
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mariana Renovato | + 2052 word(s) | 2052 | 2021-08-10 08:08:28 |
Video Upload Options
Extracellular vesicles (EVs) are crucial elements that sustain the communication between tumor cells and their microenvironment, and have emerged as a widespread mechanism of tumor formation and metastasis. In obesity, the adipose tissue becomes hypertrophic and hyperplastic, triggering increased production of pro-inflammatory adipokines, such as tumor necrosis factor α, interleukin 6, interleukin 1, and leptin.
1. Introduction
Chronic inflammation, a well-established characteristic of obesity, is a central component of tumor development and progression [1][2][3] and is characterized by metabolic dysregulation and/or reprogramming [4]. The role of adipose tissue in cancer is reinforced by the following attributes: (i) adipocytes are major components of the tumor microenvironment from tumors that are capable of metastasizing to the abdominal areas in breast, gastric, ovarian, and colon cancers; (ii) adipose tissues and the tumor microenvironment share the presence of inflammatory cells, especially macrophages [3]; (iii) adipose tissues are capable of recruiting immune cells [5] ; (iv) secretion of pro-angiogenic molecules [6] ; (v) adipose tissues provide excess substrate for adenosine triphosphate (ATP) production and lipid membrane generation [4][7][8]; (vi) adipose tissues secrete large amounts of extracellular vesicles (EVs), thereby mediating cell-to-cell communication [9][10].
In 2011, Hanahan and Weinberg revisited the hallmarks and proposed that tumors consist of more than just proliferative cancer cells; in fact, they are complex tissues composed of distinct cells types capable of interacting with each other in the so-called tumor microenvironment, which plays an active role in tumorigenesis, allowing the development of certain hallmark characteristics. Consequently, two other emerging hallmarks including dysregulation of cellular metabolism and evasion of immune destruction were added to this list [11].
It is well established that EVs derived from tumor cells can transform non-tumor cells or confer them with metastatic ability. EVs derived from MDA-MB-231 breast tumor cells induced epithelial-mesenchymal transition (EMT) in MCF-10 breast epithelial cells [12]. EVs derived from another triple-negative breast tumor cell (HCC1806) induced proliferation and drug resistance in MCF-10 cells through the alteration of genes and microRNAs (miRNAs) related to proliferation, invasion, and migration pathways [13]. Shen et al. showed that highly metastatic HO8910PM ovarian tumor cells can transfer EVs enriched with CD44 to poorly metastatic HO8910 cells, thereby increasing their malignancy [14]. Furthermore, Sakha et al. observed that exosomes derived from the highly metastatic human oral cancer cell line HOC313-LM can transfer microRNA (miR)-1246 to the poorly metastatic cancer cell line HOC313-P, leading to an increase in its mobility and invasion [15]. However, the focus of our review is to highlight the crosstalk between EVs derived from adipose tissues (AT-EVs) and tumor cells.
Importantly, AT-EVs modulate several cell biological capabilities that are characteristic hallmarks of cancer ( Figure 1 ).
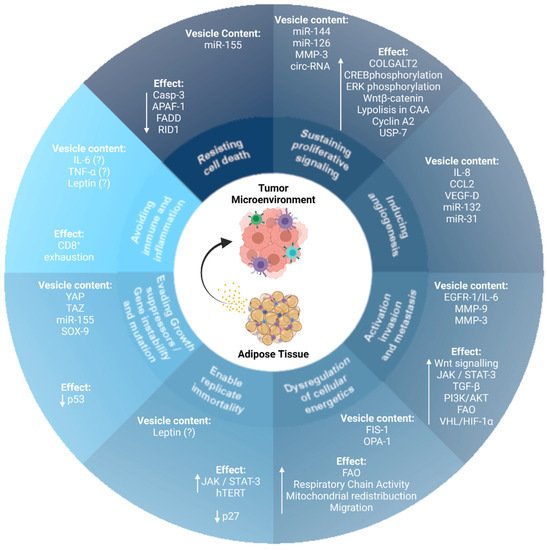
2. AT-EVs and the Hallmarks of Cancer
An important hallmark of cancer cells is their ability to evade apoptosis, both in situations of nutrient deprivation and in the presence of chemotherapeutic drugs [11]. In this context, Wang et al. showed that human exosomes derived from mesenchymal stem cell (MSC)-differentiated adipocytes protected breast cancer cells from apoptosis induced by serum starvation and treatment with the chemotherapeutic drug, 5-fluorouracil. Furthermore, the authors showed that adipocyte-conditioned medium devoid of exosomes was unable to protect breast cancer cells from apoptosis [16]. In contrast, Reza et al. demonstrated that exosomes from adipose tissue-derived MSCs (ADMSCs) contain several miRNAs that induce the expression of the pro-apoptotic protein B-cell lymphoma 2 (Bcl-2) associated protein X (Bax), and caspase-9 in SKOV-3 and A2870 ovarian cancer cells, thereby impairing cell proliferation, wound repair, colony-forming capacity, and cell survival [17].
In the tumor microenvironment, interactions between the stroma and tumor cells are fundamental for tumor progression [18]. Exosomes play a key role in this communication, linking the diseased and healthy cells [19]. As mentioned above, exosomal crosstalk between adipose tissue and cancer cells may be a significant aspect of tumor progression by reprogramming the surrounding cells [20].
Invasion and metastasis are multistep processes that involve a multitude of successful biological changes in which cancer cells migrate to distant sites, triggering the formation of a premetastatic niche at secondary sites beyond the primary tumor [21]. The metastatic niche recruits immune cells and is enriched in molecular components that induce EMT in the cancer cells. Within the primary tumor, local invasion occurs first, followed by the intravasation of cancer cells into the blood and lymphatic vessels, and extravasation into the parenchyma of distant tissues, with the formation of micrometastasis, which grows into macroscopic tumors [11].
Lin et al. demonstrated that human ADMSC-derived exosomes increased the migration of the breast cancer MCF7 cells through the upregulation of the Wnt signaling pathway, and treatment of the cells with exosome-depleted original ADMSC-conditioned medium resulted in significantly decreased migratory capacity [22]. Wu et al. treated breast cancer MCF-7 cells with human ADMSC-derived exosomes and demonstrated that this microenvironment increased migration and invasion of the tumor cells and enhanced EMT by crosstalk between two signaling pathways: TGF-β/Smad and PI3K/AKT [23]. Furthermore, the uptake of exosomes secreted by CAA by melanoma cells allows the exchange of enzymes implicated in FAO and increases their migratory capacity [24].
Adipose tissue can trigger modifications in distant tumor cells by exchange of EVs through circulation ( Figure 2 ).
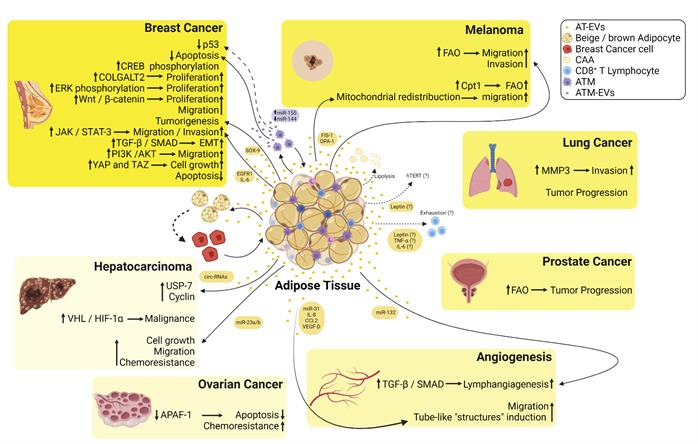
Figure 2. Summary of the crosstalk between the EVs derived from adipose tissues (AT-EVs) and several types of cancers. EVs released from adipose tissue macrophages are enriched in miR-155, whereas miR-144 is downregulated inducing inhibition of p53 and decreased apoptosis in breast cancer cells. AT-EVs increase phosphorylation of cAMP response element-binding (CREB) protein increasing the proliferation of breast cancer cells. AT-EVs increase the proliferation of breast cancer cells via induction of procollagen galactosyltransferase 2 (COLGALT2). AT-EVs increase extracellular signal-regulated kinase (ERK) and Wnt/β-catenin signaling pathways inducing an increase in the proliferation of breast cancer cells. AT-EVs enriched in SRY-box transcription factor 9 (SOX-9) induce tumorigenesis in breast cancer cells. AT-EVs from diabetic patients are enriched in epidermal growth factor receptor 1 (EGFR1)/interleukin 6 (IL-6) and induce an increase in migration and invasion of breast cancer cells via Janus kinase (JAK)/Signal transducer and activator of transcription 3 (STAT-3) signaling. AT-EVs increase TGF-β/SMAD signaling pathway triggering epithelial-mesenchymal transition (EMT) in non-metastatic breast cancer cells. AT-EVs induce PI3K/AKT signaling in MDA-MB-231 cells, thus increasing their migratory capacity. AT-EVs induce yes-associated protein 1 (YAP) and taffazin (TAZ) inducing cell growth in breast cancer cells. AT-EVs decrease apoptosis in breast cancer cells. AT-EVs enriched in mitochondrial fission protein 1 (FIS-1) and mitochondrial dynamin-like GTPase (OPA-1) induce mitochondrial redistribution toward the edge of melanoma cells increasing their migratory capacity. AT-EVs and EVs derived from cancer-associated adipocytes (CAA) fuel free fatty acids to melanoma cells and increase fatty acid oxidation (FAO) increasing their migratory and invasive capacities. AT-EVs increase metalloproteinase 3 (MMP-3) in lung cancer cells increasing their invasive. AT-EVs increase tumor progression of lung cancer. AT-EVs increase FAO and induce tumor progression in prostate cancer. AT-EVs enriched in circulating RNAs (circ-RNAs) induce hypophosphorylation of ubiquitin-specific-processing protease 7 (USP-7) and an increase in cyclin in hepatocarcinoma cells. AT-EVs induce malignancy of hepatocarcinoma cells via hypoxia-inducible factor 1 α (HIF-1α) binding to the von-Hippel Lindau tumor suppressor (VHL). AT-EVs decrease apoptosis and increase chemoresistance in ovarian cancer cells via inhibition of the apoptotic peptidase activator factor 1 (APAF-1). AT-EVs enriched in miR-31, interleukin 8 (IL-8), C-C motif chemokine ligand 2 (CCL2), and vascular endothelial growth factor-D (VEGF-D) induce the formation of tube-like structures by endothelial cells. AT-EVs enriched in miR-132 induce lymphangiogenesis via transforming growth factor β (TGF-β)/Smad signaling. CAA undergo increased lipolysis and differentiate toward beige/brown adipocytes phenotype fueling tumor cells with EVs. Leptin released from AT is possibly within EVs and induces an increase in telomerase reverse transcriptase (hTERT) activity inducing resistance to apoptosis and senescence. Leptin, TNF-α, and IL-6 may be released from AT-EVs inducing CD8+ T cells exhaustion.
3. Dual Roles of AT-EVs in Tumor Cells: miRNAs
MiRs are involved in physiological and pathological processes [25]. They are transported inside EVs that provide protection against degradation (different from when they are released directly into the body fluids or the circulation), and mediate intercellular communication with cells in distant organs, in a systemic manner [26][27].
However, how exosomes containing miRNAs affect cancer cells is not well established. Singh et al. showed that exosomes derived from MDA-MB-231 breast cells are internalized by HMLE breast epithelial cells, inducing invasive ability. These results suggest that non-invasive cells can internalize miR-enriched exosomes derived from tumor cells that then change their characteristics [28]. In another study, Li et al. observed that exosomes containing miR-1246 are shuttled from MDA-MB-231 cells to epithelial breast cells. Furthermore, these exosomes induce cell proliferation and migration in non-tumoral cells. These effects are associated with miR-1246, which targets CCNG2 expression [29].
MiR plays an important role in several types of cancers [30][31][32], and studies have shown that miR-EVs derived from hypoxic HepG2 cancer cells, which are enriched in miR-23, modulate the microenvironment to facilitate tumor progression by increasing angiogenesis, a hallmark of cancer. However, not only are tumor cells capable of releasing miR-EVs, but miR-EVs released by adipose tissue have been gaining prominence in cancer progression [33].
In contrast, miR-EVs can exert a dual role, and also exhibit antitumor action. Takahara demonstrated that miR-134, which was previously described as a tumor suppressor, is present in ATMSC-derived EVs and suppresses the growth of prostate cancer cells by inducing apoptosis via increased activity of B-cell lymphoma-extra large (Bcl-xL) [34]. Eexosomes derived from human adipose-derived mesenchymal stem cells are enriched in miRNAs with anti-cancer activities. These exosomes impair ovarian cancer cell proliferation by inducing apoptosis and blocking the cell cycle through the upregulation of different pro-apoptotic molecules [17]. Furthermore, it has been demonstrated that intra-tumor injection of ATMSC-Exos enriched in miR-122 enhanced the chemosensitivity of HCC cells to chemotherapeutic agents in vivo [35].
4. Perspectives on Immune Cells
Similar to macrophages, tumor-associated neutrophils (TANs) may exhibit two different phenotypes: cytotoxic (N1) and immunosuppressive (N2) [36][37][38]. N1 produces high amounts of TNF-α and ROS, and has an increased phagocytic capacity, resulting in a decrease in tumor growth [36]. In contrast, N2 presents a pro-tumor profile that contributes to tumor growth, angiogenesis, and metastasis [39][40]. Furthermore, the contribution of TANs to tumor development is dependent on the tumor stage [41].
Our group has shown that human adipose tissue releases microparticles derived from pre-adipocytes, adipocytes, neutrophils, and other leukocytes [5], which affects breast cancer cells [10], and could affect the tumor microenvironment.
Mahmoudi et al. showed that ADMSCs produce exosomes that are internalized by human neutrophils. These EVs decrease neutrophil apoptosis and increase phagocytic capacity [42]. In agreement, these data suggest that ADMSC-derived exosomes shift neutrophil polarization toward an N1-like profile, which may be a potentially interesting strategy for cancer treatment.
As mentioned previously, PD-L1, present in many cancer cells, binds to the PD-1 receptor on T cells, leading to suppression of antigen-derived activation and checkpoint response in these cells [43]. Patients with melanoma and head and neck cancers have exosome-enriched PD-L1 in plasma samples [44][45]. In addition to PD-L1-enriched exosomes being considered as a biomarker for cancer, we could consider the use of anti-PD-1 therapy [44]. In this context, Zhou et al. observed that human exosomes derived from AT-MSCs are rich in miR-424-5p when compared to exosomes derived from MSCs. The authors treated MDA-MB-231 breast cancer cells with exosomes derived from AT-MSCs enriched with miR-424-5p and observed downregulation of PD-L1 expression. Tumor cells that received these exosomes presented high levels of apoptosis when co-cultured with T cells. Finally, when miR-424-5p was delivered via exosomes intratumorally in mice, breast cancer grew slowly, and tumor growth was strongly suppressed [46]. Taking these data into consideration, treatment with exosomes derived from AT-MSCs enriched with miR-424-5p represents an interesting strategy to induce cytotoxic T cells in tumor cells.
References
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449.
- Renehan, A.G.; Zwahlen, M.; Egger, M. Adiposity and cancer risk: New mechanistic insights from epidemiology. Nat. Rev. Cancer 2015, 15, 484–498.
- Robado de Lope, L.; Alcibar, O.L.; Amor Lopez, A.; Hergueta-Redondo, M.; Peinado, H. Tumour-adipose tissue crosstalk: Fuelling tumour metastasis by extracellular vesicles. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373.
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016, 34, 4277–4283.
- Renovato-Martins, M.; Matheus, M.E.; De Andrade, I.R.; Moraes, J.A.; Da Silva, S.V.; Citelli Dos Reis, M.; De Souza, A.A.; Da Silva, C.C.; Bouskela, E.; Barja-Fidalgo, C. Microparticles derived from obese adipose tissue elicit a pro-inflammatory phenotype of CD16(+), CCR5(+) and TLR8(+) monocytes. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 139–151.
- Nieman, K.M.; Romero, I.L.; Van Houten, B.; Lengyel, E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim. Biophys. Acta 2013, 1831, 1533–1541.
- Guo, J.Y.; White, E. Autophagy is required for mitochondrial function, lipid metabolism, growth, and fate of KRAS(G12D)-driven lung tumors. Autophagy 2013, 9, 1636–1638.
- Rodriguez-Enriquez, S.; Hernandez-Esquivel, L.; Marin-Hernandez, A.; El Hafidi, M.; Gallardo-Perez, J.C.; Hernandez-Resendiz, I.; Rodriguez-Zavala, J.S.; Pacheco-Velazquez, S.C.; Moreno-Sanchez, R. Mitochondrial free fatty acid beta-oxidation supports oxidative phosphorylation and proliferation in cancer cells. Int. J. Biochem. Cell Biol. 2015, 65, 209–221.
- Cho, J.A.; Park, H.; Lim, E.H.; Lee, K.W. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int. J. Oncol. 2012, 40, 130–138.
- Ramos-Andrade, I.; Moraes, J.; Brandao-Costa, R.M.; Vargas da Silva, S.; de Souza, A.; da Silva, C.; Renovato-Martins, M.; Barja-Fidalgo, C. Obese adipose tissue extracellular vesicles raise breast cancer cell malignancy. Endocr. Relat. Cancer 2020, 27, 571–582.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Galindo-Hernandez, O.; Serna-Marquez, N.; Castillo-Sanchez, R.; Salazar, E.P. Extracellular vesicles from MDA-MB-231 breast cancer cells stimulated with linoleic acid promote an EMT-like process in MCF10A cells. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 299–310.
- Ozawa, P.M.M.; Alkhilaiwi, F.; Cavalli, I.J.; Malheiros, D.; de Souza Fonseca Ribeiro, E.M.; Cavalli, L.R. Extracellular vesicles from triple-negative breast cancer cells promote proliferation and drug resistance in non-tumorigenic breast cells. Breast Cancer Res. Treat. 2018, 172, 713–723.
- Shen, X.; Wang, C.; Zhu, H.; Wang, Y.; Wang, X.; Cheng, X.; Ge, W.; Lu, W. Exosome-mediated transfer of CD44 from high-metastatic ovarian cancer cells promotes migration and invasion of low-metastatic ovarian cancer cells. J. Ovarian Res. 2021, 14, 38.
- Sakha, S.; Muramatsu, T.; Ueda, K.; Inazawa, J. Exosomal microRNA miR-1246 induces cell motility and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Sci. Rep. 2016, 6, 38750.
- Wang, S.; Su, X.; Xu, M.; Xiao, X.; Li, X.; Li, H.; Keating, A.; Zhao, R.C. Exosomes secreted by mesenchymal stromal/stem cell-derived adipocytes promote breast cancer cell growth via activation of Hippo signaling pathway. Stem Cell Res. Ther. 2019, 10, 117.
- Reza, A.; Choi, Y.J.; Yasuda, H.; Kim, J.H. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci Rep. 2016, 6, 38498.
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 84.
- Rios-Colon, L.; Arthur, E.; Niture, S.; Qi, Q.; Moore, J.T.; Kumar, D. The Role of Exosomes in the Crosstalk between Adipocytes and Liver Cancer Cells. Cells 2020, 9, 1988.
- Quan, M.; Kuang, S. Exosomal Secretion of Adipose Tissue during Various Physiological States. Pharm. Res. 2020, 37, 221.
- Liu, Y.; Cao, X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell 2016, 30, 668–681.
- Lin, R.; Wang, S.; Zhao, R.C. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol. Cell Biochem. 2013, 383, 13–20.
- Wu, S.; Wang, Y.; Yuan, Z.; Wang, S.; Du, H.; Liu, X.; Wang, Q.; Zhu, X. Human adiposederived mesenchymal stem cells promote breast cancer MCF7 cell epithelialmesenchymal transition by cross interacting with the TGFbeta/Smad and PI3K/AKT signaling pathways. Mol. Med. Rep. 2019, 19, 177–186.
- Lazar, I.; Clement, E.; Dauvillier, S.; Milhas, D.; Ducoux-Petit, M.; LeGonidec, S.; Moro, C.; Soldan, V.; Dalle, S.; Balor, S.; et al. Adipocyte Exosomes Promote Melanoma Aggressiveness through Fatty Acid Oxidation: A Novel Mechanism Linking Obesity and Cancer. Cancer Res. 2016, 76, 4051–4057.
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandao, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673.
- Chen, X.; Liang, H.; Zhang, J.; Zen, K.; Zhang, C.Y. Secreted microRNAs: A new form of intercellular communication. Trends Cell Biol. 2012, 22, 125–132.
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20.
- Singh, R.; Pochampally, R.; Watabe, K.; Lu, Z.; Mo, Y.Y. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol. Cancer 2014, 13, 256.
- Li, X.J.; Ren, Z.J.; Tang, J.H.; Yu, Q. Exosomal MicroRNA MiR-1246 Promotes Cell Proliferation, Invasion and Drug Resistance by Targeting CCNG2 in Breast Cancer. Cell. Physiol. Biochem. 2017, 44, 1741–1748.
- Garzon, R.; Calin, G.A.; Croce, C.M. MicroRNAs in Cancer. Annu. Rev. Med. 2009, 60, 167–179.
- Kogure, A.; Kosaka, N.; Ochiya, T. Cross-talk between cancer cells and their neighbors via miRNA in extracellular vesicles: An emerging player in cancer metastasis. J. Biomed. Sci. 2019, 26, 7.
- Vu, L.T.; Gong, J.; Pham, T.T.; Kim, Y.; Le, M.T.N. microRNA exchange via extracellular vesicles in cancer. Cell Prolif. 2020, 53, e12877.
- Zhou, Y.; Tan, C. miRNAs in Adipocyte-Derived Extracellular Vesicles: Multiple Roles in Development of Obesity-Associated Disease. Front. Mol. Biosci. 2020, 7, 171.
- Wang, S.; Bian, C.; Yang, Z.; Bo, Y.; Li, J.; Zeng, L.; Zhou, H.; Zhao, R.C. miR-145 inhibits breast cancer cell growth through RTKN. Int. J. Oncol. 2009, 34, 1461–1466.
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015, 8, 122.
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194.
- Piccard, H.; Muschel, R.J.; Opdenakker, G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit. Rev. Oncol. Hematol. 2012, 82, 296–309.
- Guimaraes-Bastos, D.; Frony, A.C.; Barja-Fidalgo, C.; Moraes, J.A. Melanoma-derived extracellular vesicles skew neutrophils into a pro-tumor phenotype. J. Leukoc. Biol. 2021.
- Jablonska, J.; Leschner, S.; Westphal, K.; Lienenklaus, S.; Weiss, S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J. Clin. Investig. 2010, 120, 1151–1164.
- Gong, L.; Cumpian, A.M.; Caetano, M.S.; Ochoa, C.E.; De la Garza, M.M.; Lapid, D.J.; Mirabolfathinejad, S.G.; Dickey, B.F.; Zhou, Q.; Moghaddam, S.J. Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Mol. Cancer 2013, 12, 154.
- Mishalian, I.; Bayuh, R.; Levy, L.; Zolotarov, L.; Michaeli, J.; Fridlender, Z.G. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol. Immunother. 2013, 62, 1745–1756.
- Mahmoudi, M.; Taghavi-Farahabadi, M.; Rezaei, N.; Hashemi, S.M. Comparison of the effects of adipose tissue mesenchymal stromal cell-derived exosomes with conditioned media on neutrophil function and apoptosis. Int. Immunopharmacol. 2019, 74, 105689.
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264.
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386.
- Theodoraki, M.N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical Significance of PD-L1(+) Exosomes in Plasma of Head and Neck Cancer Patients. Clin. Cancer Res. 2018, 24, 896–905.
- Zhou, Y.; Yamamoto, Y.; Takeshita, F.; Yamamoto, T.; Xiao, Z.; Ochiya, T. Delivery of miR-424-5p via Extracellular Vesicles Promotes the Apoptosis of MDA-MB-231 TNBC Cells in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 844.




