.; Naash, M.I. Non-viral therapeutic approaches to ocular diseases: An overview and future directions. J. Control Release 2015, 219, 471–487.
- Rai, R.; Alwani, S.; Badea, I. Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers 2019, 11, 745.
- Koirala, A.; Makkia, R.S.; Conley, S.M.; Cooper, M.J.; Naash, M.I. S/MAR-containing DNA nanoparticles promote persistent RPE gene expression and improvement in RPE65-associated LCA. Hum. Mol. Genet. 2013, 22, 1632–1642.
- Touchard, E.; Benard, R.; Bigot, K.; Laffitte, J.D.; Buggage, R.; Bordet, T.; Behar-Cohen, F. Non-viral ocular gene therapy, pEYS606, for the treatment of non-infectious uveitis: Preclinical evaluation of the medicinal product. J. Control Release 2018, 285, 244–251.
- Argyros, O.; Wong, S.P.; Niceta, M.; Waddington, S.N.; Howe, S.J.; Coutelle, C.; Miller, A.D.; Harbottle, R.P. Persistent episomal transgene expression in liver following delivery of a scaffold/matrix attachment region containing non-viral vector. Gene Ther. 2008, 15, 1593–1605.
- Han, Z.; Conley, S.M.; Naash, M.I. Gene therapy for Stargardt disease associated with ABCA4 gene. Adv. Exp. Med. Biol. 2014, 801, 719–724.
- Cooray, S.; Howe, S.J.; Thrasher, A.J. Retrovirus and lentivirus vector design and methods of cell conditioning. Methods Enzymol. 2012, 507, 29–57.
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272.
- Lukashev, A.N.; Zamyatnin, A.A., Jr. Viral Vectors for Gene Therapy: Current State and Clinical Perspectives. Biochemistry 2016, 81, 700–708.
- DiCarlo, J.E.; Mahajan, V.B.; Tsang, S.H. Gene therapy and genome surgery in the retina. J. Clin. Invest. 2018, 128, 2177–2188.
- Audo, I.S.; Weleber, R.G.; Stout, T.; Lauer, A.K.; Pennesi, M.E.; Mohand-Said, S.; Barale, P.O.; Buggage, R.; Wilson, D.J.; Sahel, J.A. Early findings in a phase I/IIa clinical program for stargardt disease (STGD1, MIM # 248200). Invest. Ophthalmol. Vis. Sci. 2015, 56, 3819.
- Seimetz, D.; Heller, K.; Richter, J. Approval of First CAR-Ts: Have we Solved all Hurdles for ATMPs? Cell Med. 2019, 11, 2155179018822781.
- Appaiahgari, M.B.; Vrati, S. Adenoviruses as gene/vaccine delivery vectors: Promises and pitfalls. Expert Opin. Biol. Ther. 2015, 15, 337–351.
- Wilson, J.M. Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol. Genet. Metab. 2009, 96, 151–157.
- Wold, W.S.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433.
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018, 20, e3015.
- Yang, Y.; Jooss, K.U.; Su, Q.; Ertl, H.C.; Wilson, J.M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996, 3, 137–144.
- Atchison, R.W.; Casto, B.C.; Hammon, W.M. Adenovirus-Associated Defective Virus Particles. Science 1965, 149, 754–756.
- Wu, Z.; Yang, H.; Colosi, P. Effect of genome size on AAV vector packaging. Mol. Ther. 2010, 18, 80–86.
- Seimetz, D.; Heller, K.; Richter, J. Approval of First CAR-Ts: Have we Solved all Hurdles for ATMPs? Cell Med. 2019, 11, 2155179018822781.
- Appaiahgari, M.B.; Vrati, S. Adenoviruses as gene/vaccine delivery vectors: Promises and pitfalls. Expert Opin. Biol. Ther. 2015, 15, 337–351.
- Wilson, J.M. Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol. Genet. Metab. 2009, 96, 151–157.
- Wold, W.S.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433.
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018, 20, e3015.
- Yang, Y.; Jooss, K.U.; Su, Q.; Ertl, H.C.; Wilson, J.M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996, 3, 137–144.
- Berns, K.I.; Adler, S. Separation of two types of adeno-associated virus particles containing complementary polynucleotide chains. J. Virol 1972, 9, 394–396.
- Atchison, R.W.; Casto, B.C.; Hammon, W.M. Adenovirus-Associated Defective Virus Particles. Science 1965, 149, 754–756.
- Wu, Z.; Yang, H.; Colosi, P. Effect of genome size on AAV vector packaging. Mol. Ther. 2010, 18, 80–86.
- Chan, Y.K.; Wang, S.K.; Chu, C.J.; Copland, D.A.; Letizia, A.J.; Costa Verdera, H.; Chiang, J.J.; Sethi, M.; Wang, M.K.; Neidermyer, W.J., Jr.; et al. Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci. Transl. Med. 2021, 13.




