
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Karolina Dorobisz | + 1920 word(s) | 1920 | 2021-08-02 10:09:20 |
Video Upload Options
Estrogens are a group of steroid hormones that recently have gained even more attention in the eyes of scientists. There is an ongoing discussion in the scientific community about their relevance as environmental contaminants and the danger they pose to animal health and welfare. In available literature we can find many examples of their negative effects and mechanisms that are involved with such phenomena.
1. Introduction
Currently we can observe a growing interest in the state of the environment, methods of its protection, and the impact that the pollutants present in it have on the health of living organisms. Our knowledge of already present and emerging types of pollution is still expanding, but at an insufficient pace. Sex hormones are one of the groups of pollutants that have recently attracted the attention of scientists. The available literature indicates that the most important of them in terms of environmental impact are hormones belonging to the group of estrogens. Estrogens are a group of sex hormones that include estrone (E1), estradiol (E2), estriol (E3), estetrol (E4)—produced only during pregnancy, and often synthetic ethinyloestradiol (EE2). Estrogens are also called female hormones and they play crucial a role in female organisms, but it should be taken into consideration that they are also necessary for proper functioning of male organisms [1].
Estrogens mainly imply their effects by interaction with isoforms of the estrogen receptor (ER)—ERα and ERβ which then bind these hormones in the cytoplasm of cells and transport this complex to the cell nucleus. As a result, the activation of response elements in gene promoters begins the transcription process. Aforementioned receptors can be divided into nuclear estrogen receptors (nERs) and membrane estrogen receptors (mERs). Beside the “traditional” estrogen action, additional ways have been described—influence through cell signal transduction tied with mERs rather than genomic activity process. The available literature discusses the differences in the affinity and mechanism of action of these receptors, however, as shown by the latest studies, there is a high degree of functional and structural similarity between mERs and nERs [2]. Recently, additional receptors, namely, ER-X and Erx were described in the literature but additional research on them and mechanisms involved with their action are highly advised.
There are many potential sources of contamination of the environment with estrogens, such as animal farms, slaughterhouses, or large urban agglomerations ( Figure 1 ) [3][4]. Estrogens present in excreta, due to natural or artificial processes (like hormone therapy or contraception) contaminate, by wastewater or fertilization, water and soil. An additional factors determining the degree of risk resulting from the presence of sex hormones in the environment are their half-life, which varies depending on the physico-chemical conditions, the microbiological richness of contaminated waters and soil, from 12 h to even 180 days in the case of water reservoirs without a stabilized population of microorganisms [5]. Removal of estrogen from the aquatic environment is important, however, it is difficult to achieve even with the use of modern filtration methods [6][7][8]. As evidenced by recent research, microbial degradation of estrogens can be led by many bacteria strains i.e., Rhodococcus, Novosphingobium , Acinetobacter , Agromyces , and Sphingomonas , thus showing possible safe and inexpensive ways for the reduction of threat involved with such pollution [9][10][11][12][13]. It is worth noting that some fungi, mainly species belonging to Aspergillus genus are also reported to perform aerobic degradation of estrogens [9]. Another threat related to the presence of hormones in the aquatic environment may be the processes of their accumulation in bottom sediments, from which they can be released again under appropriate physical and chemical conditions [9][10][11]. Another factor of risk can be their bioaccumulation in living organisms [9][11][14][15].
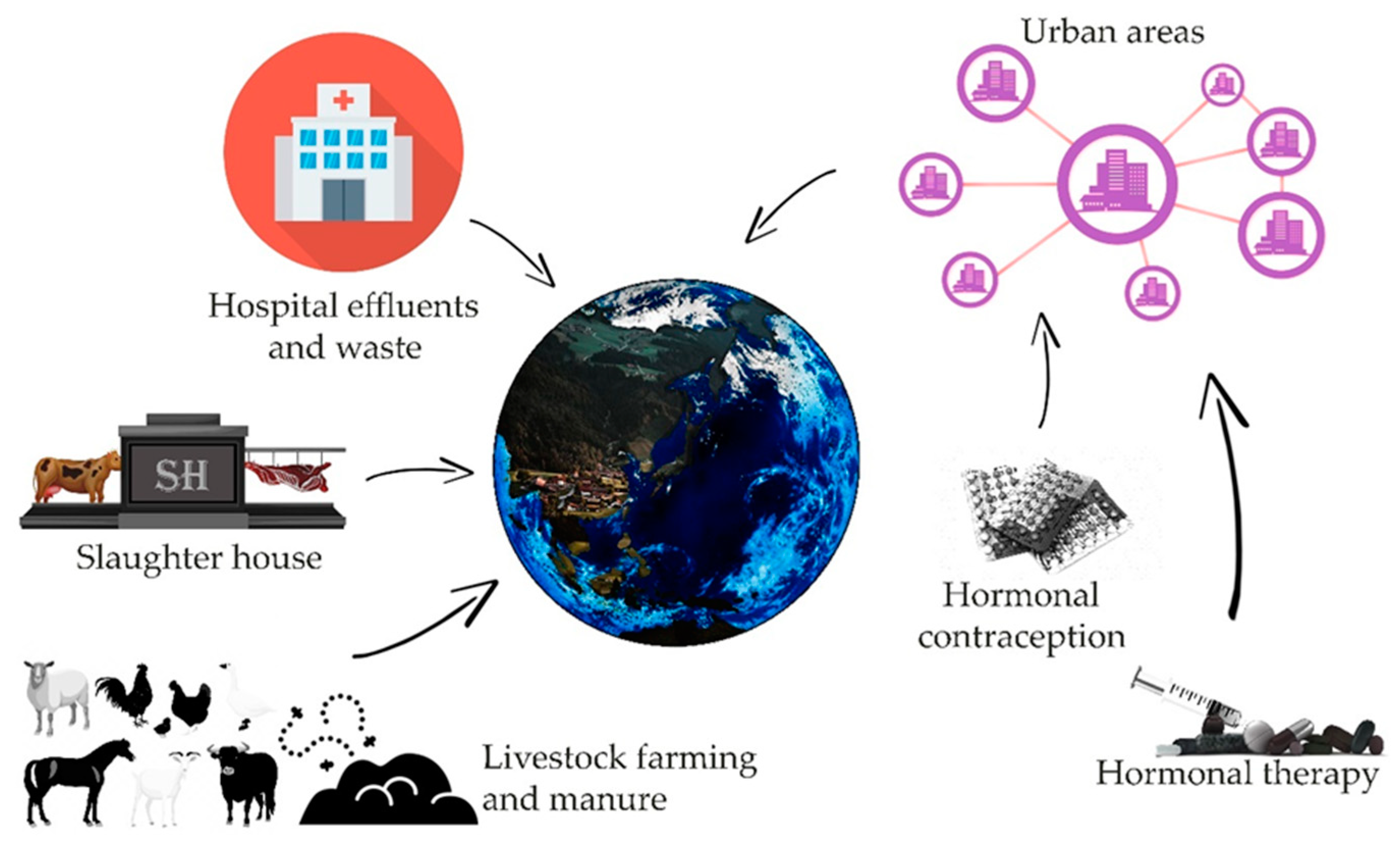 Figure 1. Sources of estrogens present in the environment and their simplified pathways leading to the environment (based on [4][5][6][7][8]).
Figure 1. Sources of estrogens present in the environment and their simplified pathways leading to the environment (based on [4][5][6][7][8]).
Occurring in the environment, they can lead to many negative consequences for health or the functioning of organisms directly or indirectly related to it. Those effect include feminization, dysregulation of natural processes related to reproduction, deterioration of the general condition of organisms, disturbances in the regulation of apoptotic processes [16], or even promoting processes leading to cancerogenesis [17][18].
2. Impact of Estrogens Present in Environment on Health and Welfare of Animals
2.1. Invertebrates
Invertebrates are one of the groups most vulnerable to environmental estrogens contamination; it is related to the periodic exposure of their juvenile forms, often related to the aquatic environment, or the constant exposure of these organisms to the effects of these compounds. In the case of invertebrates, attention should be paid to a slightly different functioning of the endocrine system, both in terms of biochemistry and the mechanisms of regulation themselves [19][20], however, it does not change the fact that the presence of both natural and synthetic estrogens can affect many aspects of their lives. There are a lot of evidence in the available literature confirming the negative influence of the presence of sex hormones in invertebrates. Bovier et al. showed that the addition of EE2 solutions to the medium administered to individuals belonging to the model invertebrate species Drosophila melanogaster statistically significantly reduced the survival and fertility parameters of the studied insects [21].
2.2. Fish
2.3. Amphibians
Among the terrestrial vertebrates, amphibians are the group most closely associated with the aquatic environment. Hence, potential exposure to estrogen contamination appears to pose a relatively greater threat to them. Most estrogens get into the environment with surface runoff or in sewage leachate, where their concentrations may be at levels that are hazardous to the health of amphibians [27][28]. In recent years, researchers have increasingly suggested that the current global decline in the amphibian population is related to the increase in pollutants, especially those of the nature of steroid hormones [29][30][31]. This phenomenon is increasingly dangerous because of the wide range of estrogenic effects on various development stages. The observed effects of pollution may lead to behavioral or sensory changes, as well as physiological changes, disrupting ontogenesis at its various stages and even being lethal [32]. In the research, the most commonly used amphibians are Anura, with the clawed frog Xenopus laevis adopted as the model species; also, numerous representatives of the genus Rana and Bufo.
2.4. Reptiles
2.5. Birds
2.6. Mammals
Estrogens can enter mammals in many ways, not only by ingesting water contaminated with them or through the skin during contact with it [46], but also through the food they eat, an example of which may be the accumulation of estrogens along with trophic levels in consumed foods, e.g., in plants [47][48], fish [49] or even as indicated in the literature in milk, however, in this case the literature reports are contradictory [50]. A very important phenomenon in the context of the threat posed by estrogens is the fact that very often mixtures of these are found in the environment, which in addition to the additive effect of these compounds may also show the synergistic effect mentioned previously [26][51]. There are a number of potential negative consequences of their action on mammals such as reproductive disorders and lowering the general condition of the body associated with their negative impact at their excessively normal concentrations [52]. There are many reports in the literature indicating an increased risk of carcinogenesis under the influence of estrogens [5][17][50][53]. Słowikowski et al. [54] describe two mechanisms that can lead to this, i.e., by destroying the structure of proteins or the structure of the genetic code. The effect of estrogens has been associated with cancers of the prostate, lung, endometrium, and breast [5][17][50][53].
3. Conclusions
References
- Hammes, S.R.; Levin, E.R. Impact of estrogens in males and androgens in females. J. Clin. Investig. 2019, 129, 1818–1826.
- Boonyaratanakornkit, V.; Edwards, D.P. Receptor Mechanisms Mediating Non-Genomic Actions of Sex Steroids. In Seminars in Reproductive Medicine; Thieme Medical Publishers: New York, NY, USA, 2007; Volume 25, pp. 139–153.
- Rechsteiner, D.; Schrade, S.; Zähner, M.; Müller, M.; Hollender, J.; Bucheli, T.D. Occurrence and Fate of Natural Estrogens in Swiss Cattle and Pig Slurry. J. Agric. Food Chem. 2020, 68, 5545–5554.
- Yang, S.; Yu, W.; Yang, L.; Du, B.; Chen, S.; Sun, W.; Jiang, H.; Xie, M.; Tang, J. Occurrence and Fate of Steroid Estrogens in a Chinese Typical Concentrated Dairy Farm and Slurry Irrigated Soil. J. Agric. Food Chem. 2021, 69, 67–77.
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119.
- Huang, Y.; Li, W.; Qin, L.; Xie, X.; Gao, B.; Sun, J.; Li, A. Distribution of endocrine-disrupting chemicals in colloidal and soluble phases in municipal secondary effluents and their removal by different advanced treatment processes. Chemosphere 2019, 219, 730–739.
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions—A review. Water Res. 2018, 133, 182–207.
- Silva, L.G.R.; Costa, E.P.; Starling, M.C.V.M.; dos Santos Azevedo, T.; Bottrel, S.E.C.; Pereira, R.O.; Sanson, A.L.; Afonso, R.J.C.F.; Amorim, C.C. LED irradiated photo-Fenton for the removal of estrogenic activity and endocrine disruptors from wastewater treatment plant effluent. Environ. Sci. Pollut. Res. 2021, 28, 24067–24078.
- Aris, A.Z.; Shamsuddin, A.S.; Praveena, S.M. Occurrence of 17α-ethynylestradiol (EE2) in the environment and effect on ex-posed biota: A review. Environ. Int. 2014, 69, 104–119.
- Shore, L.S.; Shemesh, M. Estrogen as an Environmental Pollutant. Bull. Environ. Contam. Toxicol. 2016, 97, 447–448.
- Müller, A.-K.; Markert, N.; Leser, K.; Kämpfer, D.; Crawford, S.E.; Schäffer, A.; Segner, H.; Hollert, H. Assessing endocrine disruption in freshwater fish species from a “hotspot” for estrogenic activity in sediment. Environ. Pollut. 2020, 257, 113636.
- Abdellah, Y.A.Y.; Zang, H.; Li, C. Steroidal Estrogens During Composting of Animal Manure: Persistence, Degradation, and Fate, a Review. Water Air Soil Pollut. 2020, 231, 1–19.
- Pratush, A.; Ye, X.; Yang, Q.; Kan, J.; Peng, T.; Wang, H.; Huang, T.; Xiong, G.; Hu, Z. Biotransformation strategies for steroid estrogen and androgen pollution. Appl. Microbiol. Biotechnol. 2020, 104, 2385–2409.
- Almeida, Â.; Silva, M.G.; Soares, A.M.V.M.; Freitas, R. Concentrations levels and effects of 17alpha-Ethinylestradiol in fresh-water and marine waters and bivalves: A review. Environ. Res. 2020, 185, 109316.
- He, K.; Hain, E.; Timm, A.; Blaney, L. Bioaccumulation of estrogenic hormones and UV-filters in red swamp crayfish (Pro-cambarus clarkii). Sci. Total Environ. 2021, 764, 142871.
- Amenyogbe, E.; Chen, G.; Wang, Z.; Lu, X.; Lin, M.; Lin, A.Y. A Review on Sex Steroid Hormone Estrogen Receptors in Mammals and Fish. Int. J. Endocrinol. 2020, 2020, 1–9.
- Henderson, B.E.; Feigelson, H.S. Hormonal carcinogenesis. Carcinogenesis 2000, 21, 427–433.
- Bohra, A.; Bhateja, S. Carcinogenesis and sex hormones: A review. Endocrinol. Metab. Syndr. 2015, 4, 1–14.
- Ford, A.T.; Leblanc, G.A. Endocrine Disruption in Invertebrates: A Survey of Research Progress. Environ. Sci. Technol. 2020, 54, 13365–13369.
- Castro, L.F.C.; Santos, M.M. To bind or not to bind: The taxonomic scope of nuclear receptor mediated endocrine disruption in invertebrate phyla. Environ. Sci. Technol. 2014, 48, 5361–5363.
- Bovier, T.F.; Rossi, S.; Mita, D.G.; Digilio, F.A. Effects of the synthetic estrogen 17-α-ethinylestradiol on Drosophila melanogaster: Dose and gender dependence. Ecotoxicol. Environ. Saf. 2018, 162, 625–632.
- Zubizarreta, L.; Silva, A.C.; Quintana, L. The estrogenic pathway modulates non-breeding female aggression in a teleost fish. Physiol. Behav. 2020, 220, 112883.
- Cabas, I.; Chaves-Pozo, E.; Mulero, V.; García-Ayala, A. Role of estrogens in fish immunity with special emphasis on GPER1. Dev. Comp. Immunol. 2018, 89, 102–110.
- Szwejser, E.; Kemenade, B.L.V.-V.; Maciuszek, M.; Chadzinska, M. Estrogen-dependent seasonal adaptations in the immune response of fish. Horm. Behav. 2017, 88, 15–24.
- Müller, A.-K.; Markert, N.; Leser, K.; Kämpfer, D.; Schiwy, S.; Riegraf, C.; Buchinger, S.; Gan, L.; Abdallah, A.T.; Denecke, B.; et al. Bioavailability and impacts of estrogenic compounds from suspended sediment on rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2021, 231, 105719.
- Thrupp, T.J.; Runnalls, T.J.; Scholze, M.; Kugathas, S.; Kortenkamp, A.; Sumpter, J.P. The consequences of exposure to mixtures of chemicals: Something from ‘nothing’ and ‘a lot from a little’ when fish are exposed to steroid hormones. Sci. Total Environ. 2018, 619, 1482–1492.
- Hoffmann, F.; Kloas, W. Estrogens Can Disrupt Amphibian Mating Behavior. PLoS ONE 2012, 7, e32097.
- Lambert, M.R.; Giller, G.S.J.; Barber, L.B.; Fitzgerald, K.C.; Skelly, D.K. Suburbanization, estrogen contamination, and sex ratio in wild amphibian populations. Proc. Natl. Acad. Sci. USA 2015, 112, 11881–11886.
- Kidd, K.A.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. USA 2007, 104, 8897–8901.
- Park, B.J.; Kidd, K. Effects of the synthetic estrogen ethinylestradiol on early life stages of mink frogs and green frogs in the wild and in situ. Environ. Toxicol. Chem. 2005, 24, 2027–2036.
- Hayes, T.B.; Case, P.; Chui, S.; Chung, D.; Haeffele, C.; Haston, K.; Lee, M.; Mai, V.P.; Marjuoa, Y.; Parker, J.; et al. Pesticide Mixtures, Endocrine Disruption, and Amphibian Declines: Are We Underestimating the Impact? Environ. Health Perspect. 2006, 114, 40–50.
- Ghali, K.; Leuenberger, J.; Ansermet, M.; Perrin, N.; Dufresnes, C. Toxic effects of estradiol E2 on development in the European tree frog (Hyla arborea). Herpetol. Notes 2016, 9, 249–253.
- Croteau, M.C.; Hogan, N.; Gibson, J.C.; Lean, D.; Trudeau, V.L. Toxicological threats to amphibians and reptiles in urban environments. Urban Herpetol. 2008, 3, 197–209.
- Matthews, J.; Celius, T.; Halgren, R.; Zacharewski, T. Differential estrogen receptor binding of estrogenic substances: A species comparison. J. Steroid Biochem. Mol. Biol. 2000, 74, 223–234.
- Kim, Y.S.; Stumpf, W.E.; Sar, M.; Christine, M.; Vargas, M. Estrogen and Androgen Target Cells in the Brain of Fishes, Reptiles and Birds: Phylogeny and Ontogeny. Am. Zool. 1978, 18, 425–433.
- Niranjan, M.K.; Koiri, R.K.; Srivastava, R. Expression of estrogen receptor alpha in response to stress and estrogen antagonist tamoxifen in the shell gland of Gallus gallus domesticus: Involvement of anti-oxidant system and estrogen. Stress 2021, 24, 261–272.
- Fry, D.M. Reproductive effects in birds exposed to pesticides and industrial chemicals. Environ. Health Perspect. 1995, 103, 165–171.
- Ottinger, M.A.; Quinn, M.J., Jr.; Lavoie, E.; Abdelnabi, M.A.; Thompson, N.; Hazelton, J.L.; Wu, J.M.; Beavers, J.; Jaber, M. Consequences of endocrine disrupting chemicals on reproductive endocrine function in birds: Establishing reliable end points of exposure. Domest. Anim. Endocrinol. 2005, 29, 411–419.
- Vézina, F.; Salvante, K.G.; Williams, T.D. The metabolic cost of avian egg formation: Possible impact of yolk precursor pro-duction? J. Exp. Biol. 2003, 206, 4443–4451.
- Dutta, S.; Inamdar, S.; Tso, J.; Aga, D.S.; Sims, J.T. Free and Conjugated Estrogen Exports in Surface-Runoff from Poultry Litter–Amended Soil. J. Environ. Qual. 2010, 39, 1688–1698.
- Hakk, H.; Millner, P.; Larsen, G. Decrease in Water-Soluble 17β-Estradiol and Testosterone in Composted Poultry Manure with Time. J. Environ. Qual. 2005, 34, 943–950.
- Bird, M.D.; Karavitis, J.; Kovacs, E.J. Sex differences and estrogen modulation of the cellular immune response after injury. Cell. Immunol. 2008, 252, 57–67.
- Ottinger, M.A.; Lavoie, E.; Thompson, N.; Barton, A.; Whitehouse, K.; Barton, M.; Abdelnabi, M.; Quinn, M., Jr.; Panzica, G.; Viglietti-Panzica, C. Neuroendocrine and behavioral effects of embryonic exposure to endocrine disrupting chemicals in birds. Brain Res. Rev. 2008, 57, 376–385.
- Nichols, D.J.; Daniel, T.C.; Moore, P.A., Jr.; Edwards, D.R.; Pote, D.H. Runoff of Estrogen Hormone 17β-Estradiol from Poultry Litter Applied to Pasture; Wiley Online Library: Hoboken, NJ, USA, 1997.
- Soma, K.K.; Alday, N.A.; Hau, M.; Schlinger, B.A. Dehydroepiandrosterone metabolism by 3β-hydroxysteroid dehydrogen-ase/Δ5–Δ4 isomerase in adult zebra finch brain: Sex difference and rapid effect of stress. Endocrinology 2004, 145, 1668–1677.
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine disrupting chemicals: Exposure, effects on human health, mecha-nism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord. 2020, 21, 127–147.
- Adeel, M.; Zain, M.; Fahad, S.; Rizwan, M.; Ameen, A.; Yi, H.; Baluch, M.A.; Lee, J.Y.; Rui, Y. Natural and synthetic estrogens in leafy vegetable and their risk associated to human health. Environ. Sci. Pollut. Res. 2018, 25, 36712–36723.
- Chen, X.; Li, Y.; Jiang, L.; Hu, B.; Wang, L.; An, S.; Zhang, X. Uptake, accumulation, and translocation mechanisms of steroid estrogens in plants. Sci. Total Environ. 2021, 753, 141979.
- Zhou, X.; Yang, Z.; Luo, Z.; Li, H.; Chen, G. Endocrine disrupting chemicals in wild freshwater fishes: Species, tissues, sizes and human health risks. Environ. Pollut. 2019, 244, 462–468.
- Snoj, T.; Majdič, G. Mechanisms in endocrinology: Estrogens in consumer milk: Is there a risk to human reproductive health? Eur. J. Endocrinol. 2018, 179, R275–R286.
- Ribeiro, E.; Ladeira, C.; Viegas, S. EDCs Mixtures: A Stealthy Hazard for Human Health? Toxics 2017, 5, 5.
- Kasonga, T.K.; Coetzee, M.A.A.; Kamika, I.; Ngole-Jeme, V.M.; Momba, M.N.B. Endocrine-disruptive chemicals as contami-nants of emerging concern in wastewater and surface water: A review. J. Environ. Manag. 2021, 277, 111485.
- Barakat, R.; Oakley, O.; Kim, H.; Jin, J.; Ko, C.J. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2016, 49, 488–496.
- Słowikowski, B.K.; Lianeri, M.; Jagodziński, P.P. Exploring estrogenic activity in lung cancer. Mol. Biol. Rep. 2017, 44, 35–50.


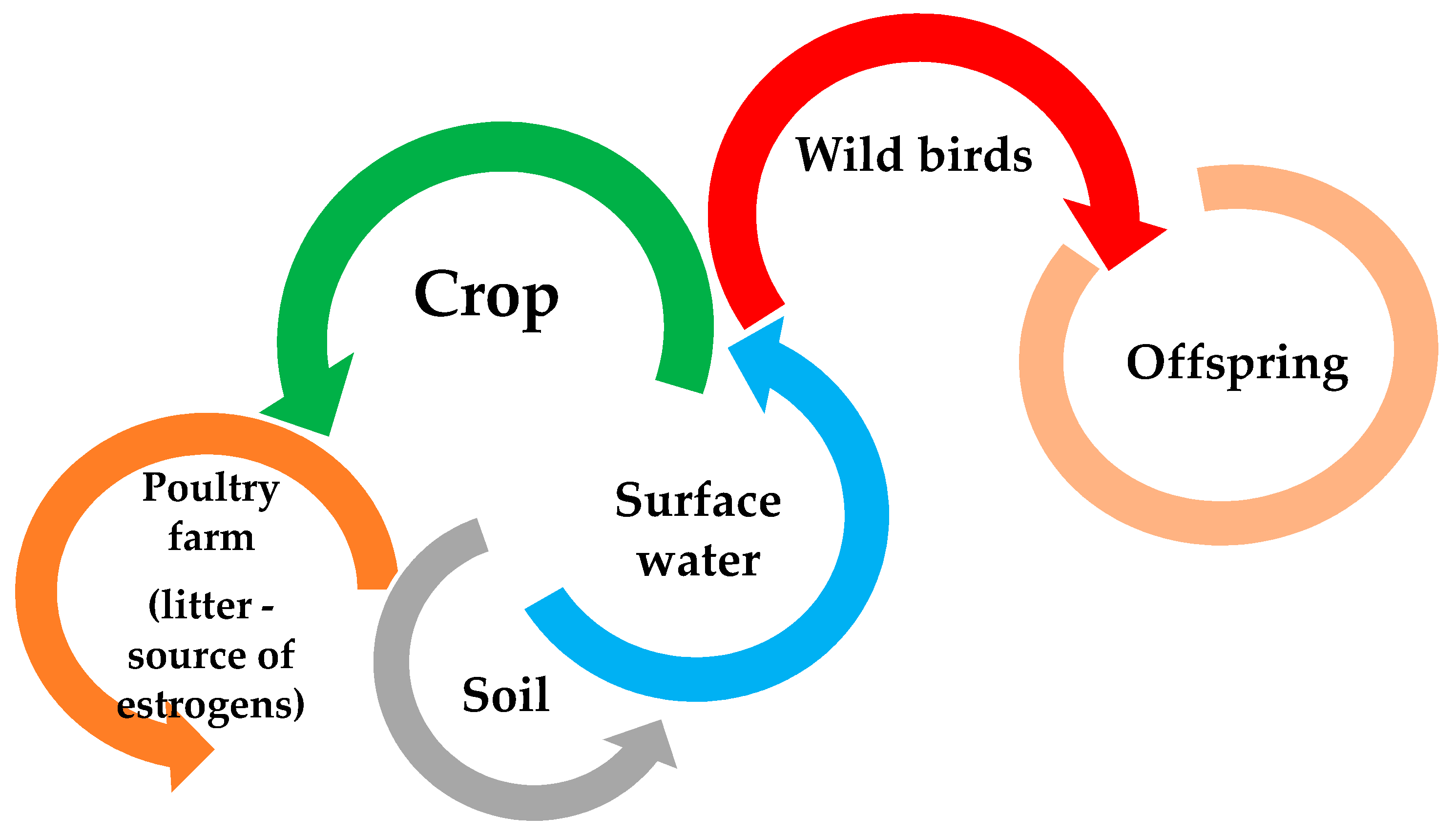 Figure 2. Estrogen contamination route (based on [
Figure 2. Estrogen contamination route (based on [

