
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jiaqi Shi | + 1565 word(s) | 1565 | 2021-08-10 08:15:30 | | | |
| 2 | Lindsay Dong | -1 word(s) | 1564 | 2021-08-12 04:48:55 | | |
Video Upload Options
Pancreatic ductal adenocarcinoma (PDAC) is a malignancy with a poor prognosis and low survival rates. PDAC is characterized by a fibroinflammatory tumor microenvironment enriched by abundant fibroblasts and a variety of immune cells, contributing to its aggressiveness. Neutrophils are essential infiltrating immune cells in the PDAC microenvironment. Recent studies have identified several cellular mechanisms by which neutrophils are recruited to tumor lesions and promote tumorigenesis.
1. Introduction
Neutrophils are the most abundant immune cells in circulation and form an essential part of the innate immune system to respond against infection and inflammatory insults. Recent studies discovered an early homogeneous neutrophil progenitor subset in human bone marrow defined by surface markers CD71 and CD117 [1]. However, the role of neutrophils in tumor development is still unclear. Some studies have proposed classifying tumor-associated neutrophils (TANs) into two polarization states, tumor-suppressing N1 neutrophils and tumor-promoting N2 neutrophils [2]. On the other hand, exposure to TGF-β transforms neutrophils to the N2 phenotype [2]. N2 neutrophils have been reported to have strong immunosuppressive and tumor-promoting functions, including the promotion of tumor metastases and angiogenesis [3][4].
One way the tumor evades the host immune system is through a group of heterogeneous immature myeloid cells, called myeloid-derived suppressor cells (MDSCs). MDSCs suppress immune responses by multiple signaling pathways. MDSCs can induce antigen-specific CD8+ T cell tolerance via generating reactive oxygen species (ROS) and peroxynitrite [5]. In addition, MDSCs suppress natural killer (NK) cell cytotoxicity by inhibiting the activation of Stat5 [6]. Markers for MDSCs include CD33 and CD11b in humans and Gr-1 and Cd11b in mice [7]. Based on phenotypic features, MDSCs can be characterized into two major types, monocytic MDSCs (M-MDSCs) and granulocytic MDSCs (G-MDSCs) [8]. Mouse M-MDSCs are Ly6G−Ly6 Chigh, and G-MDSCs are Ly6G+ Ly6 Clow [9]. In humans, CD66b and CD15 are markers for M-MDSCs and G-MDSCs [9]. However, it is believed that the MDSCs described in most studies are, in fact, a subset of neutrophils [10][11] due to a significant overlap in the expression of functional molecules or surface molecules between neutrophils and G-MDSC [12].
2. Interactions between Neutrophil and Tumor
2.1. Tumor Cells Attract Neutrophils
Neutrophils can be recruited to the PDAC microenvironment via multiple tumor-secreted factors (Figure 1).
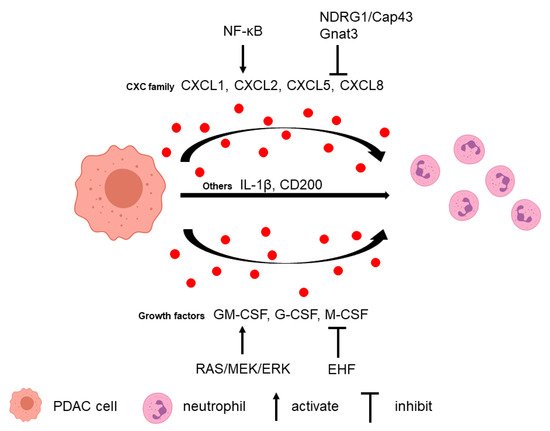
2.2. Neutrophils Promote Tumor Cell Survival and Metastasis
Several studies in PDAC have found neutrophil depletion could inhibit tumor growth and metastasis. Depleting G-MDSC in the KPC mouse model using anti-Ly6G antibody, 1A8, resulted in an increase in cleaved caspase-3 (CC3)-positive tumor cells, which suggested that depletion of G-MDSC increased tumor epithelial apoptosis [16].
3. Interactions between Neutrophil and TME
3.1. T Cell
In addition to tumor cells, T cells in the TME contribute to the recruitment of neutrophils (Figure 2).
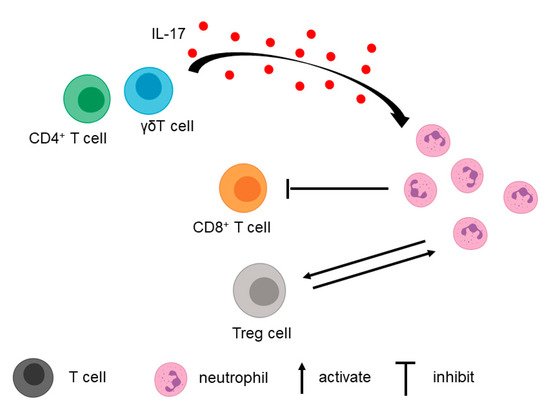
3.2. Fibroblasts, Macrophages, and Extracellular Matrix
4. Neutrophil Extracellular Trap in PDAC
The formation of neutrophil extracellular trap (NET) is a cellular function of neutrophils. Release of NET, called NETosis, is considered a defense mechanism to trap and kill bacteria and other pathogens.
Many studies have demonstrated that pancreatic cancer cells induce NET formation. For example, the conditioned media from PDAC cell line AsPC-1 was able to induce NET formation, and NETs could promote AsPC-1 cell migration and invasion as well as angiogenesis in vitro [25]. Another group found that conditioned media from KPC cells previously exposed to IL-17 induced NETosis in mouse neutrophils in vitro [26]. Moreover, serum from PDAC patients significantly increased NET formation and reduced NET degradation [26]. Tumor-derived protein tissue inhibitor of metalloproteinases-1 (TIMP-1), which correlated with poor prognosis in PDAC, directly triggered the formation of NETs. This effect depended on the interaction of TIMP-1 with its receptor CD63 and subsequent ERK signaling [27]. In addition to tumor cells, CAF-conditioned media can also induce ROS-dependent NETosis by secreting Amyloid β A4 protein. In turn, NET formation promotes CAF expansion, contractility, and deposition of matrix components supportive of tumor growth.
Recent studies suggested that NETs contribute to PDAC development. Electron microscope images of co-cultured pancreatic cancer cells with neutrophils showed that NETs could capture pancreatic cancer cells by their spider web-like structures [28].
5. Clinical Implications of Neutrophil and NET in PDAC
5.1. Prognosis in PDAC
The neutrophil-to-lymphocyte ratio (NLR) offers critical prognostic information in PDAC as well as breast cancer, lung cancer, and other types of cancer [29][30]. Studies have demonstrated that increased NLR correlates with a poor prognosis in patients with resectable and unresectable pancreatic cancer [22][31][32][33].
Intratumoral neutrophils are also correlated with PDAC patient outcomes. CD177, a marker for neutrophils, is negatively correlated with the overall survival of PDAC patients [34].
A recent study showed that plasma NET levels using SYTOX-positive areas could also predict the survival of PDAC patients [27].
5.2. Neutrophil as a Potential Therapeutic Target in PDAC
The existing treatment of PDAC patients includes surgical resection, chemoradiation therapy, and immunotherapy, but only a small proportion of patients benefit from available therapies. Based on an increasing understanding of the role of TME in PDAC, neutrophils have emerged as a potential therapeutic target.
The majority of neutrophil targeting preclinical studies use CXCR2 inhibitors or Ly6G antibodies [22]. This approach is in line with the main classes of current and past drug targets being receptors, among others [35]. In addition, the preference of CXCR2 as a target might lie in the fact that CXCR2 blockage not only inhibits the CXCL5/CXCR2 axis but also blocks the effect of other ligands of CXCR2, including CXCL1–3 and CXCL6–8. In breast cancer, CXCR2 inhibitors were demonstrated to be safe and tolerable, and relevant clinical trials are currently underway (NCT02370238) [36]. However, ligands, including cytokines, have become more important drug targets in the past two decades, largely due to the increased antibody-based therapies. Although no study was found in PDAC, CXCL5 inhibition emerged as a new strategy to attenuate tumor angiogenesis and thus slow tumor progression [37].
6. Conclusions
References
- Dinh, H.Q.; Eggert, T.; Meyer, M.A.; Zhu, Y.P.; Olingy, C.E.; Llewellyn, R.; Wu, R.; Hedrick, C.C. Coexpression of CD71 and CD117 Identifies an Early Unipotent Neutrophil Progenitor Population in Human Bone Marrow. Immunity 2020, 53, 319–334.
- Fridlender, Z.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.; Albelda, S. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194.
- Shojaei, F.; Wu, X.; Zhong, C.; Yu, L.; Liang, X.; Yao, J.; Blanchard, D.; Bais, C.; Peale, F.; van Bruggen, N.; et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 2007, 450, 825–831.
- Schmielau, J.; Finn, O. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001, 61, 4756–4760.
- Nagaraj, S.; Gupta, K.; Pisarev, V.; Kinarsky, L.; Sherman, S.; Kang, L.; Herber, D.L.; Schneck, J.; Gabrilovich, D.I. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 2007, 13, 828–835.
- Liu, C.; Yu, S.; Kappes, J.; Wang, J.; Grizzle, W.E.; Zinn, K.R.; Zhang, H.G. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood 2007, 109, 4336–4342.
- Marvel, D.; Gabrilovich, D. Myeloid-derived suppressor cells in the tumor microenvironment: Expect the unexpected. J. Clin. Investig. 2015, 125, 3356–3364.
- Youn, J.I.; Nagaraj, S.; Collazo, M.; Gabrilovich, D.I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 2008, 181, 5791–5802.
- Youn, J.I.; Gabrilovich, D.I. The biology of myeloid-derived suppressor cells: The blessing and the curse of morphological and functional heterogeneity. Eur. J. Immunol. 2010, 40, 2969–2975.
- Coffelt, S.; Wellenstein, M.; de Visser, K. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446.
- Shaul, M.E.; Fridlender, Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620.
- Fridlender, Z.G.; Sun, J.; Mishalian, I.; Singhal, S.; Cheng, G.; Kapoor, V.; Horng, W.; Fridlender, G.; Bayuh, R.; Worthen, G.S.; et al. Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PLoS ONE 2012, 7, e31524.
- Hoffman, M.T.; Kemp, S.B.; Salas-Escabillas, D.J.; Zhang, Y.; Steele, N.G.; The, S.; Long, D.; Benitz, S.; Yan, W.; Margolskee, R.F.; et al. The Gustatory Sensory G-Protein GNAT3 Suppresses Pancreatic Cancer Progression in Mice. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 349–369.
- Iida-Norita, R.; Kawamura, M.; Suzuki, Y.; Hamada, S.; Masamune, A.; Furukawa, T.; Sato, Y. Vasohibin-2 plays an essential role in metastasis of pancreatic ductal adenocarcinoma. Cancer Sci. 2019, 110, 2296–2308.
- Chao, T.; Furth, E.; Vonderheide, R. CXCR2-Dependent Accumulation of Tumor-Associated Neutrophils Regulates T-cell Immunity in Pancreatic Ductal Adenocarcinoma. Cancer Immunol. Res. 2016, 4, 968–982.
- Stromnes, I.; Brockenbrough, J.; Izeradjene, K.; Carlson, M.; Cuevas, C.; Simmons, R.; Greenberg, P.; Hingorani, S. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut 2014, 63, 1769–1781.
- Das, S.; Shapiro, B.; Vucic, E.A.; Vogt, S.; Bar-Sagi, D. Tumor Cell-Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res. 2020, 80, 1088–1101.
- Li, C.; Cui, L.; Yang, L.; Wang, B.; Zhuo, Y.; Zhang, L.; Wang, X.; Zhang, Q.; Zhang, S. Pancreatic Stellate Cells Promote Tumor Progression by Promoting an Immunosuppressive Microenvironment in Murine Models of Pancreatic Cancer. Pancreas 2020, 49, 120–127.
- Miller-Ocuin, J.; Liang, X.; Boone, B.; Doerfler, W.; Singhi, A.; Tang, D.; Kang, R.; Lotze, M.; Zeh, H. DNA released from neutrophil extracellular traps (NETs) activates pancreatic stellate cells and enhances pancreatic tumor growth. Oncoimmunology 2019, 8, e1605822.
- Mayer, P.; Dinkic, C.; Jesenofsky, R.; Klauss, M.; Schirmacher, P.; Dapunt, U.; Hackert, T.; Uhle, F.; Hänsch, G.M.; Gaida, M.M. Changes in the microarchitecture of the pancreatic cancer stroma are linked to neutrophil-dependent reprogramming of stellate cells and reflected by diffusion-weighted magnetic resonance imaging. Theranostics 2018, 8, 13–30.
- Hu, H.; Hang, J.J.; Han, T.; Zhuo, M.; Jiao, F.; Wang, L.W. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016, 37, 8657–8664.
- Nywening, T.M.; Belt, B.A.; Cullinan, D.R.; Panni, R.Z.; Han, B.J.; Sanford, D.E.; Jacobs, R.C.; Ye, J.; Patel, A.A.; Gillanders, W.E.; et al. Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 2018, 67, 1112–1123.
- Sinha, P.; Clements, V.K.; Bunt, S.K.; Albelda, S.M.; Ostrand-Rosenberg, S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 2007, 179, 977–983.
- Bausch, D.; Pausch, T.; Krauss, T.; Hopt, U.T.; Fernandez-del-Castillo, C.; Warshaw, A.L.; Thayer, S.P.; Keck, T. Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis 2011, 14, 235–243.
- Jung, H.S.; Gu, J.; Kim, J.E.; Nam, Y.; Song, J.W.; Kim, H.K. Cancer cell-induced neutrophil extracellular traps promote both hypercoagulability and cancer progression. PLoS ONE 2019, 14, e0216055.
- Zhang, Y.; Chandra, V.; Riquelme Sanchez, E.; Dutta, P.; Quesada, P.; Rakoski, A.; Zoltan, M.; Arora, N.; Baydogan, S.; Horne, W.; et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. 2020, 217, e20190354.
- Schoeps, B.; Eckfeld, C.; Prokopchuk, O.; Böttcher, J.; Häußler, D.; Steiger, K.; Demir, I.; Knolle, P.; Soehnlein, O.; Jenne, D.; et al. TIMP-1 triggers neutrophil extracellular trap formation in pancreatic cancer. Cancer Res. 2021.
- Kajioka, H.; Kagawa, S.; Ito, A.; Yoshimoto, M.; Sakamoto, S.; Kikuchi, S.; Kuroda, S.; Yoshida, R.; Umeda, Y.; Noma, K.; et al. Targeting neutrophil extracellular traps with thrombomodulin prevents pancreatic cancer metastasis. Cancer Lett. 2021, 497, 1–13.
- Chen, C.; Yang, H.; Cai, D.; Xiang, L.; Fang, W.; Wang, R. Preoperative peripheral blood neutrophil-to-lymphocyte ratios (NLR) and platelet-to-lymphocyte ratio (PLR) related nomograms predict the survival of patients with limited-stage small-cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 866–877.
- Orditura, M.; Galizia, G.; Diana, A.; Saccone, C.; Cobellis, L.; Ventriglia, J.; Iovino, F.; Romano, C.; Morgillo, F.; Mosca, L.; et al. Neutrophil to lymphocyte ratio (NLR) for prediction of distant metastasis-free survival (DMFS) in early breast cancer: A propensity score-matched analysis. ESMO Open 2016, 1, e000038.
- Garcea, G.; Ladwa, N.; Neal, C.P.; Metcalfe, M.S.; Dennison, A.R.; Berry, D.P. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J. Surg. 2011, 35, 868–872.
- Stotz, M.; Gerger, A.; Eisner, F.; Szkandera, J.; Loibner, H.; Ress, A.L.; Kornprat, P.; AlZoughbi, W.; Seggewies, F.S.; Lackner, C.; et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br. J. Cancer 2013, 109, 416–421.
- Suzuki, R.; Takagi, T.; Hikichi, T.; Konno, N.; Sugimoto, M.; Watanabe, K.O.; Nakamura, J.; Waragai, Y.; Kikuchi, H.; Takasumi, M.; et al. Derived neutrophil/lymphocyte ratio predicts gemcitabine therapy outcome in unresectable pancreatic cancer. Oncol. Lett. 2016, 11, 3441–3445.
- Wang, Y.; Fang, T.; Huang, L.; Wang, H.; Zhang, L.; Wang, Z.; Cui, Y. Neutrophils infiltrating pancreatic ductal adenocarcinoma indicate higher malignancy and worse prognosis. Biochem. Biophys. Res. Commun. 2018, 501, 313–319.
- Attwood, M.M.; Jonsson, J.; Rask-Andersen, M.; Schiöth, H.B. Soluble ligands as drug targets. Nat. Rev. Drug Discov. 2020, 19, 695–710.
- Schott, A.F.; Goldstein, L.J.; Cristofanilli, M.; Ruffini, P.A.; McCanna, S.; Reuben, J.M.; Perez, R.P.; Kato, G.; Wicha, M. Phase Ib Pilot Study to Evaluate Reparixin in Combination with Weekly Paclitaxel in Patients with HER-2-Negative Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5358–5365.
- Zhang, W.; Wang, H.; Sun, M.; Deng, X.; Wu, X.; Ma, Y.; Li, M.; Shuoa, S.M.; You, Q.; Miao, L. CXCL5/CXCR2 axis in tumor microenvironment as potential diagnostic biomarker and therapeutic target. Cancer Commun. 2020, 40, 69–80.




