Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marta Garaulet | + 2301 word(s) | 2301 | 2021-08-04 06:20:10 | | | |
| 2 | Peter Tang | Meta information modification | 2301 | 2021-08-12 03:57:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Garaulet, M. Eating Timing and Obesity/Metabolic Risk. Encyclopedia. Available online: https://encyclopedia.pub/entry/13054 (accessed on 07 February 2026).
Garaulet M. Eating Timing and Obesity/Metabolic Risk. Encyclopedia. Available at: https://encyclopedia.pub/entry/13054. Accessed February 07, 2026.
Garaulet, Marta. "Eating Timing and Obesity/Metabolic Risk" Encyclopedia, https://encyclopedia.pub/entry/13054 (accessed February 07, 2026).
Garaulet, M. (2021, August 11). Eating Timing and Obesity/Metabolic Risk. In Encyclopedia. https://encyclopedia.pub/entry/13054
Garaulet, Marta. "Eating Timing and Obesity/Metabolic Risk." Encyclopedia. Web. 11 August, 2021.
Copy Citation
Eating is fundamental to survival. Animals choose when to eat depending on food availability. The timing of eating can synchronize different organs and tissues that are related to food digestion, absorption, or metabolism, such as the stomach, gut, liver, pancreas, or adipose tissue. Studies performed in experimental animal models suggest that food intake is a major external synchronizer of peripheral clocks.
circadian rhythms
food timing
melatonin
nutrigenetic
obesity
weight loss
1. Introduction
Obesity treatment has undergone numerous changes, but problems of attrition and variability in response remain. Up to the 1960s, hypocaloric diets were the only recommended treatment, while the 1970s saw the introduction of behavioral therapy (BT), promoting changes in lifestyle and eating habits as an alternative therapy [1][2]. Since then, many studies have underlined the importance of BT together with dietetic treatment in all forms of weight control. Despite the many widely attested benefits associated with weight loss, the usefulness of dietetic treatment has been questioned [3] since some studies have shown that as many as 80% of patients abandon treatments before achieving their goal [4].
Weight loss and attrition in response to behavioral–dietary interventions show a wide range of inter-individual variation [5][6]. While the type of diet [7], exercise level [8], and emotional factors [6] contribute to differences in weight-loss effectiveness, little is known about additional causal factors. We have discovered that the timing of food intake is an emerging factor that may predict the success of weight loss therapies. Not only “what” but also “when” we eat may have a significant role in obesity treatment [9].
We found that eating the main meal late (after 3 p.m.), was predictive of difficulty in weight loss [9]. In addition, the distribution of energy intake across meals may be an important factor. As Jakubowicz et al. have shown, those subjects assigned to a small breakfast and a large dinner lost significantly less weight than those assigned to a large breakfast and a small dinner [10]. Furthermore, we have shown that food timing may affect other circadian-related variables that can predict weight loss [5][11]. We have reported genetic contributions to these factors, finding that circadian-related single-nucleotide polymorphisms (SNPs) are associated with weight loss effectiveness [12][13], adherence [14], and food timing [15]. We have also observed food timing and genetic interactions of SNPs in obesity that may predict weight loss and weight loss trajectory. Together, these observations suggest that eating at the wrong time may negatively influence the success of obesity treatments and several mechanisms may be involved in the obesogenic effect of eating late.
2. Lunch Timing Affects Weight Loss Effectiveness
One of the first studies that have highlighted the potential impact of food timing on metabolism has been conducted by the group of Turek in 2009 [16]. In that study, those mice that were fed with a high-fat diet during the “right” feeding time (during the dark period in rodents) gained less weight than those fed with a similar high fat diet but during the “wrong” period (light period in rodents, when feeding is normally reduced). This study inspired our group together with Dr. Scheer to develop a similar observational study in humans, in order to determine whether food timing influences body weight during a dietary treatment to obesity [15].
For this purpose, 420 obese subjects who attended different nutritional clinics in Spain to lose weight were classified regarding the timing of the main meal of the day (lunch in Spain). Results showed that late lunch eaters (after 3 p.m.) lost less weight during the treatment than early lunch eaters (before 3 p.m.), in spite of having similar age, appetite hormones, energy intake and expenditure, sleep duration or macronutrients distribution (Figure 1) [15]. It was remarkable that late eaters were more evening type, i.e., evening types stay up late at night, rise at a later time in the morning, and perform best mentally and physically in the late afternoon or evening [17] and carried the risk variant at CLOCK rs4580704 more frequently [15]. This study encouraged us to delve into the importance of meal timing on metabolism, obesity and weight loss, and opened a new door for further studies in this field that has been named “Chrononutrition”.
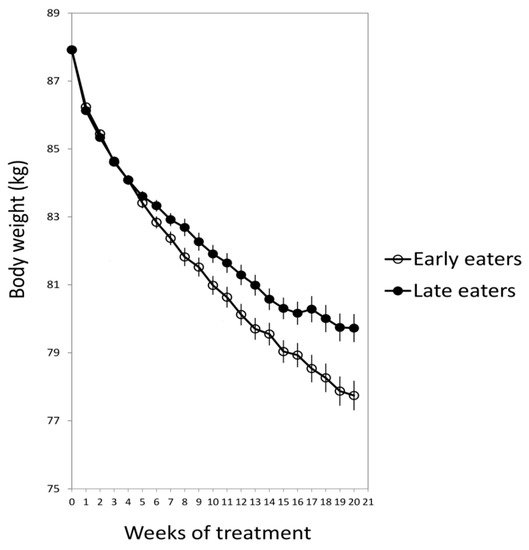
Figure 1. The weight loss evolution of late and early lunch eaters during the 20 weeks of treatment. Adapted from Garaulet et al., 2013 [15].
3. How Does the Timing of Food Intake Affect Metabolism?
In order to understand the mechanisms that underlie the difficulties of late eaters in losing weight, we developed a crossover randomized trial in 32 young women studied under two lunch-timing conditions: Early eating (lunch at 1 p.m.) and late eating (lunch at 4:30 p.m.). Volunteers received standardized meals during both meal interventions. Late eating decreased glucose tolerance, resting energy expenditure, and carbohydrate oxidation as compared to early eating. Besides, the cortisol profile was blunted for late eating as compared to early eating, similarly to that found under acute stress situations [11]. Eating late also affected the daily rhythms of peripheral temperature, towards a similar pattern to that found in overweight/obesity women which was related to metabolic alterations [18].
In order to assess whether microbiota composition and diversity were implicated in the metabolic effects of late eating, we carried out a second randomized and crossover study in 10 healthy normal-weight women [19]. We showed the impact of food timing on human salivary microbiota. There was a significant diurnal rhythm in saliva diversity across both early and late eating conditions (1 p.m. and 4 p.m., respectively) [19]. Moreover, late eating inverted the daily rhythm of salivary microbiota diversity as compared to early eating. This may have deleterious effects on the metabolism of the host [19]. It has been demonstrated that saliva bacteria, such as oral Fusobacteria, which changed with food timing, have an impact on the intestine and are related to Chron´s disease and intestinal inflammatory diseases [20]. Nevertheless, further studies should analyze the impact on obesity and weight loss of this inverted rhythm of saliva microbiota when eating late. Indeed, in spite of the significant amount of scientific studies which associate dysbiosis with obesity, even the causative role of the microbiome in obesity remains debated [21].
4. Timing of Food Intake Does Affect Everyone or it Depends on Genetics?
Once we knew that food timing affected BMI and weight loss efficiency, we wondered whether eating late affected everybody or by contrast the effects of meal timing on weight loss changed depending on the individual´s genetic background. In order to address this question, we selected PERILIPIN1 (PLIN1), a candidate gene for obesity that encodes an adipocyte-associated protein (PLIN1) which influences body weight, fat accumulation, and lipolysis and which has been shown to display circadian rhythms in murine adipose tissue, although it has not been demonstrated in humans yet [22].
PLIN1 promotes fat storage in adipose tissue by limiting the lipolytic activity of hormone-sensitive lipase. Our results indicated that eating late was related to less total weight loss and slower weight loss rate only in major carriers (AA) of a particular genetic variant in PLIN1 (14995A > T) which constitute a 44% of the population who attended the nutritional clinics. Whereas food timing did not influence weight loss among T carriers. This study demonstrates that not everybody is affected in the same manner by eating late, and that genetics may play an important role in interindividual differences in weight loss depending on the timing of food intake. In the following section, we will discuss another example of the interaction between food timing, dinner timing, and genetics, which is related to one genetic variant in the melatonin receptor 1b (MTNR1B) [23]. These are two examples, but further studies should be performed in larger populations to discover other novel genetic variants that may interact with food timing for weight loss.
5. Late Dinner
Spain is one of the countries in which people have dinner the latest in Europe. Spaniards usually have dinner around 10 p.m., thus eating later than Italians (9 p.m.), Frenchmen (8 p.m.), Germans (7 p.m.) and finally Swedes, who have dinner around 6 p.m.
While our studies showed that the timing of lunch, and not the timing of breakfast or dinner, was related to weight loss effectiveness, other studies have demonstrated that having a late dinner or eating late at night associates with increased risks of obesity [24][25][26][27], dyslipidemia [25][28], hyperglycemia [29], and metabolic syndrome [26][27].
Previous research has shown that eating in misalignment with the biological clock, such as eating late at night and shift work, is associated with increased risk for diabetes [30]. Glucose metabolism shows clear diurnal variation, and small changes in meal timing, i.e., the distribution of caloric intake across the normal wake episode, appear to influence insulin resistance [11][31]. Indeed, a 12-week experimental study in overweight/obese women with metabolic syndrome randomized into two iso-caloric weight loss groups showed that subjects with the highest caloric intake during dinner had greater insulin resistance than those with the highest caloric intake during breakfast [10]. This suggested that reduced intake at dinner was beneficial and might be a useful alternative for the management of the metabolic syndrome. A large epidemiological study performed in a Japanese adult population (n = 61,364) of late-night-dinner eaters demonstrated that late-night-dinner eating was robustly associated with hyperglycemia independent of relevant confounders including BMI [29] (Table 1). Studies performed in laboratory conditions have found that inversion of the sleep/wake and fasting/feeding cycle, i.e., being awake and eating during their biological night, caused multiple metabolic changes including increased postprandial glucose and insulin concentrations [32][33]. In fact, postprandial responses of some of these healthy subjects during their biological night were equivalent to the responses of prediabetic individuals [33].
6. Breakfast
The metabolic effects of breakfast are an open question in the nutritional field depending on several aspects, such as food composition, caloric and nutritional content, and timing of intake.
6.1. Contradictory Results in Breakfast Skipping and Weight Loss
One of the first studies on the effect of caloric distribution along the day and weight loss was the already mentioned study [10] that showed that subjects who had high caloric breakfasts (700 kcal) and low caloric dinners (300 kcal) lost significantly more weight than those who had low caloric breakfasts and high caloric dinners. In both cases, subjects followed a 12-week weight loss diet of approximately 1400 kcal, maintaining the same caloric intake for lunch and during the day. This study suggested that we should recommend a high caloric breakfast in order to lose weight [10].
In this line, it has been published that skipping breakfast is associated to unhealthy behaviors, poorer diets, and lower physical activity [34][35][36][37] and also with a higher metabolic risk, i.e., higher body mass index (BMI), larger waist circumference, higher fasting insulin and increased cholesterol and LDL levels [34][35][36][37]. This situation has also been linked to higher risk of diabetes type 2 and cardiometabolic factors independent of dietary quality [38][39][40][41][42]. One potential explanation for this deleterious effect is that skipping breakfast may be difficult to compensate later in the day, and people who do not eat the first meal of the day are reported to have higher daily intakes of fat, energy and cholesterol and lower intakes of vitamins, minerals and fiber than breakfast eaters [43]. In addition, some studies have shown a correlation with the timing of other meals [44][45][46][47].
However, in a more recent systematic review published in 2019 [48] which revises 13 trials comparing breakfast consumption with no breakfast consumption, it has been concluded that the addition of breakfast might not be a good strategy for weight loss, regardless of established breakfast habit.
Although this review must be interpreted with caution due to the relatively low quality of the included studies, the apparent contradiction among studies about the beneficial or deleterious effects of breakfast, encouraged us, together with Dr. Saxena and Dr. Dashti, to develop a Genome-Wide Association Study (GWAS) of breakfast skipping in UK Biobank (approx. 200,000 participants) and to replicate the results in other European populations (Twin UK and CHARGE) [49]. In this study, we identified six genetic variants that associated with skipping breakfast and that were implicated in caffeine, carbohydrate metabolism, and circadian clock regulation.
Using Mendelian randomization (MR), we provided evidence suggesting that genetically determined breakfast skipping was causally associated with obesity in this large population of 200,000 participants. A limitation of observational studies is that they only allow us to look for associations between one behavior and one disease, but we cannot assess causality or directionality. For example, although studies have found an association between skipping breakfast and obesity, we cannot discard the possibility that skipping breakfast is a consequence of obesity and not a cause. Randomized controlled trials (RCT) are able to address causality, however this type of studies is difficult to perform and usually limited in statistical power, due to the rather low number of participants. Our MR findings suggest that skipping breakfast could be causal of obesity. However, results should be interpreted cautiously in light of various MR limitations including that DNA’ does not contain all the information needed to specify the phenotype [50][51][52] among others [53][54][55][56].
The timing of breakfast is another relevant aspect of metabolism since it is directly connected to fasting duration at night, which has been reported to be crucial for metabolism. Previously, our research group had demonstrated in a twin study that breakfast timing has a high heritability (56%), lunch timing presents a lower heritability (38%), and that dinner timing is not driven by genetics (0%) but determined by environmental factors [57].
Unlike lunch and dinner, which are recommended early in the day, it has been proved that to have breakfast too early may be deleterious due to melatonin levels, which may still be high in the early morning. This could be particularly problematic in MTNR1B subjects, carriers of the common MTNR1B T2D risk variant G, not only because melatonin signaling is higher due to an increased receptor expression, but also because in these subjects, the duration of elevated melatonin levels may be extended with a delayed decline in the morning [58]. This effect increases the probability of concurrence with food intake in the morning, which therefore increases also the metabolic risk, as previously discussed.
References
- Marquez-Ibanez, B.; Armendariz-Anguiano, A.L.; Bacardi-Gascon, M.; Jimenez-Cruz, A. Review of controled clinical trials of behavioral treatment for obesity. Nutr. Hosp. 2008, 23, 1–5.
- Council on Scientific Affairs. Treatment of obesity in adults. JAMA 1988, 260, 2547–2551.
- Kassirer, J.P.; Angell, M. Losing weight—An ill-fated New Year’s resolution. N. Engl. J. Med. 1998, 338, 52–54.
- Kramer, F.M.; Jeffery, R.W.; Forster, J.L.; Snell, M.K. Long-term follow-up of behavioral treatment for obesity: Patterns of weight regain among men and women. Int. J. Obes. 1989, 13, 123–136.
- Bandin, C.; Martinez-Nicolas, A.; Ordovas, J.M.; Madrid, J.A.; Garaulet, M. Circadian rhythmicity as a predictor of weight-loss effectiveness. Int. J. Obes. 2014, 38, 1083.
- Corbalan, M.D.; Morales, E.M.; Canteras, M.; Espallardo, A.; Hernandez, T.; Garaulet, M. Effectiveness of cognitive-behavioral therapy based on the Mediterranean diet for the treatment of obesity. Nutrition 2009, 25, 861–869.
- Dansinger, M.L.; Gleason, J.A.; Griffith, J.L.; Selker, H.P.; Schaefer, E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA 2005, 293, 43–53.
- Anderson, J.W.; Konz, E.C.; Frederich, R.C.; Wood, C.L. Long-term weight-loss maintenance: A meta-analysis of US studies. Am. J. Clin. Nutr. 2001, 74, 579–584.
- Garaulet, M.; Gomez-Abellan, P. Timing of food intake and obesity: A novel association. Physiol. Behav. 2014, 134, 44–50.
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512.
- Bandin, C.; Scheer, F.A.; Luque, A.J.; Avila-Gandia, V.; Zamora, S.; Madrid, J.A.; Gomez-Abellan, P.; Garaulet, M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int. J. Obes. 2015, 39, 828–833.
- Garaulet, M.; Corbalan, M.D.; Madrid, J.A.; Morales, E.; Baraza, J.C.; Lee, Y.C.; Ordovas, J.M. CLOCK gene is implicated in weight reduction in obese patients participating in a dietary programme based on the Mediterranean diet. Int. J. Obes. 2010, 34, 516–523.
- Garaulet, M.; Esteban Tardido, A.; Lee, Y.C.; Smith, C.E.; Parnell, L.D.; Ordovas, J.M. SIRT1 and CLOCK 3111T>C combined genotype is associated with evening preference and weight loss resistance in a behavioral therapy treatment for obesity. Int. J. Obes. 2012, 36, 1436.
- Garaulet, M.; Corbalan-Tutau, M.D.; Madrid, J.A.; Baraza, J.C.; Parnell, L.D.; Lee, Y.C.; Ordovas, J.M. PERIOD2 variants are associated with abdominal obesity, psycho-behavioral factors, and attrition in the dietary treatment of obesity. J. Am. Diet. Assoc. 2010, 110, 917–921.
- Garaulet, M.; Gomez-Abellan, P.; Alburquerque-Bejar, J.J.; Lee, Y.C.; Ordovas, J.M.; Scheer, F.A. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. 2013, 37, 604–611.
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005, 308, 1043–1045.
- Randler, C. Differences between smokers and nonsmokers in morningness-eveningness. Soc. Behav. Personal. 2008, 36, 673–680.
- Corbalan-Tutau, M.D.; Madrid, J.A.; Ordovas, J.M.; Smith, C.E.; Nicolas, F.; Garaulet, M. Differences in daily rhythms of wrist temperature between obese and normal-weight women: Associations with metabolic syndrome features. Chronobiol. Int. 2011, 28, 425–433.
- Collado, M.C.; Engen, P.A.; Bandin, C.; Cabrera-Rubio, R.; Voigt, R.M.; Green, S.J.; Naqib, A.; Keshavarzian, A.; Scheer, F.; Garaulet, M. Timing of food intake impacts daily rhythms of human salivary microbiota: A randomized, crossover study. FASEB J. 2018, 32, 2060–2072.
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vazquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392.
- Harley, I.T.; Karp, C.L. Obesity and the gut microbiome: Striving for causality. Mol. Metab. 2012, 1, 21–31.
- Garaulet, M.; Vera, B.; Bonnet-Rubio, G.; Gomez-Abellan, P.; Lee, Y.C.; Ordovas, J.M. Lunch eating predicts weight-loss effectiveness in carriers of the common allele at PERILIPIN1: The ONTIME (Obesity, Nutrigenetics, Timing, Mediterranean) study. Am. J. Clin. Nutr. 2016, 104, 1160–1166.
- Lopez-Minguez, J.; Saxena, R.; Bandin, C.; Scheer, F.A.; Garaulet, M. Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: A randomized, cross-over study. Clin. Nutr. 2018, 37, 1133–1140.
- Xiao, Q.; Garaulet, M.; Scheer, F. Meal timing and obesity: Interactions with macronutrient intake and chronotype. Int. J. Obes. 2019, 43, 1701–1711.
- Yoshida, J.; Eguchi, E.; Nagaoka, K.; Ito, T.; Ogino, K. Association of night eating habits with metabolic syndrome and its components: A longitudinal study. BMC Public Health 2018, 18, 1366.
- Kutsuma, A.; Nakajima, K.; Suwa, K. Potential Association between Breakfast Skipping and Concomitant Late-Night-Dinner Eating with Metabolic Syndrome and Proteinuria in the Japanese Population. Scientifica (Cairo) 2014, 2014, 253581.
- Berg, C.; Lappas, G.; Wolk, A.; Strandhagen, E.; Toren, K.; Rosengren, A.; Thelle, D.; Lissner, L. Eating patterns and portion size associated with obesity in a Swedish population. Appetite 2009, 52, 21–26.
- Chen, H.J.; Chuang, S.Y.; Chang, H.Y.; Pan, W.H. Energy intake at different times of the day: Its association with elevated total and LDL cholesterol levels. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 390–397.
- Nakajima, K.; Suwa, K. Association of hyperglycemia in a general Japanese population with late-night-dinner eating alone, but not breakfast skipping alone. J. Diabetes Metab. Disord. 2015, 14, 16.
- Mattson, M.P.; Allison, D.B.; Fontana, L.; Harvie, M.; Longo, V.D.; Malaisse, W.J.; Mosley, M.; Notterpek, L.; Ravussin, E.; Scheer, F.A.; et al. Meal frequency and timing in health and disease. Proc. Natl. Acad. Sci. USA 2014, 111, 16647–16653.
- Morgan, L.M.; Shi, J.W.; Hampton, S.M.; Frost, G. Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. Br. J. Nutr. 2012, 108, 1286–1291.
- Morris, C.J.; Yang, J.N.; Garcia, J.I.; Myers, S.; Bozzi, I.; Wang, W.; Buxton, O.M.; Shea, S.A.; Scheer, F.A. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA 2015, 112, E2225–E2234.
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458.
- Ruxton, C.H.; Kirk, T.R. Breakfast: A review of associations with measures of dietary intake, physiology and biochemistry. Br. J. Nutr. 1997, 78, 199–213.
- Song, W.O.; Chun, O.K.; Obayashi, S.; Cho, S.; Chung, C.E. Is consumption of breakfast associated with body mass index in US adults? J. Am. Diet. Assoc. 2005, 105, 1373–1382.
- Timlin, M.T.; Pereira, M.A.; Story, M.; Neumark-Sztainer, D. Breakfast eating and weight change in a 5-year prospective analysis of adolescents: Project EAT (Eating Among Teens). Pediatrics 2008, 121, e638–e645.
- Van der Heijden, A.A.; Hu, F.B.; Rimm, E.B.; van Dam, R.M. A prospective study of breakfast consumption and weight gain among U.S. men. Obesity 2007, 15, 2463–2469.
- Uzhova, I.; Fuster, V.; Fernandez-Ortiz, A.; Ordovas, J.M.; Sanz, J.; Fernandez-Friera, L.; Lopez-Melgar, B.; Mendiguren, J.M.; Ibanez, B.; Bueno, H.; et al. The Importance of Breakfast in Atherosclerosis Disease: Insights from the PESA Study. J. Am. Coll. Cardiol. 2017, 70, 1833–1842.
- Smith, K.J.; Gall, S.L.; McNaughton, S.A.; Blizzard, L.; Dwyer, T.; Venn, A.J. Skipping breakfast: Longitudinal associations with cardiometabolic risk factors in the Childhood Determinants of Adult Health Study. Am. J. Clin. Nutr. 2010, 92, 1316–1325.
- Mekary, R.A.; Giovannucci, E.; Willett, W.C.; van Dam, R.M.; Hu, F.B. Eating patterns and type 2 diabetes risk in men: Breakfast omission, eating frequency, and snacking. Am. J. Clin. Nutr. 2012, 95, 1182–1189.
- Reutrakul, S.; Hood, M.M.; Crowley, S.J.; Morgan, M.K.; Teodori, M.; Knutson, K.L. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol. Int. 2014, 31, 64–71.
- Mekary, R.A.; Giovannucci, E.; Cahill, L.; Willett, W.C.; van Dam, R.M.; Hu, F.B. Eating patterns and type 2 diabetes risk in older women: Breakfast consumption and eating frequency. Am. J. Clin. Nutr. 2013, 98, 436–443.
- Timlin, M.T.; Pereira, M.A. Breakfast frequency and quality in the etiology of adult obesity and chronic diseases. Nutr. Rev. 2007, 65, 268–281.
- Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798.
- De Castro, J.M. The time of day of food intake influences overall intake in humans. J. Nutr. 2004, 134, 104–111.
- Kant, A.K.; Graubard, B.I. Within-person comparison of eating behaviors, time of eating, and dietary intake on days with and without breakfast: NHANES 2005–2010. Am. J. Clin. Nutr. 2015, 102, 661–670.
- Jakubowicz, D.; Froy, O.; Wainstein, J.; Boaz, M. Meal timing and composition influence ghrelin levels, appetite scores and weight loss maintenance in overweight and obese adults. Steroids 2012, 77, 323–331.
- Sievert, K.; Hussain, S.M.; Page, M.J.; Wang, Y.; Hughes, H.J.; Malek, M.; Cicuttini, F.M. Effect of breakfast on weight and energy intake: Systematic review and meta-analysis of randomised controlled trials. BMJ 2019, 364, 142.
- Dashti, H.S.; Merino, J.; Lane, J.M.; Song, Y.; Smith, C.E.; Tanaka, T.; McKeown, N.M.; Tucker, C.; Sun, D.; Bartz, T.M.; et al. Genome-wide association study of breakfast skipping links clock regulation with food timing. Am. J. Clin. Nutr. 2019, 110, 473–484.
- Lewontin, R.C. The analysis of variance and the analysis of causes. 1974. Int. J. Epidemiol. 2006, 35, 520–525.
- Lewontin, R.C. Biological determinism. Tanner Lect. Human Values 1983, 4, 147–183.
- Lewontin, R.C.; Rose, S.P.R.; Kamin, L.J. Not in Our Genes: Biology, Ideology, and Human Nature; Pantheon Books: New York, NY, USA, 1984.
- Archer, E. The childhood obesity epidemic as a result of nongenetic evolution: The maternal resources hypothesis. Mayo Clin. Proc. 2015, 90, 77–92.
- Maestripieri, D.; Mateo, J.M. Maternal Effects in Mammals; University of Chicago Press: Chicago, IL, USA, 2009.
- Waddington, C.H. Canalization of development and the inheritance of acquired characters. Nature 1942, 150, 563.
- Archer, E.; Lavie, C.J.; Hill, J.O. The Contributions of ‘Diet’, ‘Genes’, and Physical Activity to the Etiology of Obesity: Contrary Evidence and Consilience. Prog. Cardiovasc. Dis. 2018, 61, 89–102.
- Lopez-Minguez, J.; Dashti, H.S.; Madrid-Valero, J.J.; Madrid, J.A.; Saxena, R.; Scheer, F.; Ordonana, J.R.; Garaulet, M. Heritability of the timing of food intake. Clin. Nutr. 2019, 38, 767–773.
- Lane, J.M.; Vlasac, I.; Anderson, S.G.; Kyle, S.D.; Dixon, W.G.; Bechtold, D.A.; Gill, S.; Little, M.A.; Luik, A.; Loudon, A.; et al. Genome-wide association analysis identifies novel loci for chronotype in 100, 420 individuals from the UK Biobank. Nat. Commun. 2016, 7, 10889.
More
Information
Subjects:
Nutrition & Dietetics
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
12 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




