
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hammad Ullah | + 4248 word(s) | 4248 | 2021-08-04 04:13:42 | | | |
| 2 | Peter Tang | Meta information modification | 4248 | 2021-08-12 04:36:37 | | |
Video Upload Options
Colorectal carcinogenesis is the second most common cause of mortality across all types of malignancies, followed by hepatic and stomach cancers. Chemotherapy and radiotherapy are key approaches to treating cancer patients, but these carry major concerns, such as a high risk of side effects, poor accessibility, and the non-selective nature of chemotherapeutics. A number of natural products have been identified as countering various forms of cancer with fewer side effects. The potential impact of vitamins and minerals on long-term health, cognition, healthy development, bone formation, and aging has been supported by experimental and epidemiological studies. Successful treatment may thus be highly influenced by the nutritional status of patients. An insufficient diet could lead to detrimental effects on immune status and tolerance to treatment, affecting the ability of chemotherapy to destroy cancerous cells.
1. Introduction
2. Micronutrients and Cancer: What We Need to Know?
3. A Possible Link of Micronutrients with GI and Hepatic Cancers
|
Micronutrients |
Underlined Cancer Types |
RDA |
Dietary Sources |
References |
|---|---|---|---|---|
|
Vitamin D |
Colorectal cancer, HCC |
15 µg |
Egg yolks, tuna, salmon, sardines, mushrooms, cow’s milk, soy milk, orange juice, and fortified foods. |
|
|
Vitamin A |
Gastric cancer, Colorectal cancer, HCC |
900 µg |
Liver (animals and fishes) and egg yolk. Provitamin A carotenoids obtained from plant sources including deep green, yellow and orange fruits and vegetables such as carrots, spinach, broccoli, mangoes, turnips, and sweet potatoes. |
|
|
Vitamin E |
Upper GI cancers, Colon cancer, HCC |
15 mg |
Vegetable oils (cotton seed oil, wheat germ oil, corn germ oil, and peanut oil). All green plants contain some concentration of tocopherol but some green leafy vegetables and rose hips contain more than wheat germ. |
|
|
Vitamin C |
Intestinal metaplasia, HCC |
90 mg |
Fruits (especially citrus fruits) and vegetables (especially peppers andpotatoes). |
|
|
Zinc |
Esophageal tumors, Gastric cancers, Colon cancer, HCC |
11 mg |
Oysters, red meat, nuts, whole grains, poultry, and dairy products. |
|
|
Selenium |
Colon cancer, HCC |
0.055 mg |
Brazil nuts, seafoods, meats, grains, dairy products, eggs, and organ meats. |
4. Molecular Mechanisms of Micronutrients
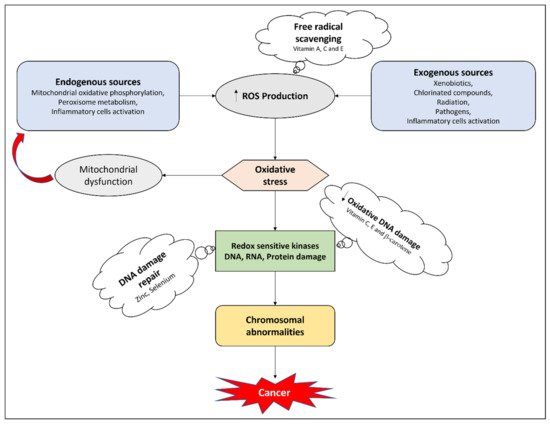
4.1. Antioxidant Effects
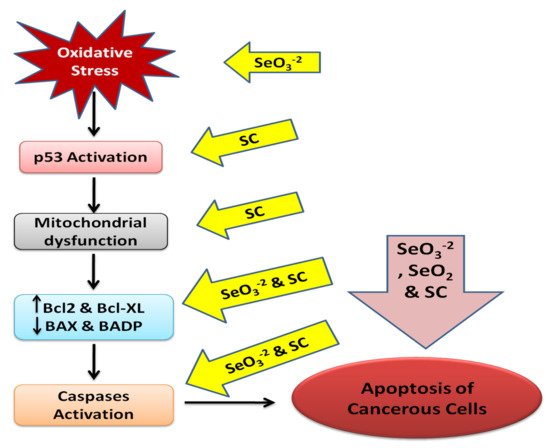
4.2. Apoptosis Targeting
4.3. Anti-Proliferative Mechanisms
4.4. Anti-Angiogenic Effects
References
- WHO Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 12 February 2021).
- Wild, C.; Weiderpass, E.; Stewart, B. World Cancer Report: Cancer Research for Cancer Prevention; IARC: Lyon, France, 2020; pp. 23–33.
- O’Connor, A.; McNamara, D.; O’Moráin, C.A. Surveillance of gastric intestinal metaplasia for the prevention of gastric cancer. Cochrane Database Syst. Rev. 2013, 9, CD009322.
- MacFarlane, A.J.; Stover, P.J. Convergence of genetic, nutritional and inflammatory factors in gastrointestinal cancers. Nutr. Rev. 2007, 65, S157–S166.
- Jayasekara, H.; English, D.R.; Haydon, A.; Hodge, A.M.; Lynch, B.M.; Rosty, C.; Williamson, E.J.; Clendenning, M.; Southey, M.C.; Jenkins, M.A. Associations of alcohol intake, smoking, physical activity and obesity with survival following colorectal cancer diagnosis by stage, anatomic site and tumor molecular subtype. Int. J. Cancer 2018, 142, 238–250.
- Tuan, J.; Chen, Y.-X. Dietary and lifestyle factors associated with colorectal cancer risk and interactions with microbiota: Fiber, red or processed meat and alcoholic drinks. Gastrointest. Tumors 2016, 3, 17–24.
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117.
- Alter, M.J. Epidemiology of hepatitis C virus infection. World J. Gastroenterol. 2007, 13, 2436.
- Marengo, A.; Rosso, C.; Bugianesi, E. Liver cancer: Connections with obesity, fatty liver, and cirrhosis. Annu. Rev. Med. 2016, 67, 103–117.
- White, D.L.; Kanwal, F.; El–Serag, H.B. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin. Gastroenterol. Hepatol. 2012, 10, 1342–1359.
- El–Serag, H.B.; Hampel, H.; Javadi, F. The association between diabetes and hepatocellular carcinoma: A systematic review of epidemiologic evidence. Clin. Gastroenterol. Hepatol. 2006, 4, 369–380.
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211.
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.-J.; Xiao, J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: Updates and future directions. Biotechnol. Adv. 2020, 38, 107385.
- Malhotra, V.; Perry, M.C. Classical chemotherapy: Mechanisms, toxicities and the therapeutic window. Cancer Biol. Ther. 2003, 2, 1–3.
- Gibson, R.J.; Keefe, D.M. Cancer chemotherapy-induced diarrhoea and constipation: Mechanisms of damage and prevention strategies. Support Care Cancer 2006, 14, 890–900.
- Davis, M.P.; Hallerberg, G. A systematic review of the treatment of nausea and/or vomiting in cancer unrelated to chemotherapy or radiation. J. Pain Symptom Manag. 2010, 39, 756–767.
- Can, G.; Demir, M.; Erol, O.; Aydiner, A. A comparison of men and women’s experiences of chemotherapy-induced alopecia. Eur. J. Oncol. Nurs. 2013, 17, 255–260.
- Morrison, V.A. Immunosuppression associated with novel chemotherapy agents and monoclonal antibodies. Clin. Infect. Dis. 2014, 59, S360–S364.
- Fu, H.; Chen, B.; Hong, S.; Guo, Y. Acupuncture therapy for the treatment of myelosuppression after chemotherapy: A literature review over the past 10 years. J. Acupunct. Meridian Stud. 2015, 8, 122–126.
- Rodgers, G.M.; Becker, P.S.; Blinder, M.; Cella, D.; Chanan-Khan, A.; Cleeland, C.; Coccia, P.F.; Djulbegovic, B.; Gilreath, J.A.; Kraut, E.H. Cancer-and chemotherapy-induced anemia. J. Natl. Compr. Cancer Netw. 2012, 10, 628–653.
- Nesher, L.; Rolston, K.V. Neutropenic enterocolitis, a growing concern in the era of widespread use of aggressive chemotherapy. Clin. Infect. Dis. 2013, 56, 711–717.
- Lorenzi, E.; Simonelli, M.; Santoro, A. Infertility risk and teratogenicity of molecularly targeted anticancer therapy: A challenging issue. Crit. Rev. Oncol. Hematol. 2016, 107, 1–13.
- Martin, A.; Schneiderman, J.; Helenowski, I.B.; Morgan, E.; Dilley, K.; Danner-Koptik, K.; Hatahet, M.; Shimada, H.; Cohn, S.L.; Kletzel, M. Secondary malignant neoplasms after high-dose chemotherapy and autologous stem cell rescue for high-risk neuroblastoma. Pediatr. Blood Cancer 2014, 61, 1350–1356.
- Carrier, X.; Gaur, S.; Philipovskiy, A. Tumor lysis syndrome after a single dose of atezolizumab with Nab-Paclitaxel: A case report and review of literature. Am. J. Med. Case Rep. 2020, 21, e925248-1.
- Liu, Z.; Huang, P.; Law, S.; Tian, H.; Leung, W.; Xu, C. Preventive effect of curcumin against chemotherapy-induced side-effects. Front. Pharmacol. 2018, 9, 1374.
- Tong, Y.; Wang, K.; Sheng, S.; Cui, J. Polydatin ameliorates chemotherapy-induced cognitive impairment (chemobrain) by inhibiting oxidative stress, inflammatory response, and apoptosis in rats. Biosci. Biotechnol. Biochem. 2020, 84, 1201–1210.
- Waghray, D.; Zhang, Q. Inhibit or evade multidrug resistance p-glycoprotein in cancer treatment: Miniperspective. J. Med. Chem. 2017, 61, 5108–5121.
- Luqmani, Y. Mechanisms of drug resistance in cancer chemotherapy. Med. Princ. Pract. 2005, 14, 35–48.
- Saam, J.; Critchfield, G.C.; Hamilton, S.A.; Roa, B.B.; Wenstrup, R.J.; Kaldate, R.R. Body surface area–based dosing of 5-fluoruracil results in extensive interindividual variability in 5-fluorouracil exposure in colorectal cancer patients on FOLFOX regimens. Clin. Colorectal Cancer 2011, 10, 203–206.
- Gamelin, E.; Delva, R.; Jacob, J.; Merrouche, Y.; Raoul, J.L.; Pezet, D.; Dorval, E.; Piot, G.; Morel, A.; BoisdronCelle, M. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: Results of a multicenter randomized trial of patients with metastatic colorectal cancer. J. Clin. Oncol. 2013, 31, 3612.
- Hoeft, B.; Weber, P.; Eggersdorfer, M. Micronutrients—A global perspective on intake, health benefits and economics. Int. J. Vitam. Nutr. Res. 2012, 82, 316–320.
- Godswill, A.G.; Somtochukwu, I.V.; Ikechukwu, A.O.; Kate, E.C. Health benefits of micronutrients (vitamins and minerals) and their Associated Deficiency Diseases: A systematic review. Int. J. Food Sci. 2020, 3, 1–32.
- Gröber, U.; Holzhauer, P.; Kisters, K.; Holick, M.F.; Adamietz, I.A. Micronutrients in oncological intervention. Nutrients 2016, 8, 163.
- Micke, O.; Bruns, F.; Glatzel, M.; Schönekaes, K.; Micke, P.; Mücke, R.; Büntzel, J. Predictive factors for the use of complementary and alternative medicine (CAM) in radiation oncology. Eur. J. Integr. Med. 2009, 1, 19–25.
- Zirpoli, G.R.; Brennan, P.M.; Hong, C.-C.; McCann, S.E.; Ciupak, G.; Davis, W.; Unger, J.M.; Budd, G.T.; Hershman, D.L.; Moore, H.C. Supplement use during an intergroup clinical trial for breast cancer (S0221). Breast Cancer Res. Treat. 2013, 137, 903–913.
- D’Andrea, G.M. Use of antioxidants during chemotherapy and radiotherapy should be avoided. Cancer J. Clin. 2005, 55, 319–321.
- Lawenda, B.D.; Kelly, K.M.; Ladas, E.J.; Sagar, S.M.; Vickers, A.; Blumberg, J.B. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J. Natl. Cancer Inst. 2008, 100, 773–783.
- Yasueda, A.; Urushima, H.; Ito, T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: A systematic review. Integr. Cancer Ther. 2016, 15, 17–39.
- Block, K.I.; Koch, A.C.; Mead, M.N.; Tothy, P.K.; Newman, R.A.; Gyllenhaal, C. Impact of antioxidant supplementation on chemotherapeutic toxicity: A systematic review of the evidence from randomized controlled trials. Int. J. cancer 2008, 123, 1227–1239.
- Nechuta, S.; Lu, W.; Chen, Z.; Zheng, Y.; Gu, K.; Cai, H.; Zheng, W.; Shu, X.O. Vitamin supplement use during breast cancer treatment and survival: A prospective cohort study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 262–271.
- Gröber, U.; Kisters, K.; Adamietz, I.A. Vitamin D in oncology: Update 2015. Med. Monatsschr. Pharm. 2015, 38, 512–516.
- Russell, S.T.; Tisdale, M.J. The role of glucocorticoids in the induction of zinc-α 2-glycoprotein expression in adipose tissue in cancer cachexia. Br. J. cancer 2005, 92, 876–881.
- Büntzel, J.; Bruns, F.; Glatzel, M.; Garayev, A.; Mücke, R.; Kisters, K.; Schäfer, U.; Schönekaes, K.; Micke, O. Zinc concentrations in serum during head and neck cancer progression. Anticancer Res. 2007, 27, 1941–1943.
- Churilla, T.M.; Brereton, H.D.; Klem, M.; Peters, C.A. Vitamin D deficiency is widespread in cancer patients and correlates with advanced stage disease: A community oncology experience. Nutr. Cancer 2012, 64, 521–525.
- Cruciani, R.; Dvorkin, E.; Homel, P.; Culliney, B.; Malamud, S.; Shaiova, L.; Fleishman, S.; Lapin, J.; Klein, E.; Lesage, P. L-carnitine supplementation for the treatment of fatigue and depressed mood in cancer patients with carnitine deficiency: A preliminary analysis. Ann. N. Y. Acad. Sci. 2004, 1033, 168–176.
- Mayland, C.R.; Bennett, M.I.; Allan, K. Vitamin C deficiency in cancer patients. Palliat. Med. 2005, 19, 17–20.
- Babaknejad, N.; Sayehmiri, F.; Sayehmiri, K.; Rahimifar, P.; Bahrami, S.; Delpesheh, A.; Hemati, F.; Alizadeh, S. The relationship between selenium levels and breast cancer: A systematic review and meta-analysis. Biol. Trace Elem. Res. 2014, 159, 1–7.
- Bhagat, S.S.; Ghone, R.A.; Suryakar, A.N.; Hundekar, P.S. Lipid peroxidation and antioxidant vitamin status in colorectal cancer patients. Indian J. Physiol. Pharmacol. 2011, 55, 72–76.
- Lin, C.-C.; Yin, M.-C. B vitamins deficiency and decreased anti-oxidative state in patients with liver cancer. Eur. J. Nutr. 2007, 46, 293–299.
- Stefanini, M. Cutaneous bleeding related to zinc deficiency in two cases of advanced cancer. Cancer 1999, 86, 866–870.
- Jatoi, A.; Williams, B.; Nichols, F.; Marks, R.; Aubry, M.-C.; Wampfler, J.; Finke, E.E.; Yang, P. Is voluntary vitamin and mineral supplementation associated with better outcome in non-small cell lung cancer patients?: Results from the Mayo Clinic lung cancer cohort. Lung Cancer 2005, 49, 77–84.
- Sieja, K.; Talerczyk, M. Selenium as an element in the treatment of ovarian cancer in women receiving chemotherapy. Gynecol. Oncol. 2004, 93, 320–327.
- Pathak, A.K.; Bhutani, M.; Guleria, R.; Bal, S.; Mohan, A.; Mohanti, B.K.; Sharma, A.; Pathak, R.; Bhardwaj, N.K.; Prasad, K.N. Chemotherapy alone vs. chemotherapy plus high dose multiple antioxidants in patients with advanced non small cell lung cancer. J. Am. Coll. Nutr. 2005, 24, 16–21.
- Prasad, K.N. Multiple dietary antioxidants enhance the efficacy of standard and experimental cancer therapies and decrease their toxicity. Integr. Cancer Ther. 2004, 3, 310–322.
- Ströhle, A.; Zänker, K.; Hahn, A. Nutrition in oncology: The case of micronutrients. Oncol. Rep. 2010, 24, 815–828.
- Norman, H.A.; Butrum, R.R.; Feldman, E.; Heber, D.; Nixon, D.; Picciano, M.F.; Rivlin, R.; Simopoulos, A.; Wargovich, M.J.; Weisburger, E.K. The role of dietary supplements during cancer therapy. J. Nutr. 2003, 133, 3794S–3799S.
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M. Nutrition and physical activity guidelines for cancer survivors. Cancer J. Clin. 2012, 62, 242–274.
- Marmot, M.; Atinmo, T.; Byers, T.; Chen, J.; Hirohata, T.; Jackson, A.; James, W.; Kolonel, L.; Kumanyika, S.; Leitzmann, C. Food, Nutrition, Physical activity, and the Prevention of Cancer: A Global Perspective; World Cancer Research Fund/American Institute for Cancer Research: Washington, DC, USA, 2007; pp. 4–29.
- Ja Kim, H.; Lee, S.S.; Choi, B.Y.; Kim, M.K. Nitrate intake relative to antioxidant vitamin intake affects gastric cancer risk: A case-control study in Korea. Nutr. Cancer 2007, 59, 185–191.
- Donma, O.; Donma, M.M.; Sonmez, S. Metal speciation, phytochemicals and Helicobacter pylori infection. Med. Hypotheses 2006, 67, 545–549.
- Abreu, M.T.; Peek, R.M., Jr. Gastrointestinal malignancy and the microbiome. Gastroenterology 2014, 146, 1534–1546.e3.
- Noto, J.M.; Peek, R.M., Jr. Micronutrients: A double-edged sword in microbe-induced gastric carcinogenesis. Trends Cancer 2015, 1, 136–144.
- Jenab, M.; Riboli, E.; Ferrari, P.; Sabate, J.; Slimani, N.; Norat, T.; Friesen, M.; Tjønneland, A.; Olsen, A.; Overvad, K. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Carcinogenesis 2006, 27, 2250–2257.
- Jenab, M.; Riboli, E.; Ferrari, P.; Friesen, M.; Sabate, J.; Norat, T.; Slimani, N.; Tjønneland, A.; Olsen, A.; Overvad, K. Plasma and dietary carotenoid, retinol and tocopherol levels and the risk of gastric adenocarcinomas in the European prospective investigation into cancer and nutrition. Br. J. Cancer 2006, 95, 406–415.
- Larsson, S.C.; Bergkvist, L.; Näslund, I.; Rutegård, J.; Wolk, A. Vitamin A, retinol, and carotenoids and the risk of gastric cancer: A prospective cohort study. Am. J. Clin. Nutr. 2007, 85, 497–503.
- Pelucchi, C.; Tramacere, I.; Bertuccio, P.; Tavani, A.; Negri, E.; La Vecchia, C. Dietary intake of selected micronutrients and gastric cancer risk: An Italian case-control study. Ann. Oncol. 2009, 20, 160–165.
- Lazarević, K.; Nagorni, A.; Bogdanović, D.; Rančić, N.; Stošić, L.; Milutinović, S. Dietary micronutrients and gastric cancer: Hospital based study. Cent. Eur. J. Med. 2011, 6, 783–787.
- Sun, Z.; Zhu, Y.; Wang, P.P.; Roebothan, B.; Zhao, J.; Zhao, J.; Dicks, E.; Cotterchio, M.; Buehler, S.; Campbell, P.T. Reported intake of selected micronutrients and risk of colorectal cancer: Results from a large population-based case–control study in Newfoundland, Labrador and Ontario, Canada. Anticancer Res. 2012, 32, 687–696.
- Da Costa, P.M.; Martins, I.; Neves, J.; Cortez-Pinto, H.; Velosa, J. Serum vitamin D levels correlate with the presence and histological grading of colorectal adenomas in peri and postmenopausal women. Clin. Nutr. 2019, 38, 1390–1397.
- Yaprak, G.; Gemici, C.; Temizkan, S.; Ozdemir, S.; Dogan, B.C.; Seseogullari, O.O. Osteoporosis development and vertebral fractures after abdominal irradiation in patients with gastric cancer. BMC Cancer 2018, 18, 1–6.
- McCullough, M.L.; Zoltick, E.S.; Weinstein, S.J.; Fedirko, V.; Wang, M.; Cook, N.R.; Eliassen, A.H.; Zeleniuch-Jacquotte, A.; Agnoli, C.; Albanes, D. Circulating vitamin D and colorectal cancer risk: An international pooling project of 17 cohorts. J. Natl. Cancer Inst. 2019, 111, 158–169.
- Sacco, R.; Conte, C.; Marceglia, S.; Mismas, V.; Bresci, G.; Romano, A.; Eggenhoffner, R.; Giacomelli, L. Beneficial and detrimental effects of natural dietary products on the risk of hepatocellular carcinoma, and their roles in its management. Hepatoma Res. 2016, 2, 53–61.
- Kozeniecki, M.; Ludke, R.; Kerner, J.; Patterson, B. Micronutrients in liver disease: Roles, risk factors for deficiency, and recommendations for supplementation. Nutr. Clin. Pract. 2020, 35, 50–62.
- Schütte, K.; Schulz, C.; Malfertheiner, P. Nutrition and hepatocellular cancer. Gastrointest. Tumors 2015, 2, 188–194.
- Fedirko, V.; Duarte-Salles, T.; Bamia, C.; Trichopoulou, A.; Aleksandrova, K.; Trichopoulos, D.; Trepo, E.; Tjønneland, A.; Olsen, A.; Overvad, K. Prediagnostic circulating vitamin D levels and risk of hepatocellular carcinoma in European populations: A nested case-control study. Hepatology 2014, 60, 1222–1230.
- Grüngreiff, K.; Reinhold, D.; Wedemeyer, H. The role of zinc in liver cirrhosis. Ann. Hepatol. 2016, 15, 7–16.
- Ebara, M.; Fukuda, H.; Hatano, R.; Saisho, H.; Nagato, Y.; Suzuki, K.; Nakajima, K.; Yukawa, M.; Kondo, F.; Nakayama, A. Relationship between copper, zinc and metallothionein in hepatocellular carcinoma and its surrounding liver parenchyma. J. Hepatol. 2000, 33, 415–422.
- Alberino, F.; Gatta, A.; Amodio, P.; Merkel, C.; Di Pascoli, L.; Boffo, G.; Caregaro, L. Nutrition and survival in patients with liver cirrhosis. Nutrition 2001, 17, 445–450.
- Maqbool, M.A.; Aslam, M.; Akbar, W.; Iqbal, Z. Biological importance of vitamins for human health: A review. J. Agric. Basic Sci. 2017, 2, 50–58.
- Chiang, K.C.; Yeh, C.N.; Chen, M.F.; Chen, T.C. Hepatocellular carcinoma and vitamin D: A review. J. Gastroenterol. Hepatol. 2011, 26, 1597–1603.
- Zuo, S.; Wu, L.; Wang, Y.; Yuan, X. Long non-coding RNA MEG3 activated by vitamin d suppresses glycolysis in colorectal cancer via promoting c-myc degradation. Front. Oncol. 2020, 10, 274.
- Zhang, W.; Shu, X.-O.; Li, H.; Yang, G.; Cai, H.; Ji, B.-T.; Gao, J.; Gao, Y.-T.; Zheng, W.; Xiang, Y.-B. Vitamin intake and liver cancer risk: A report from two cohort studies in China. J. Natl. Cancer Inst. 2012, 104, 1174–1182.
- Taylor, P.R.; Qiao, Y.-L.; Abnet, C.C.; Dawsey, S.M.; Yang, C.S.; Gunter, E.W.; Wang, W.; Blot, W.J.; Dong, Z.-W.; Mark, S.D. Prospective study of serum vitamin E levels and esophageal and gastric cancers. J. Natl. Cancer Inst. 2003, 95, 1414–1416.
- Barnett, K.T.; Fokum, F.D.; Malafa, M.P. Vitamin E succinate inhibits colon cancer liver metastases. J. Surg. Res. 2002, 106, 292–298.
- Correa, P.; Fontham, E.T.; Bravo, J.C.; Bravo, L.E.; Ruiz, B.; Zarama, G.; Realpe, J.L.; Malcom, G.T.; Li, D.; Johnson, W.D. Chemoprevention of gastric dysplasia: Randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J. Natl. Cancer Inst. 2000, 92, 1881–1888.
- Lv, H.; Wang, C.; Fang, T.; Li, T.; Lv, G.; Han, Q.; Yang, W.; Wang, H. Vitamin C preferentially kills cancer stem cells in hepatocellular carcinoma via SVCT-2. NPJ Precis. Oncol. 2018, 2, 1–13.
- García-Closas, R.; Berenguer, A.; Tormo, M.J.; Sánchez, M.J.; Quiros, J.R.; Navarro, C.; Arnaud, R.; Dorronsoro, M.; Chirlaque, M.D.; Barricarte, A. Dietary sources of vitamin C, vitamin E and specific carotenoids in Spain. Br. J. Nutr. 2004, 91, 1005–1011.
- Dani, V.; Goel, A.; Vaiphei, K.; Dhawan, D. Chemopreventive potential of zinc in experimentally induced colon carcinogenesis. Toxicol. Lett. 2007, 171, 10–18.
- Fong, L.Y.; Nguyen, V.T.; Farber, J.L. Esophageal cancer prevention in zinc-deficient rats: Rapid induction of apoptosis by replenishing zinc. J. Natl. Cancer Inst. 2001, 93, 1525–1533.
- Ji, J.H.; Shin, D.G.; Kwon, Y.; Cho, D.H.; Lee, K.B.; Park, S.S.; Yoon, J. Clinical correlation between gastric cancer type and serum selenium and zinc levels. J. Gastric Cancer 2012, 12, 217.
- Costello, L.C.; Franklin, R.B. The status of zinc in the development of hepatocellular cancer: An important, but neglected, clinically established relationship. Cancer Biol. Ther. 2014, 15, 353–360.
- Rollison, D.E.; Cole, A.L.; Tung, K.-H.; Slattery, M.L.; Baumgartner, K.B.; Byers, T.; Wolff, R.K.; Giuliano, A.R. Vitamin D intake, vitamin D receptor polymorphisms, and breast cancer risk among women living in the southwestern US. Breast Cancer Res. Treat. 2012, 132, 683–691.
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357.
- Ames, B.N. Micronutrients prevent cancer and delay aging. Toxicol. Lett. 1998, 102, 5–18.
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L., Jr.; Valanis, B.; Williams, J.H., Jr. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155.
- Block, G. The data support a role for antioxidants in reducing cancer risk. Nutr. Rev. 1992, 50, 207–213.
- Block, G.; Patterson, B.; Subar, A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr. Cancer 1992, 18, 1–29.
- Byers, T.; Guerrero, N. Epidemiologic evidence for vitamin C and vitamin E in cancer prevention. Am. J. Clin. Nutr. 1995, 62, 1385S–1392S.
- Diplock, A.T. Will the ‘good fairies’ please prove to us that vitamin E lessens human degenerative disease? Free Radic. Res. 1997, 27, 511–532.
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922.
- Ames, B.N.; Gold, L.S. Environmental pollution, pesticides, and the prevention of cancer: Misconceptions 1. FASEB J. 1997, 11, 1041–1052.
- Gerster, H. β-carotene, vitamin E and vitamin C in different stages of experimental carcinogenesis. Eur. J. Clin. Nutr. 1995, 49, 155–168.
- Hagen, T.M.; Yowe, D.L.; Bartholomew, J.C.; Wehr, C.M.; Do, K.L.; Park, J.-Y.; Ames, B.N. Mitochondrial decay in hepatocytes from old rats: Membrane potential declines, heterogeneity and oxidants increase. Proc. Natl. Acad. Sci. USA 1997, 94, 3064–3069.
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316.
- Ames, B.N.; Gold, L.S.; Willett, W.C. The causes and prevention of cancer. Proc. Natl. Acad. Sci. USA 1995, 92, 5258–5265.
- Kukovetz, E.M.; Bratschitsch, G.; Hofer, H.P.; Egger, G.; Schaur, R.J. Influence of age on the release of reactive oxygen species by phagocytes as measured by a whole blood chemiluminescence assay. Free Radic. Biol. Med. 1997, 22, 433–438.
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer prevention: A review. J. Am. Diet. Assoc. 1996, 96, 1027–1039.
- Blot, W.J. Vitamin/mineral supplementation and cancer risk: International chemoprevention trials. Proc. Soc. Exp. Biol. Med. 1997, 216, 291–296.
- Duthie, S.J.; Ma, A.; Ross, M.A.; Collins, A.R. Antioxidant supplementation decreases oxidative DNA damage in human lymphocytes. Cancer Res. 1996, 56, 1291–1295.
- Cooney, R.V.; Harwood, P.J.; Franke, A.A.; Narala, K.; Sundström, A.-K.; Berggren, P.-O.; Mordan, L.J. Products of γ-tocopherol reaction with NO2 and their formation in rat insulinoma (RINm5F) cells. Free Radic. Biol. Med. 1995, 19, 259–269.
- Shigenaga, M.K.; Lee, H.H.; Blount, B.C.; Christen, S.; Shigeno, E.T.; Yip, H.; Ames, B.N. Inflammation and NOx-induced nitration: Assay for 3-nitrotyrosine by HPLC with electrochemical detection. Proc. Natl. Acad. Sci. USA 1997, 94, 3211–3216.
- Fleet, J.C.; DeSmet, M.; Johnson, R.; Li, Y. Vitamin D and cancer: A review of molecular mechanisms. Biochem. J. 2012, 441, 61–76.
- Jacob, R.A.; Sotoudeh, G. Vitamin C function and status in chronic disease. Nutr. Clin. Care 2002, 5, 66–74.
- Van den Berg, J.J.; Kuypers, F.A.; Roelofsen, B.; den Kamp, J.A.O. The cooperative action of vitamins E and C in the protection against peroxidation of parinaric acid in human erythrocyte membranes. Chem. Phys. Lipids 1990, 53, 309–320.
- Du, J.; Martin, S.M.; Levine, M.; Wagner, B.A.; Buettner, G.R.; Wang, S.-H.; Taghiyev, A.F.; Du, C.; Knudson, C.M.; Cullen, J.J. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin. Cancer Res. 2010, 16, 509–520.
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609.
- Burton, G.; Wronska, U.; Stone, L.; Foster, D.; Ingold, K. Biokinetics of dietary RRR-α-tocopherol in the male guinea pig at three dietary levels of vitamin C and two levels of vitamin E. Evidence that vitamin C does not “spare” vitamin E in vivo. Lipids 1990, 25, 199–210.
- Niki, E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am. J. Clin. Nutr. 1991, 54, 1119S–1124S.
- Chen, X.-j.; Duan, F.-d.; Zhang, H.-h.; Xiong, Y.; Wang, J. Sodium selenite-induced apoptosis mediated by ROS attack in human osteosarcoma U2OS cells. Biol. Trace Elem. Res. 2012, 145, 1–9.
- Pang, K.-L.; Chin, K.-Y. Emerging anticancer potentials of selenium on osteosarcoma. Int. J. Mol. Sci. 2019, 20, 5318.
- Wang, Y.; Wang, J.; Hao, H.; Cai, M.; Wang, S.; Ma, J.; Li, Y.; Mao, C.; Zhang, S. In vitro and in vivo mechanism of bone tumor inhibition by selenium-doped bone mineral nanoparticles. ACS Nano 2016, 10, 9927–9937.
- Liu, B.; Chen, Y.; Clair, D.K.S. ROS and p53: A versatile partnership. Free Radic. Biol. Med. 2008, 44, 1529–1535.
- Wang, W.; Meng, F.B.; Wang, Z.X.; Li, X.; Zhou, D.S. Selenocysteine inhibits human osteosarcoma cells growth through triggering mitochondrial dysfunction and ROS-mediated p53 phosphorylation. Cell Biol. Int. 2018, 42, 580–588.
- Jiang, P.; Du, W.; Heese, K.; Wu, M. The Bad guy cooperates with good cop p53: Bad is transcriptionally up-regulated by p53 and forms a Bad/p53 complex at the mitochondria to induce apoptosis. Mol. Cell. Biol. 2006, 26, 9071–9082.
- Stambolic, V.; MacPherson, D.; Sas, D.; Lin, Y.; Snow, B.; Jang, Y.; Benchimol, S.; Mak, T. Regulation of PTEN transcription by p53. Mol. Cell 2001, 8, 317–325.
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of poly (ADP-ribose) polymerase (PARP) cleavage in apoptosis: Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999, 274, 22932–22940.
- Chaitanya, G.V.; Alexander, J.S.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010, 8, 1–11.
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700.
- Baudet, C.; Chevalier, G.; Chassevent, A.; Canova, C.; Filmon, R.; Larra, F.; Brachet, P.; Wion, D. 1,25-Dihydroxyvitamin D3 induces programmed cell death in a rat glioma cell line. J. Neurosci. Res. 1996, 46, 540–550.
- Pitot, H.C.; Dragan, Y.P. The multistage nature of chemically induced hepatocarcinogenesis in the rat. Drug Metab. Rev. 1994, 26, 209–220.
- Bodmer, W.; Tomlinson, I. Population Genetics of Tumours, Ciba Foundation Symposium, 1996; Wiley Online Library: Hoboken, NJ, USA, 1996; pp. 181–193.
- Moreno, J.; Krishnan, A.V.; Feldman, D. Molecular mechanisms mediating the anti-proliferative effects of Vitamin D in prostate cancer. J. Steroid Biochem. Mol. Biol. 2005, 97, 31–36.
- Konety, B.R.; Getzenberg, R.H. Vitamin D and prostate cancer. Urol. Clin. 2002, 29, 95–106.
- Wyllie, A. Apoptosis: Cell death in tissue regulation. J. Pathol. 1987, 153, 313–316.
- Jensen, S.S.; Madsen, M.W.; Lukas, J.; Binderup, L.; Bartek, J. Inhibitory effects of 1α, 25-dihydroxyvitamin D3 on the G1–S phase-controlling machinery. Mol. Endocrinol. 2001, 15, 1370–1380.
- Meyer, M.B.; Goetsch, P.D.; Pike, J.W. VDR/RXR and TCF4/β-catenin cistromes in colonic cells of colorectal tumor origin: Impact on c-FOS and c-MYC gene expression. Mol. Endocrinol. 2012, 26, 37–51.
- Salehi-Tabar, R.; Nguyen-Yamamoto, L.; Tavera-Mendoza, L.E.; Quail, T.; Dimitrov, V.; An, B.-S.; Glass, L.; Goltzman, D.; White, J.H. Vitamin D receptor as a master regulator of the c-MYC/MXD1 network. Proc. Natl. Acad. Sci. USA 2012, 109, 18827–18832.
- Washington, M.N.; Kim, J.S.; Weigel, N.L. 1α,25-dihydroxyvitamin D3 inhibits C4-2 prostate cancer cell growth via a retinoblastoma protein (Rb)-independent G1 arrest. Prostate 2011, 71, 98–110.
- Li, P.; Li, C.; Zhao, X.; Zhang, X.; Nicosia, S.V.; Bai, W. p27Kip1 stabilization and G1 arrest by 1,25-dihydroxyvitamin D3 in ovarian cancer cells mediated through down-regulation of cyclin E/cyclin-dependent kinase 2 and Skp1-Cullin-F-box protein/Skp2 ubiquitin ligase. J. Biol. Chem. 2004, 279, 25260–25267.
- Bao, B.-Y.; Hu, Y.-C.; Ting, H.-J.; Lee, Y.-F. Androgen signaling is required for the vitamin D-mediated growth inhibition in human prostate cancer cells. Oncogene 2004, 23, 3350–3360.
- Sunil Kumar, B.; Singh, S.; Verma, R. Anticancer potential of dietary vitamin D and ascorbic acid: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2623–2635.
- Losso, J.N.; Bawadi, H.A. Hypoxia inducible factor pathways as targets for functional foods. J. Agric. Food Chem. 2005, 53, 3751–3768.
- Ben-Shoshan, M.; Amir, S.; Dang, D.T.; Dang, L.H.; Weisman, Y.; Mabjeesh, N.J. 1α,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol. Cancer Ther. 2007, 6, 1433–1439.
- Peyman, G.; Kivilcim, M.; Dellacroce, J.; Morales, A.M. Inhibition of corneal neovascularization by ascorbic acid in rat model. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1634.
- Mikirova, N.A.; Ichim, T.E.; Riordan, N.H. Anti-angiogenic effect of high doses of ascorbic acid. J. Transl. Med. 2008, 6, 1–10.




