Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rabin Dhakal | + 5791 word(s) | 5791 | 2021-08-11 05:56:32 | | | |
| 2 | Hanna Moussa | Meta information modification | 5791 | 2021-08-11 21:57:53 | | | | |
| 3 | Bruce Ren | -21 word(s) | 5770 | 2021-08-12 05:00:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Dhakal, R.; Moussa, H. Radiation Exposure on Caenorhabditis elegans. Encyclopedia. Available online: https://encyclopedia.pub/entry/13073 (accessed on 07 February 2026).
Dhakal R, Moussa H. Radiation Exposure on Caenorhabditis elegans. Encyclopedia. Available at: https://encyclopedia.pub/entry/13073. Accessed February 07, 2026.
Dhakal, Rabin, Hanna Moussa. "Radiation Exposure on Caenorhabditis elegans" Encyclopedia, https://encyclopedia.pub/entry/13073 (accessed February 07, 2026).
Dhakal, R., & Moussa, H. (2021, August 11). Radiation Exposure on Caenorhabditis elegans. In Encyclopedia. https://encyclopedia.pub/entry/13073
Dhakal, Rabin and Hanna Moussa. "Radiation Exposure on Caenorhabditis elegans." Encyclopedia. Web. 11 August, 2021.
Copy Citation
Knowledge regarding complex radiation responses in biological systems can be enhanced using genetically amenable model organisms. In this manuscript, we reviewed the use of the nematode, Caenorhabditis elegans (C. elegans), as a model organism to investigate radiation’s biological effects. Diverse types of experiments were conducted on C. elegans, using acute and chronic exposure to different ionizing radiation types, and to assess various biological responses. These responses differed based on the type and dose of radiation and the chemical substances in which the worms were grown or maintained.
ionizing radiation
acute and chronic exposure
dose
biological dosimeter
Caenorhabditis elegans
1. Introduction
Humans can be subjected to various types and levels of radiation exposure as they age. In addition to background radiation, humans might receive ionizing radiation (IR) from radiotherapy instruments as part of their medical treatment during their entire life [1]. In these treatments (e.g., Leukemia patient treatment), an absorbed dose of 12 to 15 Gy is delivered to the patient’s body, often given in 8 to 12 fractions in two to three treatments per day over 4–5 days a week for several weeks [2][3]. Moreover, humans at any age may be overexposed to ionizing radiation from nuclear accidents [4]. After Soviet cosmonaut, Yuri Gagarin, first orbited the Earth in a Vostok spacecraft on 12 April 1961, human activities in outer space have expanded tremendously [5]. As of 26 September 2019, a total of 565 people have gone to space, and 12 have walked on the Moon. They have spent more than 29,000 person-days in space, including over 100 person-days of spacewalk [6]. Thus, the number of people and the length of time they spent in space increased in the last century. Therefore, understanding the effects of the space environment on human beings is a priority among the stakeholders working on space exploration. Therefore, it is crucial to understand the effects of such radiation on humans as continuing exposure to IR causes biological damage, leading to severe health effects [7][8]. It is also important to lessen IR’s impact on the quality of life, human health, and life span (aging); thus, ionizing radiation is a critical research topic. The model organism, and nematode, Caenorhabditis elegans has been studied to assess the effects of radiation exposure at different levels, such as molecular, cellular, and individual, to determine the implications for human health studies [9].
Caenorhabditis elegans are worms, commonly known as C. elegans (Figure 1), they fall under phylum Nematoda; are non-hazardous, non-infectious, and free-living organisms that live in decayed vegetation (soil), where they thrive by feeding on microbes [10]. C. elegans is an ‘in vivo’ model system that is easy to culture in the laboratory because their adults are small, about 1 mm in length [11]. C. elegans consists of many sections similar both anatomically and genomically to those found in humans, such as muscles, an integrated nervous system, gut, and reproductive system, and fat body. This nematode is one of the best-suited model organisms widely used to investigate the exposure to different types of ionizing and non-ionizing radiation [12]. C. elegans consists of about 20,000 predicted protein-encoding genes, over third of which are homologous to human genes [13], and shares similarities in response to DNA damage response with humans [14].
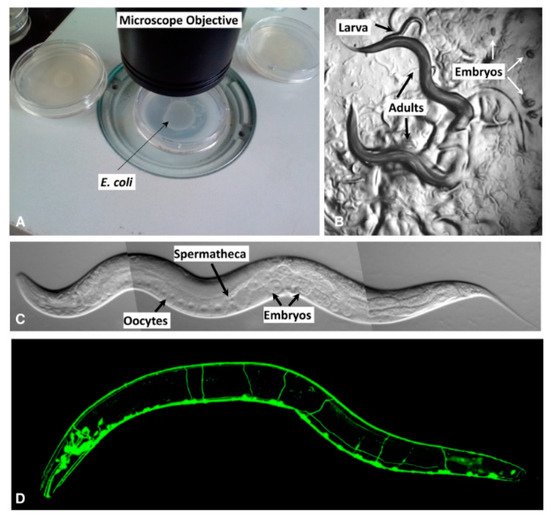
Figure 1. Different views of C. elegans in the laboratory. (A) Petri dishes with bacterial lawns on the surface of the agar (not visible in this picture). (B) Two adults move under a dissecting microscope. (C) View from a compound microscope of an adult hermaphrodite. (D) The nervous system is shown in the fluorescent image. Photo reprinted with permission of the Genetics Society of America [15][16].
C. elegans model organism has been an excellent model to conduct microbiological experiments to research apoptosis, muscle atrophy, radiation effects, metabolic diseases, and aging, given its short life span compared to humans and other mammals [17]. It has also been used to study embryogenesis, morphogenesis, nerve function, and behavior [18]. In 1976, Herman et al. conducted a remarkable investigation on ionizing radiation’s effects on C. elegans. He analyzed the chromosome rearrangement due to irradiation with X-ray to produce a system called balancing lethal mutations using chromosome balancers [19]. Subsequently, in 1985, by irradiating the worm with ionizing radiation, Hartman found various mutants of C. elegans; two of them were radiosensitive mutants, which he named rad-1 and rad-2 [20]. Later, the interest in radiation effects on C. elegans expanded to various other fields of research such as the DNA damage induced by radiation, DNA repair, aging, behavior, the effects of different types of radiation, the effects of the quality of radiation, etc. [12][21][22].
3. Acute and Chronic Irradiation in C. elegans
Research on C. elegans irradiation can be divided into the following two types based on the intensity and the length of exposure: acute and chronic exposures. Acute irradiation is a short-term exposure to a high dose, while chronic irradiation is prolonged exposure to a low dose over a determined period of time [23]. Quantitatively, exposures greater than 0.1 Gy/min would be accepted as acute, while exposures of less than 1 Gy/h would be accepted as chronic exposures by most radiobiologists; however, the intermediate levels are not well defined [24]. In nature, most of the irradiation from the solar particle events is characterized as acute irradiation, whereas irradiation from galactic cosmic rays is characterized as chronic irradiation (Figure 3) [25]. In addition to the natural irradiation of C. elegans that were flown to space for experiments, various other ground-simulated acute and chronic irradiation of C. elegans are discussed in this section. Both types of irradiation decreases the life span, increases the death ratio compared with control groups, and cause severe DNA damage, leading to genetic mutations and less fertility in C. elegans. The irradiation from solar particle events is short-term but is of high intensity; hence, it is considered that it is acute irradiation that has a high impact on the C. elegans’ biological life causing increased in the death and DNA damage and a decrease in life span, genetic mutation, and less reproduction [25]. The irradiation from galactic cosmic rays is considered a low dose, but a high exposure time; hence, it is considered chronic irradiation and it changes the biology of C elegans less than acute irradiation relatively [26].
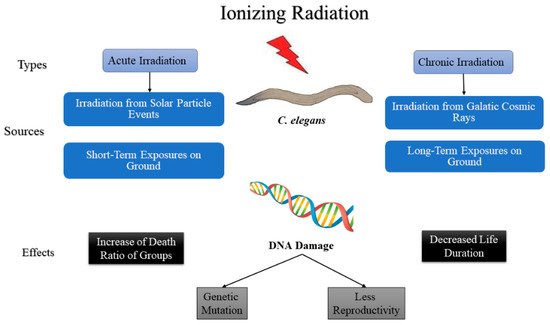
Figure 3. Diagram describing the effect of ionizing radiation on C. elegans.
After the original study evaluating the effect of ionizing radiation on C. elegans by Herman et al. in 1976 [19], much work has been conducted on the topic of acute and chronic radiation exposure. Johnson et al. exposed a wild-type and radiation-sensitive mutant to gamma rates of 0.027 Gy/min from a Cesium radionuclide (137Cs) at different developmental stages of C. elegans to monitor the life span; they found that at least 0.1 Gy is needed to reduce the mean life of C. elegans [27]. Moreover, they have also found the same conclusion drawn by Johnson et al., that dauers are the most sensitive, which may be due to arrest of development, while eight-day-old adults are the most resistant to the ionizing radiation [28][27]. Similarly, various other studies focusing on different conditions are discussed and also a summary of the irradiation with various types of ionizing radiation is presented in Table 1 and Table 2.
3.1. Space vs. Ground Experiments
The number of mutations observed in C. elegans due to the exposure to naturally occurring radiation in space is proportional to the exposure time. This enabled the C. elegans to be used as a biological dosimeter, as shown in various space experiments. For example, G. A. Nelson et al. observed the development of C. elegans as a function of gravity and space radiation exposure through a study called Genetic Molecular Dosimetry of HZE Radiation, where C. elegans were prepared and kept inside the International Microgravity Laboratory of the Discovery Space Shuttle were flown on 22 January 1994 for about eight days in space [29]. The physical dosimetry was performed with thermoluminescence dosimetry (TLD), a versatile passive dosimeter that recorded a total dose of 0.8 to 1.1 mGy in the Spacelab tunnel where protection against radiation is minimal. This research concluded that the radiation-induced mutation rate in C. elegans in space is eight times greater than in the ground control group, demonstrating a significant risk of cancer inherent in extended space travel [29].
Another experiment in space assessing the effects of space radiation on the biological growth of C. elegans has been extensively studied by Hartman et al. [30]. The fem-3 gene of C. elegans was investigated to find the mutation frequency and the nature of mutations caused by space radiation in low earth orbit during Space Shuttle flight STS-76. The Space Shuttle was flown with C. elegans on 22 March 1996, and the radiation exposure time was about nine days. Dauer larvae were screened for mutants; a total of 25 fem-3 mutants were recovered and yielded a mutation frequency of 2.1 × 10–5, which is approximately 3.3 times higher than the spontaneous rate of 6.3 × 10–6. A radiation dose of 0.268–0.306 cGy was measured during the space flight with a dose equivalent to 0.592–0.892 cGy and an average quality factor of 2.59. The mutation frequency was found to be significantly increased with the increased radiation exposure. The presence of a significant number of putative deficiencies in the recovered genes of C. elegans suggests that charged iron particles are the major mutagenic component, and the increased mutation frequency brings a significant cancer risk in extended space travel [30]. Thus, NASA began research on C. elegans as a biological dosimeter in 2004, based on the findings of previous experiments by G. A. Nelson et al. and Phil S. Hartman et al., which showed that the number of mutations observed in C. elegans is proportional to the amount of time exposed to naturally occurring radiation in space [31]. The agency launched the International Caenorhabditis elegans-First Flight (ICE-first), where C. elegans was used as a model organism to investigate the biological effects of a short duration (about 11 days) spaceflight [31]. The research focused on studying the mutational effects of spaceflight, where they utilized a genetic balancer system known as eT1 for capturing, maintaining, and recovering mutational events that occurred over several generations during spaceflight. During this experiment, C. elegans were grown for the first time in defined liquid media during the spaceflight. This research concluded that there was no significant difference in the mutation rates during the short space flight. The researchers demonstrated that the T1 balancer system can be used for longer-term biological damage measurements, as the model system was relatively simple and robust and it also could be used as a biological dosimeter [32].
Besides the space-based experiments, numerous simulated ground-based radiation tests demonstrated the same changes observed during exposure in space. Yi et al. performed a ground simulation on C. elegans where they simulated a long-duration flight and analyzed the effect of space radiation and gravitational force (G-force) on C. elegans [33]. In their experiment, they exposed C. elegans to accelerated protons and gamma rays with an equivalent dose. During long space flights of different durations, the anticipated radiation dose was calculated based on Reitz simulations and methods. They simulated the dose received by C. elegans during space flights lasting one month, six months, and two years and gave different doses to C. elegans. They analyzed whole-genome using microarray and investigated the worm phenotypes; the results of their experiment showed that protons and gamma rays provoked genetic changes related to response to DNA damage, oxidative stress, and cell death [34]. These exposures to ionizing radiation regulate (provoke or reduce) embryonic development ending in egg hatching and birth. This study predicted that accelerated protons induce a gene expression that is related to the response to DNA damage and anti-apoptosis, whereas gamma rays induce apoptosis.
Guo et al. studied the effect of a radiation-induced bystander in cells not directly irradiated in C. elegans [35]. Worms were grown in room conditions and were fed with standard E. coli OP50. Proton radiation was used in these experiments, and the dose delivered by each proton hitting the C. elegans was calculated as 0.033 Gy. The L4 stage worms were irradiated by proton beams with 1.65, 6.6, 16.5, 33, and 66 Gy and were compared with the control group. The number of dead germ cells was counted 24 h after the irradiation. The results clearly showed that bystander effects happen in C. elegans after ionizing irradiation, including effects on germ cells [35]. In a similar study, Tang et al. used a combination of irradiation with a proton beam and gamma rays to investigate the interaction between the radio-adaptive response and the radiation-induced bystander effect (RIBE) in C. elegans [36]. In this experiment, they performed whole-body exposure of a 14-h-old C. elegans to a microbeam of 1000 and 2000 protons, respectively. After two hours, the worms were irradiated with gamma radiation with 75 and 100 Gy, respectively. Their study suggested the phenomenon of RIBE, where defects such as the vulva abnormalities (non-irradiated cell) can be significantly decreased because of a signal received from the irradiated rectal valves (irradiated cell) of C. elegans; therefore, the radio-adaptive response of the C. elegans can be improved by a radiation-induced bystander effect. Xu et al. exposed C. elegans to X-rays to study the response of C. elegans to ionizing radiation [37]. In this study, the following three C. elegans cohorts were included: a control group and two groups exposed to X-ray doses of 200 and 400 Gy. After irradiation, total RNA was extracted from each group, also RNA was sequenced to compare the transcriptomes between these groups. They found that many genes expressed differentially and were enriched significantly in ionizing radiation-related biological pathways and processes [37]. This demonstrated that C. elegans genes related to various biological processes, such as behavior, regulation of growth and locomotion, positive regulation of growth, calcium ion transport, and di- and trivalent inorganic cation transport, were modulated by different doses of X-ray radiation [37]. Ionizing radiation affected the locomotion of C. elegans and influences the reaction of C. elegans to the environment. Sakashita et al. studied the effects of dietary NaCl on the learning (learning function of nervous system) of radiation-induced C. elegans [38]. The C. elegans were grown at room condition and fed with E coli until the adult stage. The C. elegans were exposed to both acute and chronic irradiation in two separate groups. The animals were irradiated with a 100 Gy dose of gamma-ray at the rate of 32 Gy/min as acute irradiation. In chronic irradiation, the animals were also irradiated with a 100 Gy dose of gamma-ray but at a rate of 0.42 Gy/min for four hours. The animals were then treated in the following three sets of NGM plates: (1) without NaCl, (2) 10 Mg NaCl, and (3) 50 mM NaCl to measure the chemotaxis toward NaCl. Their experiment showed that both acute and chronic irradiation decreases the chemotaxis of C. elegans to NaCl. They suggested that irradiation in C. elegans may have a modulatory effect on diet NaCl associated with animals learning.
3.2. Effect on Aging
The effects of exposing C. elegans to ionizing radiation are a subject of interest in radiation biology research investigating the process of aging in this organism as the life span of C. elegans is affected by the radiation exposure. This was investigated in a study by Kuzmic et al., where they show an acceleration of the aging process by exposing C. elegans to chronic irradiation. Irrespective of the dose rate and dose delivered, chronic exposure reduced the level of oxidative protein damage (carbonylation), which is a biomarker of the aging process [39]; Dubois et al. also experimented with the response of C. elegans to acute and chronic exposure [40]. After chronic exposure, 168 proteins were significantly changed in the experimental group, while 369 proteins were significantly changed following acute radiation exposure. Analysis of global protein expression in this study showed the modulation of proteins involved in regulating biological processes such as lipid transport, DNA replication, development of germ cells, apoptosis, transport of ions, and the development of cuticles. These results confirmed that chronic irradiation induced different molecular mechanisms than acute irradiation [40].
Besides the whole-body irradiation of C. elegans, partial irradiation is becoming more important in recent studies. Suzuki et al. irradiated specific regions on the C. elegans to compare animal movements with whole-body irradiation [41]. C. elegans were grown at 20 °C and were fed with E coli. Then, the three-day-old animals were irradiated with a 500 Gy dose of heavy carbon ions. The worms were irradiated within four groups. The groups were as follows: whole-body irradiation, central nervous system, CNS, irradiation or head, middle of the body irradiation, and tail irradiation. They also used active animals in their experiment and studied the motion of the animals immediately after the irradiation, which was the novelty of their experiment method. The body bend rate was calculated for five animals in each group. The results showed that the whole body irradiation reduces the mobility of the worms, while region-specific irradiation did not significantly affect the motion of the C. elegans [41]. Another study by Suzuki et al. presents evidence of the CNS’s activity in reducing the mortality in adult hermaphrodite C. elegans due to acute irradiation. Research shows that decreased mortality after irradiation of the CNS depends on the dose, and the body muscle around the CNS is also partly involved in reducing the mortality [42]. A device that makes the researcher able to partially irradiate C. elegans has recently been developed by Funayama et al. [43]. They have designed and built a device to irradiate animal models such as C. elegans with an ionized microbeam. They obtained a beam size smaller than 10 μm in their device to target specific cells in their animals under the microscope. They irradiated intracellular objects with the newly developed device. They grew C. elegans in a room temperature environment at 20 °C for three days, and the animals were cultured on nematode growth medium [43]. The CNS of the C. elegans was irradiated with 500 Gy of carbon ions. The microbeam’s size was 8 μm, which helped to irradiate the worms’ nervous system spot. Following irradiation, samples were collected, and the animals were placed on an NGM plate where they were not fed anymore. Then, they evaluated the effects of the irradiation on the locomotion of the C. elegans. The results showed that the irradiation did not change the animals’ locomotory rate, and thus, the 500 Gy had no effect on the movement of the C. elegans. They also designed a frame in which they can irradiate the C. elegans with micrometer precision, which can be used for further investigations on the heavy ion irradiation on C. elegans and cultured cells [43].
3.3. Effect on Reproduction
Radiation exposure affects C. elegans in its reproduction. Min et al. showed that C. elegans irradiated using a proton beam at the L4 larval stage grew almost generally in the adult stage, but later experienced reproductive deficiencies demonstrated by the decreased size of brood as measured by the total number of eggs that are fertilized by hermaphrodite animals; moreover, an increase in germline apoptosis caused reduced fertility [44]. The effects of a heavy-ionized beam on C. elegans germline cells were studied by Sugimoto et al. [45]. The nematodes were grown in room conditions at 20 °C for 24 h on NGM plates. When the animals entered the L4 larval stage, they were exposed to 20, 40, 60, and 100 Gy doses of carbon ion microbeams for both whole-body irradiation and germline cells. Then, the nematodes’ hatching rate was calculated at 0 to 4 h and 11 to 15 h after the irradiation. The hatching rate for the delivered dose before 4 h was 65, 20, 2, and 0 percent, respectively for the surviving eggs. The calculated rate for 11 to 15 h was about 100, 90, 60, and 0 percentages for the surviving eggs. The experiment results showed that both whole-body irradiation and germline spot irradiation decrease the hatching rate of the larva [45]. Histone-modifying enzymes have been reported to affect longevity through several generations [46][47]. Their progeny may gain trans-generationally inheritable survival benefits [48].
Min H et al. reported major increases in the number of proteins named RAD-51 and HUS-1 (RAD-51 and HUS-1 are both critical components in the DNA damage response of C. elegans) and in the number of chromosomal aberrations in gonads and oocytes, respectively, following proton beam irradiation in hermaphrodites P0. However, these defects were recovered mainly in progeny F1 and F2 [44]. Homologous recombination resects the double-strand breaks (DSBs) and produces single-stranded DNA tails. Since RAD-51 binds directly to these single-stranded DNA tails, a good estimate of the number of DSBs in a cell is given by the number of these proteins [49]. An increase in the number of RAD-51 foci (6.0 ± 1.75 per nucleus) in hermaphrodite gonad germ cells relative to those of non-irradiated hermaphrodite gonad germ cells (0.9 ± 0.61 per nucleus) was observed following irradiation at a dose of 10 Gy with a proton beam [49]. A critical component of the response to DNA damage in C. elegans is HUS-1; compared to non-irradiated gonads, the number of HUS-1 proteins was found to have increased in the irradiated hermaphrodite gonads [50]. Ryu et al. studied the roles of a homolog of p53 binding protein 1, HSR-9, in the DNA repair and the ionized irradiation response of C. elegans [51]. Standard C. elegans strains were cultured in room conditions and fed with E. coli. Furthermore, the worms were grown until the L1 stage, and then the worms were used for different experiments such as survival gem cells rate and abnormality developments in the eggs of the next generation of worms. Twenty different RNA-types of L4 stage animals were irradiated with 60 and 120 Gy doses of gamma-ray to find the germ cell survival of C. elegans. The hatching rate for the eggs was calculated at 24 and 48 h after the irradiation. Additionally, the survival rate for L1 stage worms was calculated in a separate experiment [51]. The nematodes were exposed to 25 and 50 Gy doses of acute gamma irradiation, and the hatching rate was calculated after 48 h. Furthermore, the 4-h-old eggs of C. elegans were irradiated with a 90 Gy dose of Gamma-ray searching for any abnormalities in the hatched worms after the irradiation. Both the irradiated and the control group of worms were examined until the L4 stage and abnormalities were recorded in the irradiated worms after three days. The results of these experiments showed that although p53 binding protein one homolog increases apoptosis, it does not directly influence the C. elegans response to DNA damage [51].
3.4. Effect on DNA Response
The DNA response to ionizing irradiation in C. elegans has gained much interest recently. Bertolini et al. studied DNA repair mechanism in irradiated C. elegans [52]. They used wild-type C. elegans and compared their DNA response to other mutant C. elegans strains, such as brc-1, bub-3, and san-1. The C. elegans were grown in room temperature conditions and fed E coli OP-50 and irradiated using a 137Cs gamma source. The L1 stage worms were exposed to a 60 Gy dose of ionizing irradiation, which resulted in vast DNA damage in C. elegans, but it did not decrease the worms’ reproduction. Other C. elegans were irradiated with 90 and 120 Gy doses, and results were compared to a control non irradiated group [52]. They found that the lethality increased in the bub-3 mutant compared to wild-type C. elegans. Additionally, ionized sensitivity in both bub-3 and san-1 was increased. These studies suggested that the involvement of bub-3 in response to the DNA damage in C. elegans occurs in cell cycle timing [52]. In another genetic study, Vujin et al. exposed different mutants of C. elegans, as well as wild-type N2 and wild-type NHJ1 strains to ionizing radiation [53]. The C. elegans were irradiated by 0, 25, 37.5, 50, and 75 Gy doses of X-ray in different experiments and different factors, such as ionizing radiation sensitivity or resistance, were determined. They suggested that damage in the nhj-1 sequence caused sensitivity to ionized radiation in wild-type N2 C. elegans [53]. Yang et al. showed ceramide regulated DNA damage response to mediate radiation induced germ cell apoptosis [54]. Buisset-Goussen et al. described a decrease in reproductive capacity in future generations after chronic exposure of C. elegans mother hermaphrodites to gamma radiation [55]. Barber et al. reported that the genomic instability in the progeny, which was not irradiated, was caused by irradiation of the mother C. elegans and concluded that the damage due to radiation is a long-term risk that lasts for generations [56]. Proton beam irradiation was also reported to cause transgenerational effects in three successive generations by Min et al. [44].
In another experiment, C. elegans were exposed to both chronic and acute γ-ray radiation in two different experiments by Dubois et al. [57]. They showed that acute irradiation influences the hatching of the worms, but chronic irradiation does not. C. elegans were exposed to six different doses and four doses in acute and chronic irradiation experiments. C. elegans were irradiated for 65 h during a whole life cycle from the embryo stage to the L4-YA adult stage for chronic exposure. The study’s radiation source was 137Cs, which provided a homogeneous dose rate on the plate [57]. A similar study by Maremonti et al. about the acute and chronic exposure of C. elegans to γ-ray radiation demonstrated that toxicity of chronic exposure in reproduction compared to acute exposure [58]. While acute irradiation did not significantly affect reproduction, chronic irradiation of 62 h from egg to young adult stage decreased the number of larvae hatched per adult hermaphrodite C. elegans up to 61% with a total dose of 14 Gy. These effects were mainly due to increased germ cell apoptosis, impaired sperm meiosis, with adverse effects on sperm production [58]. Later studies by Maremonti et al. showed an increase in the copy of mitochondrial DNA (mtDNA), which plays an important role in reproduction influencing germ cell development, quality, and embryonic development after chronic exposure [59]. Moreover, in their latest study, Meremonti et al. investigated effects of chronic exposure of gamma radiation on accumulation of free radical and the subsequent antioxidant responses to the apical reproductive and developmental processes in C. elegans. This research showed a reduction in the number of offspring by 20 and 40% after 96 h of gamma exposure of rate 40 100 mGy h−1 (total dose ~3.9) and 100 mGy h−1 (total doses ~9.6 Gy), respectively [60].
Table 1. Summary of Acute Irradiation of C. elegans.
| Type of Radiation and Exposure Time | Radiation Dose/Dose Rate | Findings | Year/ Studies |
|---|---|---|---|
| Gamma Radiation for about an hour | 0.027 Gy/min | ≥0.1 Gy is needed to reduce the mean life of C. elegans. Dauers are the most sensitive and 8-day-old adults are the most resistant to ionizing radiation. |
Johnson et al., 1984 [28] |
| Targeted micro bream (12C ion particles) | 20, 40, 60, and 100 Gy | Reproduction in C. elegans eggs arrested for both whole body and tip irradiation. In spot irradiation, the neighboring cells around the targeted point did not arrest the reproduction of germ cells nor did apoptosis happen. |
Sugimoto et al., 2006 [45] |
| Gamma radiation | 100 Gy (32 Gy/min) | Chemotaxis reaction of C. elegans toward NaCl decreases. The role of ionizing irradiation in associative learning of C. elegans toward NaCl is elaborated. |
Sakashita et al., 2008 [38] |
| Gamma radiation for 18 s | 6 × 10−3 to 2.8 × 10−2 Gy (for 1 month) 36 × 10–3 to 16.8 × 10–2 Gy (for 6 months) 144 × 10–3 to 67.2 × 10–2 Gy (for 2 years) |
C. elegans would experience some damage from irradiation during long-term space flight, there are changes in genes related to DNA damage response, oxidative stress, and cell death, and the gamma rays induce apoptosis. | S. Yi et al., 2013 [33] |
| Accelerated proton for 18 s | 33.6 × 10–3 to 16.8 × 10–2 Gy (for 6 months) 144 × 10–3 Gy (for 2 years) |
DNA repair mechanism was reduced due to proton exposure. Accelerated protons induce the expression of genes that are related to the DNA damage response and anti-apoptosis. |
S. Yi et al., 2013 [33] |
| Gamma radiation | 25, 50, 60, 90, 120 Gy | 53BP1 homolog, HSR-9 increases the cell death in muted C. elegans exposed to acute irradiation. HSR-9 does not involve in C. elegans cells’ response to DNA damage due to ionizing irradiation. |
Ryu et al., 2013 [51] |
| Proton particles microbeam | 1.65, 6.6, 16.5, 33 and 66 Gy (0.033 Gy per proton particle) | Proton irradiation increases the germ cell apoptosis in neighboring cells around the radiation spot. Ionized irradiation causes bystander effects in C. elegans cells |
Guo et al., 2013 [35] |
| Proton microbeam and gamma rays (137Cs) (Time not specified) |
Proton bream: 3.2 MeV with linear energy transfer rate Gamma rays: 75 and 100 Gy |
DNA damage in worms was unique and in somatic cells, which include vulval cells the DNA damage checkpoint was not active. Radio-adaptive responses of the whole C. elegans organisms were improved by bystander effect, which was induced by radiation. |
Tang et al., 2016 [36] |
| Targeted micro bream (12C ion particles) | 500 Gy | Development of a method to irradiate active C. elegans. Whole-body irradiation decreased the movement rate of C. elegans significantly. Regional irradiation on the head, middle, and tail of C. elegans did not have a significant effect on the movement rate. |
Suzuki et al., 2017 [41] |
| Gamma radiation (137Cs) | 0, 60, 90, and 120 Gy | Ionized irradiation caused significant damage in C. elegans DNA but did not reduce the reproduction cells. Sensitivity to ionized irradiation increased in C. elegans mutants compared to the wild-type strain. BUB has a role in the response of C. elegans to DNA damage. |
Bertolini et al., 2017 [52] |
| Gamma radiation (137Cs) | 2.5, 6.5, 14.4, 50, 100, and 200 Gy | Dependency of hatchability on irradiation dose was shown by the result of the decrease in significant number of progenies per individual after irradiation from and above 50 Gy, until 200 Gy. | Dubois et al., 2018 [57] |
| Gamma radiation (137Cs) | 1 Gy·min−1 0.5 Gy, 1 Gy, and 3.3 Gy |
369 proteins were found with significant differences. The molecular mechanisms induced by chronic irradiation differ from those induced by acute irradiation. |
Dubois et al., 2019 [40] |
| X-ray (600C/D linear accelerator) | 0 gray [33], 200 Gy, and 400 Gy (not specified) | Genes related to several biological processes, such as behavior, regulation growth and locomotion, positive regulation of growth, calcium ion transport, and di- and trivalent inorganic cation transport, are differentially expressed | Xu et al., 2019 [37] |
| Gamma Radiation | Dose Rate: 1445 mGy·h−1 Total dose up to 6 Gy |
Acute irradiation does not induce a significant change in reproduction. | Maremonti et al., 2019 [58] |
| Targeted micro bream (12C ion particles) | 0, 500, 100, and 1500 Gy | The decrease in mortality depends on the dose due to central nervous system (CNS) targeted irradiation and may partly be due to body-wall muscle cells around the CNS. | Suzuki et al., 2020 [42] |
| Targeted micro bream (12C ion particles) | 500 Gy | Targeted heavy ion microbeam smaller than 10 µm. The preparation and irradiation method for the device is provided. Targeted irradiation on the specific spots did not have an impact on the movement of C. elegans. |
Funayama et al., 2020 [43] |
| Gamma Radiation | 0, 5, 10, 25, and 50 Gy (3.37 Gy/min) | Germ cell apoptosis decreases when C. elegans are treated with Ceramide. Ceramide influences C. elegans’s response to DNA damage. Ceramide involves in the functioning of mitochondria in C. elegans under ionizing irradiation. |
Yang et al., 2020 [54] |
| X-ray | 0, 25, 37.5, 50, and 75 Gy | NHEJ factor in C. elegans is reported. NHJ-1 causes ionized radiation sensitivity in N2 wild-type C. elegans. |
Vujin et al., 2020 [53] |
Table 2. Summary of Chronic Irradiation of C. elegans.
| Type of Radiation & Exposure Time | Radiation Dose | Findings | Year/Studies |
|---|---|---|---|
| Low dose gamma-ray radiation for 219.5 h | 0.268 to 0.306 cGy | The mutation frequency increased significantly due to exposure to space radiation. The charged iron particles are the major mutagenic component and the increased mutation frequencies caused significant cancer risk inherent in extended space travel. |
Hartman et al., 2001 [30] |
| Low dose gamma-ray radiation for 11 days | Not specified | No significant increase in the mutation rate Introduction of eT1 balancer system for longer-term measurement of biological damage in space. |
Zhao et al., 2006 [31] |
| Gamma radiation for 4 h | 100 Gy (0.42 Gy/min) | The avoidance response of C. elegans toward NaCl decreased significantly. | Sakashita et al., 2008 [38] |
| Accelerated proton for 18 s | 33.6 × 10–3 to 16.8 × 10–2 Gy (for 6 months) 144 × 10–3 Gy (for 2 years) |
DNA repair mechanism was reduced due to proton exposure. Accelerated protons induce the expression of genes that are related to the DNA damage response and anti-apoptosis. |
Yi et al., 2013 [33] |
| Gamma Radiation (137Cs source) for 64 h |
Dose rate 7 and 52 mGy/h Dose: 0.5 and 3.3 Gy |
Life span is significantly shortened in irradiated C. elegans. There was a significant difference between different absorbed doses for the same dose rate. |
Kuzmic et al., 2019 [39] |
| Gamma Radiation (137Cs source) for 19 days |
Dose rate 7 and 52 mGy/h Dose: 3.3 and 24 Gy |
Life span is significantly shortened in irradiated C. elegans. There was a significant difference in absorbed doses in the treatments between 3.3 Gy cumulative irradiation (with 7 mGy/h) and 24 Gy cumulative irradiation (with 52 mGy/h) |
Kuzmic et al., 2019 [39] |
| Gamma Radiation (137Cs source) for 65 h |
Six dose rates 7, 14, 45, 50, 75, and 100 mGy/h Dose: 0.5, 1, 3.3 Gy |
There are no effects from irradiation on the percentage of the hatch after chronic irradiation compared to control C. elegans. | Dubois et al., 2018 [57] |
| Gamma radiation (137Cs source) for 65 h |
Dose rate: 7, 14, 50 mGy·h−1 corresponding to cumulated doses (0.5, 1, and 3.3 Gy) | 168 proteins were found with significant differences. The molecular mechanisms induced by chronic irradiation differ from those that were induced by acute irradiation. |
Dubois et al., 2019 [40] |
| Gamma Radiation for 62 h | Dose rate: 0.9 to 227 mGy·h−1 Total dose up to 228 Gy |
The number of larvae hatched was significantly decreased (by 43 and 61%, when chronically exposed from egg to young adult stage to a total dose of 6.7 Gy and 14 Gy, respectively) with increased germ cell apoptosis, impaired sperm meiosis, and adverse effects on sperm production. | Maremonti et al., 2019 [59] |
| Gamma Radiation for 72 h | Dose rate: 0.4 to 100 mGy Dose: 0.03 to 72 | Significant increase in mtDNA copy number (approx. 1.6-fold). | Maremonti et al., 2019 [59] |
| Gamma Radiation for 96 h | Dose rate: 40 and 100 mGy·h−1 Total dose: ~3.9 and 9.6 Gy |
Toxic effect in reproduction. No of offspring reduced by 20 and 40%. |
Maremonti et al., 2020 [60] |
Besides these effects on the various processes of the life cycle of C. elegans, there are several other important radiation studies that have been conducted. Weidhaas et al. developed a tissue model of reproductive cell death induced by radiation in C. elegans [61]. Huangqui et al. demonstrated the radio-adaptive response for reproductive cell death in the valval tissue in C. elegans for the first time and showed that the C. elegans is an excellent in vivo model for radiation-induced reproductive cell death [62]. Similarly, Liangwen et al. demonstrated reproductive cell death across the entire range of carbon-ion irradiation [63] These studies opened new horizons on radiation-induced reproductive cell death in C. elegans and further confirmed that this nematode is an excellent model to study human reproductive cell death from the radiation therapy that is used in cancer therapy.
It can be inferred from the study of Table 1 and Table 2 that there is a variation of biological responses by the C. elegans irradiated with different radiation types and dose rates. For a similar type of radiation, it is obvious that the higher dose of radiation affected biological responses more than that the lower dose did. Moreover, there is distinctive variation of biological responses by irradiation from low-LET and high-LET (LET- linear energy transfer). High-LETs are highly mutagenic compared to low-LETs to diploid animal cells in an in vivo based system such as the C. elegans model organism. On the other hand, biological response are also affected by the treatment of C. elegans and the environment where they were grown, before and during the irradiation. Despite all these conditions, C. elegans has been regarded as an excellent model organism for radiation biology research. However, there are also some disadvantages or limitation of the C. elegans model system. First, C. elegans, having a simple body plan, lack many defined organs or tissue such as brain, blood, defined fat tissue or cells, and internal organs found in humans. Second, C. elegans has a very short length of about 1mm, which make it difficult to conduct some biochemical experiments such as understanding tissue-specific signaling [64].
References
- Ryan, J.L. Ionizing Radiation: The Good, the Bad, and the Ugly. J. Investig. Dermatol. 2012, 132, 985–993.
- Wong, J.Y.; Filippi, A.R.; Dabaja, B.S.; Yahalom, J.; Specht, L.K. Total Body Irradiation: Guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int. J. Radiat. Oncol. 2018, 101, 521–529.
- Wills, C.; Cherian, S.; Yousef, J.; Wang, K.; Mackley, H.B. Total body irradiation: A practical review. Appl. Rad. Oncol. 2016, 5, 11–17.
- Yasunari, T.J.; Stohl, A.; Hayano, R.; Burkhart, J.; Eckhardt, S. Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Proc. Natl. Acad. Sci. USA 2011, 108, 19530–19534.
- National Geographic Partners. Early Manned Spaceflight. 2019. Available online: https://www.nationalgeographic.com/science/space/space-exploration/early-manned-spaceflight/ (accessed on 14 October 2019).
- Astronaut/Cosmonaut Statistics. Available online: http://www.worldspaceflight.com (accessed on 6 December 2019).
- Mettler, F.A. Medical effects and risks of exposure to ionizing radiation. J. Radiol. Prot. 2012, 32, N9.
- Tzortzis, M.; Svoukis, E.; Tsertos, H. A comprehensive study of natural gamma radioactivity levels and associated dose rates from surface soils in Cyprus. Radiat. Prot. Dosim. 2004, 109, 217–224.
- Hart, A.C.; Chao, M.Y. From odors to behaviors in Caenorhabditis elegans. In The Neurobiology of Olfaction; CRC Press: Boca Raton, FL, USA, 2010.
- Wood, W. The Nematode Caenorhabditis Elegans; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1998; p. 1.
- Zhao, Y.; Johnsen, R.; Baillie, D.; Rose, A. Worms in space? A model biological dosimeter. Gravit. Space Biol. Bull. Publ. Am. Soc. Gravit. Space Biol. 2005, 18, 11–16.
- Sakashita, T.; Takanami, T.; Yanase, S.; Hamada, N.; Suzuki, M.; Kimura, T.; Kobayashi, Y.; Ishii, N.; Higashitani, A. Radiation Biology of Caenorhabditis elegans: Germ Cell Response, Aging and Behavior. J. Radiat. Res. 2010, 51, 107–121.
- McKay, S.; Johnsen, R.; Khattra, J.; Asano, J.; Baillie, D.; Chan, S.; Dube, N.; Fang, L.; Goszczynski, B.; Ha, E.; et al. Gene Expression Profiling of Cells, Tissues, and Developmental Stages of the Nematode C. elegans. Cold Spring Harb. Symp. Quant. Biol. 2003, 68, 159–170.
- Bernstein, C.; Prasad, R.A.; Nfonsam, V.; Bernstein, H. DNA damage, DNA repair, and cancer, in New Research Directions in DNA Repair. IntechOpen 2013.
- Corsi, K.A.; Wightman, B.; Chalfie, M. A transparent window into biology: A primer on Caenorhabditis elegans. Genetics 2015, 200, 387–407.
- Corsi, A.; Wightman, B.; Chalfie, M. A transparent window into biology: A primer on Caenorhabditis elegans. In WormBook: The Online Review of C. elegans Biology; WormBook: Pasadena, CA, USA, 2015.
- Bouyanfif, A.; Jayarathne, S.; Koboziev, I.; Moustaid-Moussa, N. The nematode Caenorhabditis elegans as a model organism to study metabolic effects of ω-3 polyunsaturated fatty acids in obesity. Adv. Nutr. 2019, 10, 165–178.
- Chin-Sang, I.; George, S.; Ding, M.; Moseley, S.L.; Lynch, A.S.; Chisholm, A.D. The Ephrin VAB-2/EFN-1 Functions in Neuronal Signaling to Regulate Epidermal Morphogenesis in C. elegans. Cell 1999, 99, 781–790.
- Herman, R.K.; Albertson, D.G.; Brenner, S. Chromosome rearrangements in Caenorhabditis elegans. Genetics 1976, 83, 91–105.
- Hartman, P.S. Epistatic interactions of radiation-sensitive (rad) mutants of Caenorhabditis elegans. Genetics 1985, 109, 81–93.
- Hartman, P.S.; Simpson, V.J.; Johnson, T.; Mitchell, D. Radiation sensitivity and DNA repair in Caenorhabditis elegans strains with different mean life spans. Mutat. Res. Lett. 1988, 208, 77–82.
- Hartman, P.; Childress, E.; Beyer, T. Nematode development is inhibited by methyl viologen and high oxygen concentrations at a rate inversely proportional to life span. J. Gerontol. Ser. A Biol. Sci. Med Sci. 1995, 50, B322–B326.
- Hartman, P.S.; Herman, R.K. Radiation-sensitive mutants of Caenorhabditis elegans. Genetics 1982, 102, 159–178.
- Kai, M.; Suzuki, K.; Imaoka, T.; Sasatani, M.; Tanaka, S.; Yamada, Y.; Kakinuma, S. Estimation of dose-rate effectiveness factor for malignant tumor mortality: Joint analysis of mouse data exposed to chronic and acute radiation. Radiat. Res. 2020, 194, 500–510.
- Wu, H.; Huff, J.L.; Casey, R.; Kim, M.H.; Cucinotta, F.A. Risk of acute radiation syndromes due to solar particle events. In The Human Health and Performance Risks for Space Explorations; NASA Human Research Program: Houston, TX, USA, 2009; pp. 171–190.
- Cekanaviciute, E.; Rosi, S.; Costes, S.V. Central nervous system responses to simulated galactic cosmic rays. Int. J. Mol. Sci. 2018, 19, 3669.
- Johnson, T.E.; Hartman, P.S. Radiation Effects on Life Span in Caenorhabditis elegans. J. Gerontol. 1988, 43, B137–B141.
- Johnson, T.E.; Mitchell, D.H.; Kline, S.; Kemal, R.; Foy, J. Arresting development arrests aging in the nematode Caenorhabditis elegans. Mech. Ageing Dev. 1984, 28, 23–40.
- Nelson, G.; Schubert, W.; Kazarians, G.; Richards, G.; Benton, E.; Henke, R. Radiation effects in nematodes: Results from IML-1 experiments. Adv. Space Res. 1994, 14, 87–91.
- Hartman, P.S.; Hlavacek, A.; Wilde, H.; Lewicki, D.; Schubert, W.; Kern, R.G.; Kazarians, G.A.; Benton, E.V.; Benton, E.R.; Nelson, G.A. A comparison of mutations induced by accelerated iron particles versus those induced by low earth orbit space radiation in the FEM-3 gene of Caenorhabditis elegans. Mutat. Res. Mol. Mech. Mutagen. 2001, 474, 47–55.
- Zhao, Y.; Lai, K.; Cheung, I.; Youds, J.; Tarailo, M.; Tarailo, S.; Rose, A. A mutational analysis of Caenorhabditis elegans in space. Mutat. Res. 2006, 601, 19–29.
- Zhao, Y.; Jones, M.; Baillie, D.; Rose, A. Developing an integrating biological dosimeter for spaceflight. Microgravity Sci. Technol. 2007, 19, 201–204.
- Yi, S.; Kim, S.; Song, J. Analysis of the Effect of Space Radiations on the Nematode, Caenorhabditis elegans, through the Simulated Space Radiation. Int. J. Astron. Astrophys. 2013, 03, 291–302.
- Reitz, G.; Beaujean, R.; Benton, E.; Burmeister, S.; Dachev, T.; Deme, S.; Luszik-Bhadra, M.; Olko, P. Space radiation measurements on-board ISS—The DOSMAP experiment. Radiat. Prot. Dosim. 2005, 116, 374–379.
- Guo, X.; Sun, J.; Bian, P.; Chen, L.; Zhan, F.; Wang, J.; Xu, A.; Wang, Y.; Hei, T.K.; Wu, L. Radiation-Induced Bystander Signaling from Somatic Cells to Germ Cells in Caenorhabditis elegans. Radiat. Res. 2013, 180, 268–275.
- Tang, H.; Chen, L.; Chen, L.; Chen, B.; Wang, T.; Yang, A.; Zhang, F.; Wu, L.; Bian, P. Interaction between radioadaptive response and radiation-induced bystander effect in Caenorhabditis elegans: A unique role of the DNA damage checkpoint. Radiat. Res. 2016, 186, 662–668.
- Xu, Y.; Chen, L.; Liu, M.; Lu, Y.; Yue, Y.; Liu, Y.; Chen, H.; Xiu, F.; Zhang, C. High-throughput transcriptome sequencing reveals extremely high doses of ionizing radiation-response genes in Caenorhabditis elegans. Toxicol. Res. 2019, 8, 754–766.
- Sakashita, T.; Hamada, N.; Ikeda, D.D.; Yanase, S.; Suzuki, M.; Ishii, N.; Kobayashi, Y. Modulatory effect of ionizing radiation on food—NaCl associative learning: The role of γ subunit of G protein in Caenorhabditis elegans. FASEB J. 2008, 22, 713–720.
- Kuzmic, M.; Galas, S.; Lecomte-Pradines, C.; Dubois, C.; Dubourg, N.; Frelon, S. Interplay between ionizing radiation effects and aging in C. elegans. Free. Radic. Biol. Med. 2019, 134, 657–665.
- Dubois, C.; Pophillat, M.; Audebert, S.; Fourquet, P.; Lecomte, C.; Dubourg, N.; Galas, S.; Camoin, C.; Frelon, S. Differential modification of the C. elegans proteome in response to acute and chronic gamma radiation: Link with reproduction decline. Sci. Total Environ. 2019, 676, 767–781.
- Suzuki, M.; Hattori, Y.; Sakashita, T.; Yokota, Y.; Kobayasi, Y.; Funayama, T. Region-specific irradiation system with heavy-ion microbeam for active individuals of Caenorhabditis elegans. J. Radiat. Res. 2017, 58, 881–886.
- Suzuki, M.; Soh, Z.; Yamashita, H.; Tsuji, T.; Funayama, T. Targeted Central Nervous System Irradiation of Caenorhabditis elegans Induces a Limited Effect on Motility. Biology 2020, 9, 289.
- Funayama, T.; Sakashita, T.; Suzuki, M.; Yokota, Y.; Miyawaki, N.; Kashiwagi, H.; Satoh, T.; Kurashima, S. An irradiation device for biological targets using focused microbeams of cyclotron-accelerated heavy ion. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 465, 101–109.
- Min, H.; Sung, M.; Son, M.; Kawasaki, I.; Shim, Y.-H. Transgenerational effects of proton beam irradiation on Caenorhabditis elegans germline apoptosis. Biochem. Biophys. Res. Commun. 2017, 490, 608–615.
- Sugimoto, T.; Dazai, K.; Sakashita, T.; Funayama, T.; Wada, S.; Hamada, N.; Kakizaki, T.; Kobayashi, Y.; Higashitani, A. Cell cycle arrest and apoptosis in Caenorhabditis elegans germline cells following heavy-ion microbeam irradiation. Int. J. Radiat. Biol. 2006, 82, 31–38.
- Greer, E.; Maures, T.J.; Hauswirth, A.G.; Green, E.; Leeman, D.S.; Maro, G.S.; Han, S.; Banko, M.R.; Gozani, O.; Brunet, A. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nat. Cell Biol. 2010, 466, 383–387.
- Greer, E.; Maures, T.J.; Ucar, D.; Hauswirth, A.G.; Mancini, E.; Lim, J.P.; Benayoun, B.; Shi, Y.; Brunet, A. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nat. Cell Biol. 2011, 479, 365–371.
- Kishimoto, S.; Uno, M.; Okabe, E.; Nono, M.; Nishida, E. Environmental stresses induce transgenerationally inheritable survival advantages via germline-to-soma communication in Caenorhabditis elegans. Nat. Commun. 2017, 8, 14031.
- Alpi, A.; Pasierbek, P.; Gartner, A.; Loidl, J. Genetic and cytological characterization of the recombination protein RAD-51 in Caenorhabditis elegans. Chromosoma 2003, 112, 6–16.
- Hofmann, E.R.; Milstein, S.; Boulton, S.J.; Ye, M.; Hofmann, J.; Sergiou, L.; Gartner, A.; Vidal, M.; Hengartner, M. Caenorhabditis elegans HUS-1 is a DNA damage checkpoint protein required for genome stability and EGL-1-mediated apoptosis. Curr. Biol. 2002, 12, 1908–1918.
- Ryu, S.J.; Kang, S.J.; Koo, H.-S. The 53BP1 homolog in C. elegans influences DNA repair and promotes apoptosis in response to ionizing radiation. PLoS ONE 2013, 8, e64028.
- Bertolini, S.; Wang, B.; Meier, B.; Hong, Y.; Gartner, A. Caenorhabditis elegans BUB-3 and SAN-1/MAD3 spindle assembly checkpoint components are required for genome stability in response to treatment with ionizing radiation. G3 Genes Genomes Genet. 2017, 7, 3875–3885.
- Vujin, A.; Jones, S.; Zetka, M. NHJ-1 Is Required for Canonical Nonhomologous End Joining in Caenorhabditis elegans. Genetics 2020, 215, 635–651.
- Yang, Y.; Xu, G.; Xu, Y.; Cheng, X.; Xu, S.; Chen, S.; Wu, L. Ceramide mediates radiation-induced germ cell apoptosis via regulating mitochondria function and MAPK factors in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2020, 208, 111579.
- Buisset-Goussen, A.; Goussen, B.; Della-Vedova, C.; Galas, S.; Adam-Guillermin, C.; Lecomte-Pradines, C. Effects of chronic gamma irradiation: A multigenerational study using Caenorhabditis elegans. J. Environ. Radioact. 2014, 137, 190–197.
- Barber, R.C.; Hickenbotham, P.; Hatch, T.; Kelly, D.; Topchiy, N.; Almeida, G.; Jones, G.; Johnson, G.; Parry, J.M.; Rothkamm, K.; et al. Radiation-induced transgenerational alterations in genome stability and DNA damage. Oncogene 2006, 25, 7336–7342.
- Dubois, C.; Lecomte, C.; Dit Ruys, S.P.; Kuzmic, M.; Della-Vedova, C.; Dubourg, N.; Glasa, S.; Frelin, S. Precoce and opposite response of proteasome activity after acute or chronic exposure of C. elegans to γ-radiation. Sci. Rep. 2018, 8, 11349.
- Maremonti, E.; Eide, D.M.; Oughton, D.H.; Salbu, B.; Grammes, F.; Kassaye, Y.; Guedon, R.; Lecomte-Pradines, C.; Brede, D. Gamma radiation induces life stage-dependent reprotoxicity in Caenorhabditis elegans via impairment of spermatogenesis. Sci. Total Environ. 2019, 695, 133835.
- Maremonti, E.; Brede, D.A.; Olsen, A.K.; Eide, D.M.; Berg, E.S. Ionizing radiation, genotoxic stress, and mitochondrial DNA copy-number variation in Caenorhabditis elegans: Droplet digital PCR analysis. Mutat. Res. 2020, 858–860, 503277.
- Maremonti, E.; Eide, D.M.; Rossbach, L.M.; Lind, O.C.; Salbu, B.; Brede, D.A. In vivo assessment of reactive oxygen species production and oxidative stress effects induced by chronic exposure to gamma radiation in Caenorhabditis elegans. Free. Radic. Biol. Med. 2020, 152, 583–596.
- Weidhaas, J.B.; Eisenmann, D.M.; Holub, J.M.; Nallur, S.V. A Caenorhabditis elegans tissue model of radiation-induced reproductive cell death. Proc. Natl. Acad. Sci. USA 2006, 103, 9946–9951.
- Tang, H.; Chen, L.; Liu, J.; Shi, J.; Li, Q.; Wang, T.; Wu, L.; Zhan, F.; Bian, P. Radioadaptive Response for Reproductive Cell Death Demonstrated in In Vivo Tissue Model of Caenorhabditis elegans. Radiat. Res. 2016, 185, 402–410.
- Chen, L.; Tang, H.; Du, Y.; Dai, Z.; Wang, T.; Wu, L.; Zhou, L.; Bian, P. Induction of reproductive cell death in Caenorhabditis elegans across entire linear-energy-transfer range of carbon-ion irradiation. DNA Repair 2018, 63, 39–46.
- Tissenbaum, H.A. Using C. elegans for aging research. Invertebr. Reprod. Dev. 2015, 59 (Suppl. S1), 59–63.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
3 times
(View History)
Update Date:
12 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




