
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Youngok Son | + 1312 word(s) | 1312 | 2021-08-01 10:20:48 | | | |
| 2 | Vicky Zhou | Meta information modification | 1312 | 2021-08-11 03:29:04 | | |
Video Upload Options
Various SARS-CoV-2-based vaccines are in the final developmental stages and have been evaluated in a variety of in vivo models. However, despite positive outcomes in animal models, only a handful of these vaccines have made it to humans. With access to several coronavirus genome sequences, bioinformatics methods can now be adopted to investigate the developmental origins of new strains as well as the mechanism of viral entry and pathogenesis in the host.
1. Introduction
Pandemics of crippling viral infections such as influenza, smallpox, Middle East respiratory syndrome-related coronavirus (MERS), severe acute respiratory coronavirus 1 syndrome (SARS-CoV-1), Ebola, and Zika have occurred many times in human history [1]. The extreme acute respiratory coronavirus 2 syndrome ( SARS-CoV-2) recently emerged, and the World Health Organization (WHO) formally proclaimed it a pandemic on 11 March 2020. Since the first incidence of the disease was observed in the Chinese city of Wuhan, the entire world has been fighting to keep this outbreak under control. Promptly afterward, rising infection figures significantly inflated, resulting in a high number of fatalities in every corner of the globe.
The research on viruses has always been a fascinating topic, especially because of their unusual dual nature as a living and non-living entity. When a virus is in a live and receptive host, it reproduces its kind, but when it is in a non-living environment, it persists as an inanimate particle [2]. As a virulent pathogen, it exhibits a wide range of symptoms, rendering some viral infections mild and self-limiting, requiring only symptomatic care, whereas other forms, such as human immunodeficiency virus (HIV) infection, necessitate long-term medication [3]. The scientific community worldwide is working hard to develop a strategy to fight against these infectious viral diseases. In such a prevailing scenario, the design and delivery of a wide variety of innovative anti-SARS-CoV-2 therapeutics agents are desperately needed, not only to tackle COVID-19 disease but also to suppress a broad spectrum of previously resistant infectious virions and their mutants to save the human population from numerous life-threatening outbreaks. In addition, to successfully handle the COVID-19 pandemic it is important to develop safe and effective vaccines. From concept to clinical trial in humans, the main concepts and difficulties faced in the production of vaccines targeting a diverse range of pathogens need to be elucidated and tackled. In such a context a deep knowledge of the functioning of the immune system and host-pathogen interactions must be acquired for the efficient design of a novel vaccine candidate [4]. Vaccine production necessitates the identification of immunogenic epitopes followed by various phases of clinical trials to ensure the vaccine’s safety and efficacy profile.
2. Vaccines for COVID-19: Efficacy and Prospective
To avoid the substantial threat of a pandemic, investigators have been working around the clock to expedite the production and manufacture of a much-needed vaccine against COVID-19, and several of the emerging vaccine candidates have used the SARS-CoV-2 S protein as the main target [5]. Vaccine production necessitates the identification of an immunogenic epitope and the formulation of a complete vaccine after various trials have been completed to ensure the vaccine’s safety and efficacy profile. Although SARS-CoV-2 has a different genome organization, the research on SARS and MERS can help scientists to have a better understanding of the infection and immune response in the human body. Before initiating the mass vaccination stage and development process, the volunteer clinical trial is subjected to a code of conduct in terms of safety regulation of the vaccines. This clinical trial plan and approval for general use are strictly regulated by a governing body such as the Food and Drug Administration (FDA) and European Medicines Agency (EMA) [6]. The doses and schedule for the vaccine are calculated during restricted human studies and tested in phase III studies. Therefore, producing the vaccine against SARS-CoV-2 has the target time of only 12–18 months, while, historically, vaccines have taken 15–20 years to develop ( Figure 1 ).
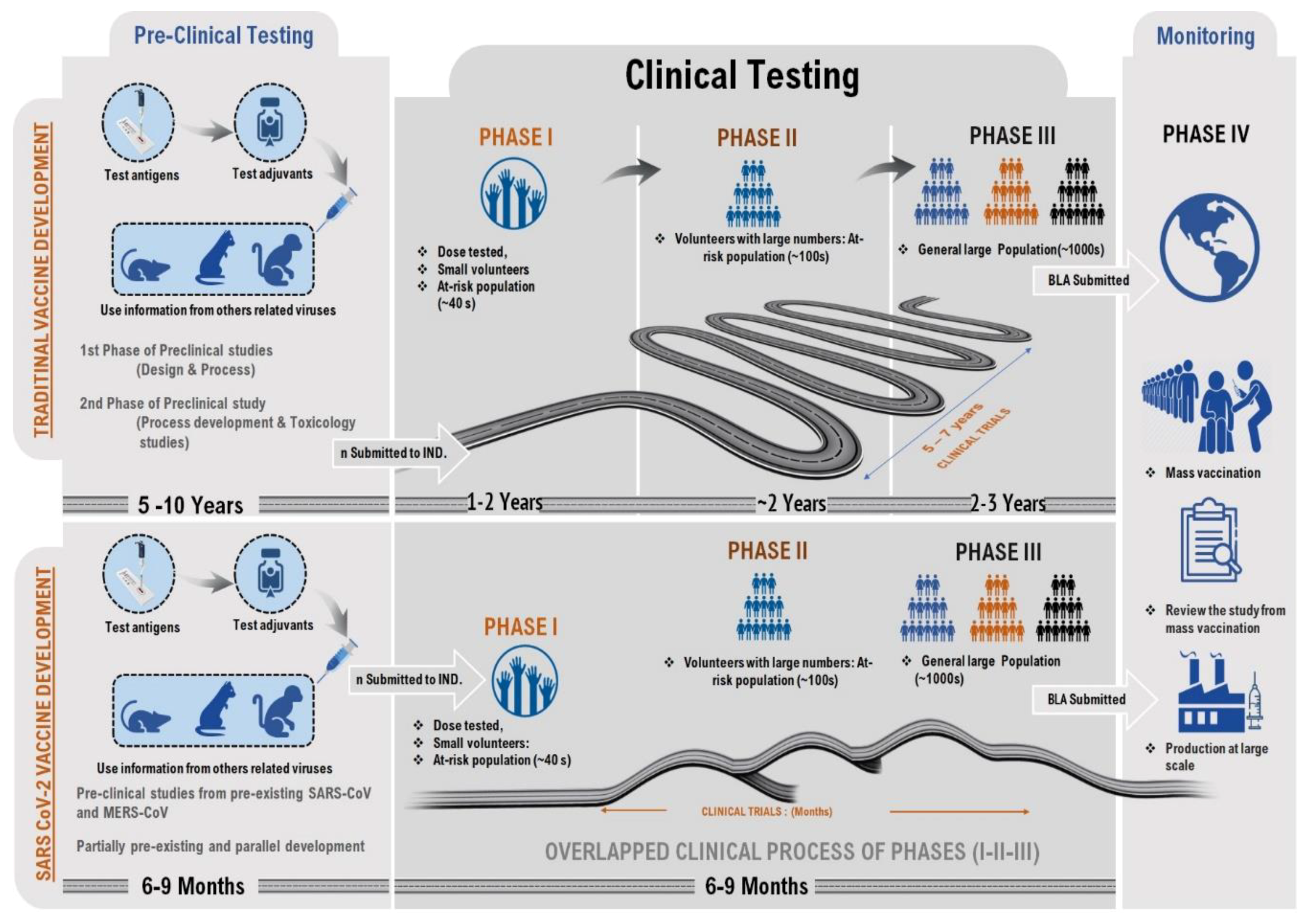 Figure 1. The comparative illustration of traditional vaccine production and CoV vaccine development. The reasons for focusing on each of these technologies for COVID-19 include their rapid expansion, scale, and manufacturing fit, and small dose size, which are all highly desirable features for a rapid pandemic response.
Figure 1. The comparative illustration of traditional vaccine production and CoV vaccine development. The reasons for focusing on each of these technologies for COVID-19 include their rapid expansion, scale, and manufacturing fit, and small dose size, which are all highly desirable features for a rapid pandemic response.
The COVID-19 vaccine candidates in development are focused on live attenuated viruses, DNA, RNA, nanoparticles, replicating and non-replicating viral vectors, immunogenic adjuvants, protein subunits, with each demonstrating its main advantages and risks before being released into the existing international market [7][8][9][10] ( Figure 2 ). The new genetic method of the mRNA vaccine against SARS-CoV-2 is based on messenger ribonucleic acid (mRNA) fragments, the genetic material that is copied from DNA and encodes proteins. It can stimulate the production of antibodies, which can neutralize the virus in laboratory samples. Another type of DNA vaccine is obtained by recombinant DNA technology encoding with the target molecule. Modified ChAdOx1, nCoV-19, and INO-4800 are some of the new DNA vaccines against coronavirus that are entering human phase I testing.
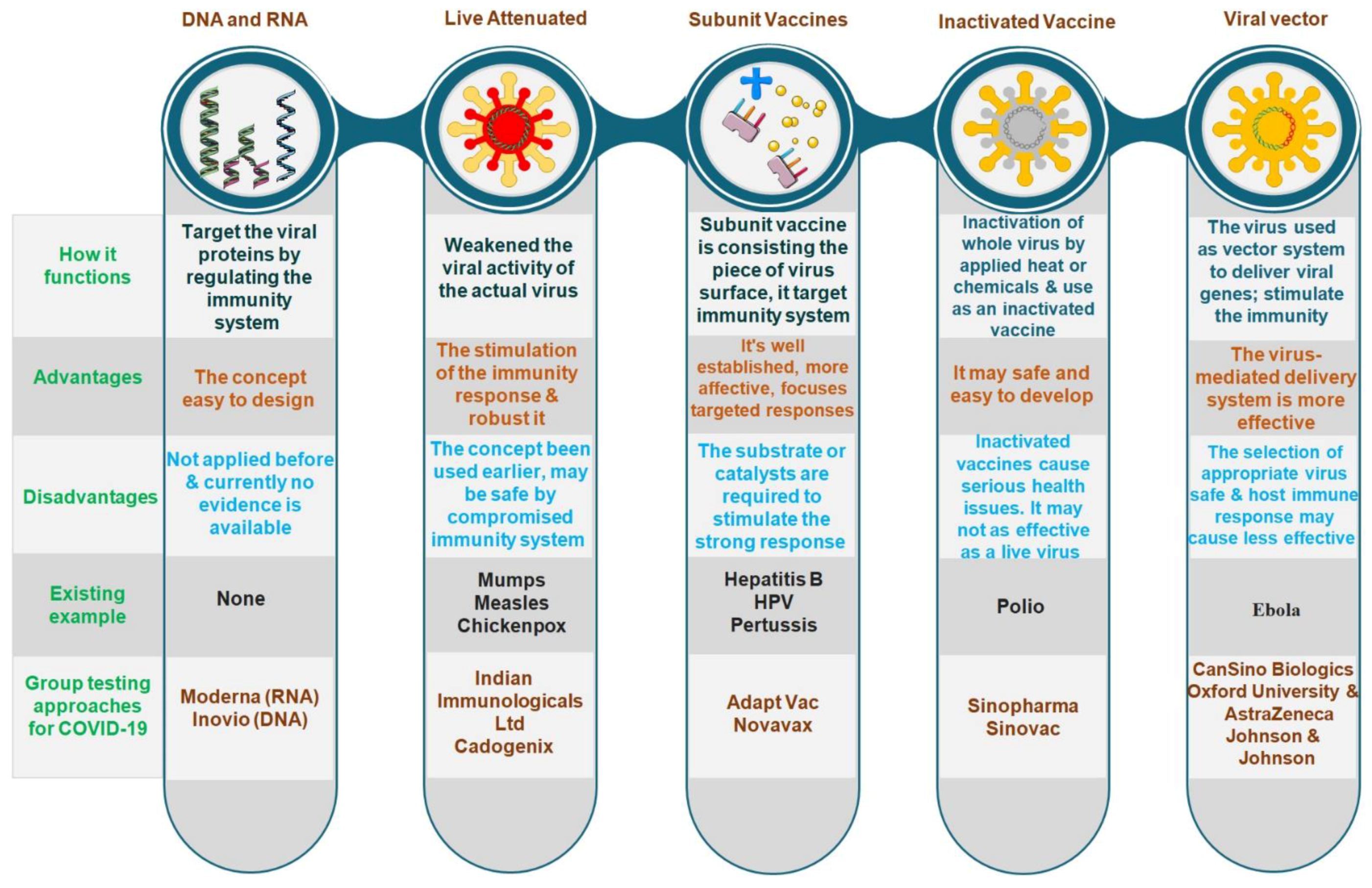 Figure 2. Types of vaccine development approaches and platforms. They differ in whether they use the entire virus or bacterium, only the germ parts that activate the immune system or only the genetic material that provides instructions for making specific proteins rather than the entire virus.
Figure 2. Types of vaccine development approaches and platforms. They differ in whether they use the entire virus or bacterium, only the germ parts that activate the immune system or only the genetic material that provides instructions for making specific proteins rather than the entire virus.
The subunit vaccines concept is based on certain antigenic determinants and increases the efficiency of the immune response against pathogenic microorganisms. In addition, the development of a vaccine based on the surface proteins that form virus-like particles (VLPs) is a more innovative approach. The vaccine candidates that are already commercialized are presented in Table 1. Vaccine developers’ products such as Moderna’s mRNA1273 and Pfizer’s BNT162b2 (mRNA-based), Sino Biotech’s CoronaVac, Sinovac’s SARS-CoV-2 vaccine (inactivated virus), Johnson & Johnson’s JNJ-78436735, ChAdOx1 nCoV-19, Sputnik V (adenovirus-based), CanSino’s Ad5-nCoV (viral vector), Inovio’s INO4800 (DNA plasmid vaccine), considered to be the front-runners, are currently gearing up to conduct phase trials and manufacture up to a billion doses.
Table 1. List of approved/authorized vaccines.
| Name of the Vaccine Candidate | Name of Developers | Platform Utilized | Country of Origin |
|---|---|---|---|
| Moderna COVID-19 Vaccine (mRNA-1273) | Moderna, BARDA, NIAID | mRNA-based vaccine | USA |
| Covaxin | Bharat Biotech, ICMR | Inactivated vaccine | India |
| Comirnaty (BNT162b2) | Pfizer, BioNTech; Fosun Pharma | mRNA-based vaccine | Multinational |
| Covishield | Oxford University and AstraZeneca | Adenovirus vaccine | UK |
| Sputnik V | Gamaleya Research Institute, Acellena Contract Drug Research, and Development | Recombinant adenovirus vaccine (rAd26 and rAd5) | Russia |
| CoronaVac | Sinovac | Inactivated vaccine (formalin with alum adjuvant) | China |
| COVID-19 Vaccine Janssen (JNJ-78436735; Ad26.COV2.S) | Janssen Vaccines (Johnson & Johnson) | Non-replicating viral vector | The Netherlands, USA |
| EpiVacCorona | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology | Peptide vaccine | Russia |
| BBIBP-CorV | Beijing Institute of Biological Products; China National Pharmaceutical Group (Sinopharm) | Inactivated vaccine | China |
3. Conclusions
References
- Ahn, D.G.; Shin, H.J.; Kim, M.H.; Lee, S.; Kim, H.S.; Myoung, J.; Kim, B.T.; Kim, S.J. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19). J. Microbiol. Biotechnol. 2020, 30, 313–324.
- Lim, Y.X.; Ng, Y.L.; Tam, J.P.; Liu, D.X. Human coronaviruses: A review of virus–host interactions. Diseases 2016, 4, 26.
- Rouse, B.T.; Sehrawat, S. Immunity and immunopathology to viruses: What decides the outcome? Nat. Rev. Immunol. 2010, 10, 514–526.
- O’Hagan, D.T.; Valiante, N.M. Recent advances in the discovery and delivery of vaccine adjuvants. Nat. Rev. Drug. Discov. 2003, 2, 727–735.
- Dhama, K.; Sharun, K.; Tiwari, R.; Dadar, M.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W. COVID-19, an emerging coronavirus infection: Advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccines Immunother. 2020, 16, 1232–1238.
- Hwang, T.J.; Ross, J.S.; Vokinger, K.N.; Kesselheim, A.S. Association between FDA and EMA expedited approval programs and therapeutic value of new medicines: Retrospective cohort study. BMJ 2020, 371, M3434.
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020, 288, 198114.
- Rawat, K.; Kumari, P.; Saha, L. COVID-19 vaccine: A recent update in pipeline vaccines, their design and development strategies. Eur. J. Pharmacol. 2021, 892, 173751.
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527.
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632.




