| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Juliana Marisol Godínez-Rubí | + 598 word(s) | 598 | 2020-07-06 08:11:02 | | | |
| 2 | Camila Xu | -76 word(s) | 522 | 2020-07-13 07:52:04 | | | | |
| 3 | Camila Xu | -31 word(s) | 491 | 2020-10-27 09:25:03 | | | | |
| 4 | Camila Xu | -31 word(s) | 491 | 2020-10-27 09:25:37 | | |
Video Upload Options
miR-615, a miRNA highly conserved across eutherian mammals. It is involved not only during embryogenesis in the regulation of growth and development, for instance during osteogenesis and angiogenesis, but also in the regulation of cell growth and the proliferation and migration of cells, acting as a tumor suppressor or tumor promoter. It, therefore, serves as a biomarker for several types of cancer and recently has also been found to be involved in reparative processes and neural repair.
1. Introduction
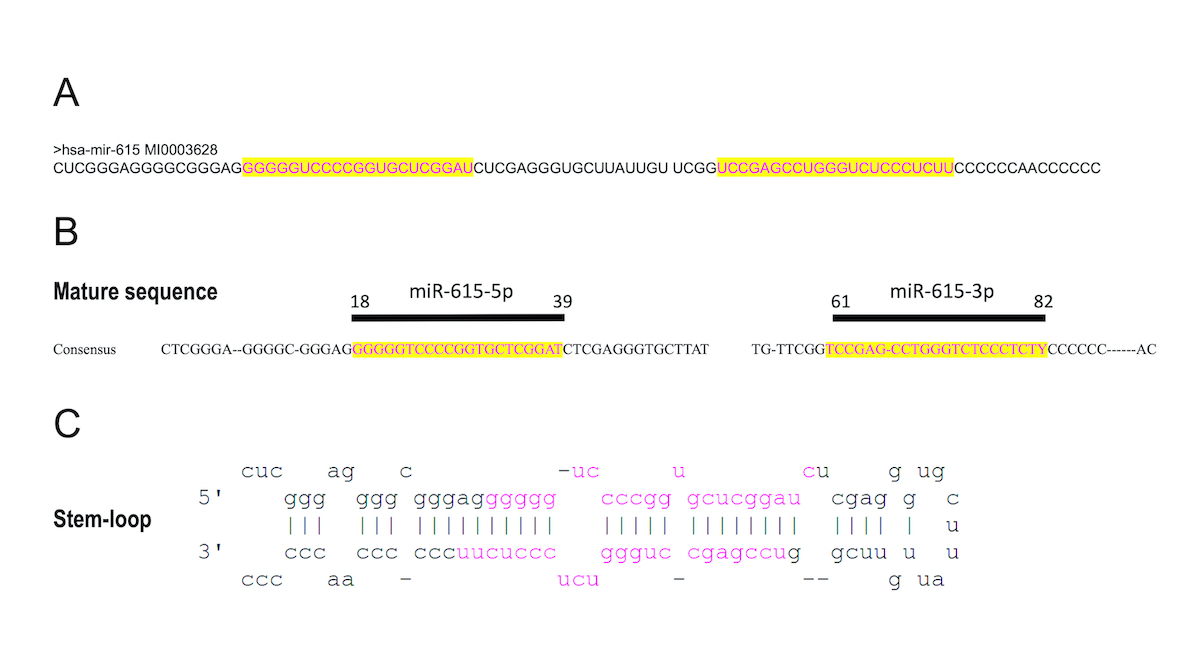
miR-615 is a microRNA located within the intron of the Hoxc5 gene (12q13.13) [1], contains two different segments (miR-615-3p and miR-615-5p of 21 nucleotides each) which can perform interference activities and function as regulatory miRNAs. This sequence forms a hairpin or stem-loop where the two sides of the stem contain the corresponding sequences miR-615-3p and miR-615-5p [2].
2. Introduction
Homeobox genes encode transcription factors that regulate the expression of several genes during embryogenesis[3]. Among them, Hox genes are very well described as specifying the anteroposterior pattern as well as the development of many organs[4]. Within this gene cluster, introns represent a hypothetically rich source of microRNAs (miRNAs). miRNAs are single stranded noncoding 18–25 nucleotide RNAs that post-transcriptionally attenuate gene expression[5]. Among them is miR-615, which is located within the intron of the Hoxc5 gene (12q13.13). miR-615 has a restricted phylogenetic distribution and is absent in non-mammalian tetrapods but highly conserved across eutherian mammals [1], which allows it to contribute in eutherian evolution and development[6].
3. Functions of miR-615
At present, more is known about the involvement of miRNA-615 in various pathologies than about its physiological function. miR-615 is highly expressed in the mouse embryo and has therefore been involved during embryogenesis in the regulation of growth and development, as suggested by target prediction and transcriptomic analyses. After analyzing miR-615 expression in several human cell lines and tissues, only very few cell lines lacked its expression [6]. The gonads, the kidney and the cerebellum are among the principal tissues in various species in which miR-615 expression occurs.
During embryonic development in rats, the expression of miR-615-3p was detected from the fourteenth day and was significantly reduced during osteogenic and adipogenic differentiation in a time-dependent manner. In bone marrow mesenchymal stem cells (BMSCs) induced with chondrogenic differentiation, the mRNA expression of specific related genes COL2A1, COL10A1, ACAN and MATN3 was significantly decreased following miR-615-3p overexpression. In addition, the expression of SOX9, a transcription factor promoting chondrogenesis, was also significantly decreased, while knockdown of miR-615-3p obtained the opposite result. This indicates that miR-615-3p inhibits the expression of chondrogenic-specific genes and may inhibit chondrogenic differentiation [7].
On the other hand, some studies on cancer cell lines have involved this miRNA in the regulation of cell growth, proliferation and migration [8], with it acting as a tumor suppressor [1] or as a tumor promoter [9][10][11].
References
- Joost M. Woltering; Antony J. Durston; MiR-10 Represses HoxB1a and HoxB3a in Zebrafish. PLOS ONE 2008, 3, e1396, 10.1371/journal.pone.0001396.
- Pablo Landgraf; Mirabela Rusu; Robert Sheridan; Alain Sewer; Nicola Iovino; Alexei Aravin; Sebastien Pfeffer; Amanda Rice; Alice O. Kamphorst; Markus Landthaler; et al.Carolina LinNicholas D. SocciLeandro HermidaValerio FulciSabina ChiarettiRobin FoàJulia SchliwkaUta FuchsAstrid NovoselRoman-Ulrich MüllerBernhard SchermerUte BisselsJason InmanQuang PhanMinchen ChienDavid B. WeirRuchi ChoksiGabriella De VitaDaniela FrezzettiHans-Ingo TrompeterVeit HornungGrace TengGunther HartmannMiklós PalkovitsRoberto Di LauroPeter WernetGiuseppe MacinoCharles E. RoglerJames W. NagleJingyue JuF. Nina PapavasiliouThomas BenzingPeter LichterWayne TamMichael J. BrownsteinAndreas BosioArndt BorkhardtJames J. RussoChris SanderMihaela ZavolanThomas Tuschl A Mammalian microRNA Expression Atlas Based on Small RNA Library Sequencing. Cell 2007, 129, 1401-1414, 10.1016/j.cell.2007.04.040.
- Peter W. H. Holland; Evolution of homeobox genes. WIREs Developmental Biology 2012, 2, 31-45, 10.1002/wdev.78.
- Jacqueline Deschamps; D. Duboule; Embryonic timing, axial stem cells, chromatin dynamics, and the Hox clock. Genes & Development 2017, 31, 1406-1416, 10.1101/gad.303123.117.
- Witold Filipowicz; Suvendra N. Bhattacharyya; Nahum Sonenberg; Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight?. Nature Reviews Genetics 2008, 9, 102-114, 10.1038/nrg2290.
- Shan Quah; Peter W. H. Holland; The Hox cluster microRNA miR-615: a case study of intronic microRNA evolution. EvoDevo 2015, 6, 1-12, 10.1186/s13227-015-0027-1.
- J-X Zhou; Z-G Tian; L-F Zhu; W-D Wu; S-L Zhou; Y-T Zhao; S Huang; MicroRNA-615-3p promotes the osteoarthritis progression by inhibiting chondrogenic differentiation of bone marrow mesenchymal stem cells.. European review for medical and pharmacological sciences 2018, 22, 6212-6220.
- W Gao; Y Gu; Z Li; H Cai; Q Peng; M Tu; Y Kondo; K Shinjo; Y Zhu; J Zhang; et al.Y SekidoB HanZ QianY Miao miR-615-5p is epigenetically inactivated and functions as a tumor suppressor in pancreatic ductal adenocarcinoma. Oncogene 2014, 34, 1629-1640, 10.1038/onc.2014.101.
- Jizhao Wang; Lin Liu; Yuchen Sun; Yumo Xue; Jingkun Qu; Shupei Pan; Huajing Li; Hangying Qu; Jiansheng Wang; Jia Zhang; et al. miR-615-3p promotes proliferation and migration and inhibits apoptosis through its potential target CELF2 in gastric cancer. Biomedicine & Pharmacotherapy 2018, 101, 406-413, 10.1016/j.biopha.2018.02.104.
- Ryota Mukai; Yoshito Tomimaru; Hiroaki Nagano; Hidetoshi Eguchi; Koshi Mimori; Akira Tomokuni; Tadafumi Asaoka; Hiroshi Wada; Koichi Kawamoto; Shigeru Marubashi; et al.Yuichiro DokiMasaki Mori miR-615-3p expression level in bone marrow is associated with tumor recurrence in hepatocellular carcinoma. Molecular and Clinical Oncology 2015, 3, 487-494, 10.3892/mco.2015.514.
- Emma Bollmann Laursen; Jacob Fredsøe; Linnéa Schmidt; Siri H. Strand; Helle Kristensen; Anne K.I. Rasmussen; Tina Fuglsang Daugaard; Peter Mouritzen; Søren Høyer; Gitte Kristensen; et al.Hein Vincent StroombergKlaus BrassoMartin Andreas RøderMichael BorreKarina D. SørensenAnne K. Ildor RasmussenAnne Karin Ildor Rasmussen Elevated miR-615-3p Expression Predicts Adverse Clinical Outcome and Promotes Proliferation and Migration of Prostate Cancer Cells.. The American Journal of Pathology 2019, 189, 2377-2388, 10.1016/j.ajpath.2019.08.007.
- Emma Bollmann Laursen; Jacob Fredsøe; Linnéa Schmidt; Siri H. Strand; Helle Kristensen; Anne K.I. Rasmussen; Tina Fuglsang Daugaard; Peter Mouritzen; Søren Høyer; Gitte Kristensen; et al.Hein Vincent StroombergKlaus BrassoMartin Andreas RøderMichael BorreKarina D. SørensenAnne K. Ildor RasmussenAnne Karin Ildor Rasmussen Elevated miR-615-3p Expression Predicts Adverse Clinical Outcome and Promotes Proliferation and Migration of Prostate Cancer Cells.. The American Journal of Pathology 2019, 189, 2377-2388, 10.1016/j.ajpath.2019.08.007.