Pancreatic ductal adenocarcinoma (PDAC) is a malignancy with a poor prognosis and low survival rates. PDAC is characterized by a fibroinflammatory tumor microenvironment enriched by abundant fibroblasts and a variety of immune cells, contributing to its aggressiveness. Neutrophils are essential infiltrating immune cells in the PDAC microenvironment. Recent studies have identified several cellular mechanisms by which neutrophils are recruited to tumor lesions and promote tumorigenesis.
- pancreatic ductal adenocarcinoma
- tumor microenvironment
- immune cells
- neutrophil extracellular traps
1. Introduction
Neutrophils are the most abundant immune cells in circulation and form an essential part of the innate immune system to respond against infection and inflammatory insults. Recent studies discovered an early homogeneous neutrophil progenitor subset in human bone marrow defined by surface markers CD71 and CD117 [4]. However, the role of neutrophils in tumor development is still unclear. Some studies have proposed classifying tumor-associated neutrophils (TANs) into two polarization states, tumor-suppressing N1 neutrophils and tumor-promoting N2 neutrophils [14]. On the other hand, exposure to TGF-β transforms neutrophils to the N2 phenotype [14]. N2 neutrophils have been reported to have strong immunosuppressive and tumor-promoting functions, including the promotion of tumor metastases and angiogenesis [17,18].
One way the tumor evades the host immune system is through a group of heterogeneous immature myeloid cells, called myeloid-derived suppressor cells (MDSCs). MDSCs suppress immune responses by multiple signaling pathways. MDSCs can induce antigen-specific CD8+ T cell tolerance via generating reactive oxygen species (ROS) and peroxynitrite [21]. In addition, MDSCs suppress natural killer (NK) cell cytotoxicity by inhibiting the activation of Stat5 [22]. Markers for MDSCs include CD33 and CD11b in humans and Gr-1 and Cd11b in mice [23]. Based on phenotypic features, MDSCs can be characterized into two major types, monocytic MDSCs (M-MDSCs) and granulocytic MDSCs (G-MDSCs) [24]. Mouse M-MDSCs are Ly6G−Ly6 Chigh, and G-MDSCs are Ly6G+ Ly6 Clow [25]. In humans, CD66b and CD15 are markers for M-MDSCs and G-MDSCs [25]. However, it is believed that the MDSCs described in most studies are, in fact, a subset of neutrophils [26,27] due to a significant overlap in the expression of functional molecules or surface molecules between neutrophils and G-MDSC [28].
2. Interactions between Neutrophil and Tumor
2.1. Tumor Cells Attract Neutrophils
Neutrophils can be recruited to the PDAC microenvironment via multiple tumor-secreted factors (Figure 1).
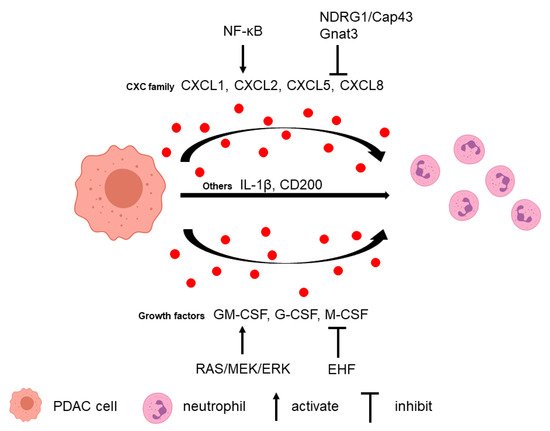
2.2. Neutrophils Promote Tumor Cell Survival and Metastasis
Several studies in PDAC have found neutrophil depletion could inhibit tumor growth and metastasis. Depleting G-MDSC in the KPC mouse model using anti-Ly6G antibody, 1A8, resulted in an increase in cleaved caspase-3 (CC3)-positive tumor cells, which suggested that depletion of G-MDSC increased tumor epithelial apoptosis [42].
3. Interactions between Neutrophil and TME
3.1. T Cell
In addition to tumor cells, T cells in the TME contribute to the recruitment of neutrophils (Figure 2).
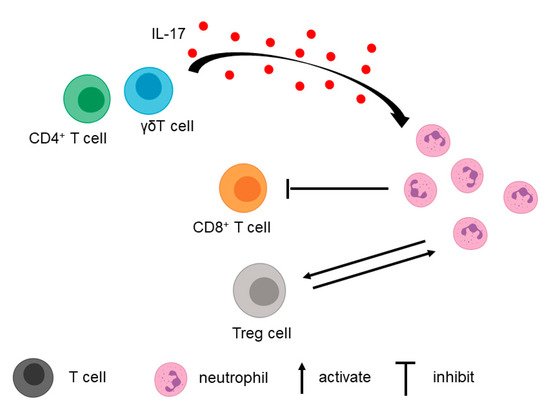
3.2. Fibroblasts, Macrophages, and Extracellular Matrix
4. Neutrophil Extracellular Trap in PDAC
The formation of neutrophil extracellular trap (NET) is a cellular function of neutrophils. Release of NET, called NETosis, is considered a defense mechanism to trap and kill bacteria and other pathogens.
Many studies have demonstrated that pancreatic cancer cells induce NET formation. For example, the conditioned media from PDAC cell line AsPC-1 was able to induce NET formation, and NETs could promote AsPC-1 cell migration and invasion as well as angiogenesis in vitro [68]. Another group found that conditioned media from KPC cells previously exposed to IL-17 induced NETosis in mouse neutrophils in vitro [51]. Moreover, serum from PDAC patients significantly increased NET formation and reduced NET degradation [51]. Tumor-derived protein tissue inhibitor of metalloproteinases-1 (TIMP-1), which correlated with poor prognosis in PDAC, directly triggered the formation of NETs. This effect depended on the interaction of TIMP-1 with its receptor CD63 and subsequent ERK signaling [69]. In addition to tumor cells, CAF-conditioned media can also induce ROS-dependent NETosis by secreting Amyloid β A4 protein. In turn, NET formation promotes CAF expansion, contractility, and deposition of matrix components supportive of tumor growth.
Recent studies suggested that NETs contribute to PDAC development. Electron microscope images of co-cultured pancreatic cancer cells with neutrophils showed that NETs could capture pancreatic cancer cells by their spider web-like structures [70].
5. Clinical Implications of Neutrophil and NET in PDAC
5.1. Prognosis in PDAC
The neutrophil-to-lymphocyte ratio (NLR) offers critical prognostic information in PDAC as well as breast cancer, lung cancer, and other types of cancer [73,74]. Studies have demonstrated that increased NLR correlates with a poor prognosis in patients with resectable and unresectable pancreatic cancer [30,75,76,77].
Intratumoral neutrophils are also correlated with PDAC patient outcomes. CD177, a marker for neutrophils, is negatively correlated with the overall survival of PDAC patients [83].
A recent study showed that plasma NET levels using SYTOX-positive areas could also predict the survival of PDAC patients [69].
5.2. Neutrophil as a Potential Therapeutic Target in PDAC
The existing treatment of PDAC patients includes surgical resection, chemoradiation therapy, and immunotherapy, but only a small proportion of patients benefit from available therapies. Based on an increasing understanding of the role of TME in PDAC, neutrophils have emerged as a potential therapeutic target.
The majority of neutrophil targeting preclinical studies use CXCR2 inhibitors or Ly6G antibodies [30]. This approach is in line with the main classes of current and past drug targets being receptors, among others [85]. In addition, the preference of CXCR2 as a target might lie in the fact that CXCR2 blockage not only inhibits the CXCL5/CXCR2 axis but also blocks the effect of other ligands of CXCR2, including CXCL1–3 and CXCL6–8. In breast cancer, CXCR2 inhibitors were demonstrated to be safe and tolerable, and relevant clinical trials are currently underway (NCT02370238) [86]. However, ligands, including cytokines, have become more important drug targets in the past two decades, largely due to the increased antibody-based therapies. Although no study was found in PDAC, CXCL5 inhibition emerged as a new strategy to attenuate tumor angiogenesis and thus slow tumor progression [87].
6. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/biom11081170
References
- Society AC. Cancer Facts & Figures 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf
- Rahib L, Smith B, Aizenberg R, Rosenzweig A, Fleshman J, Matrisian L. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer research 2014;74:2913-21
- Binnewies M, Roberts E, Kersten K, Chan V, Fearon D, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nature medicine 2018;24:541-50
- Dinh HQ, Eggert T, Meyer MA, Zhu YP, Olingy CE, Llewellyn R, et al. Coexpression of CD71 and CD117 Identifies an Early Unipotent Neutrophil Progenitor Population in Human Bone Marrow. Immunity 2020;53:319-34.e6
- Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. British journal of cancer 2013;108:914-23
- Soto-Perez-de-Celis E, Chavarri-Guerra Y, Leon-Rodriguez E, Gamboa-Dominguez A. Tumor-Associated Neutrophils in Breast Cancer Subtypes. Asian Pacific journal of cancer prevention : APJCP 2017;18:2689-93
- Galdiero M, Bianchi P, Grizzi F, Di Caro G, Basso G, Ponzetta A, et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. International journal of cancer 2016;139:446-56
- Jensen TO, Schmidt H, Møller HJ, Donskov F, Høyer M, Sjoegren P, et al. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer 2012;118:2476-85
- Yuen KC, Liu L-F, Gupta V, Madireddi S, Keerthivasan S, Li C, et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nature Medicine 2020;26:693-8
- Arvanitakis K, Mitroulis I, Germanidis G. Tumor-Associated Neutrophils in Hepatocellular Carcinoma Pathogenesis, Prognosis, and Therapy. Cancers (Basel) 2021;13
- Caruso RA, Bellocco R, Pagano M, Bertoli G, Rigoli L, Inferrera C. Prognostic value of intratumoral neutrophils in advanced gastric carcinoma in a high-risk area in northern Italy. Modern pathology 2002;15:831-7
- Fossati G, Ricevuti G, Edwards SW, Walker C, Dalton A, Rossi ML. Neutrophil infiltration into human gliomas. Acta neuropathologica 1999;98:349-54
- Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang S-P, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nature Medicine 2020;26:688-92
- Fridlender Z, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer cell 2009;16:183-94
- Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nature Reviews Cancer 2020;20:485-503
- Wang X, Qiu L, Li Z, Wang XY, Yi H. Understanding the Multifaceted Role of Neutrophils in Cancer and Autoimmune Diseases. Front Immunol 2018;9:2456
- Shojaei F, Wu X, Zhong C, Yu L, Liang X, Yao J, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 2007;450:825-31
- Schmielau J, Finn O. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer research 2001;61:4756-60
- Kalafati L, Kourtzelis I, Schulte-Schrepping J, Li X, Hatzioannou A, Grinenko T, et al. Innate Immune Training of Granulopoiesis Promotes Anti-tumor Activity. Cell 2020;183:771-85.e12
- Wang W, Liu L, Xu H, Wu C, Xiang J, Xu J, et al. Infiltrating immune cells and gene mutations in pancreatic ductal adenocarcinoma. The British journal of surgery 2016;103:1189-99
- Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med 2007;13:828-35
- Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, et al. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood 2007;109:4336-42
- Marvel D, Gabrilovich D. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. The Journal of clinical investigation 2015;125:3356-64
- Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. Journal of immunology (Baltimore, Md : 1950) 2008;181:5791-802
- Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 2010;40:2969-75
- Coffelt S, Wellenstein M, de Visser K. Neutrophils in cancer: neutral no more. Nature reviews Cancer 2016;16:431-46
- Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nature Reviews Clinical Oncology 2019;16:601-20
- Fridlender ZG, Sun J, Mishalian I, Singhal S, Cheng G, Kapoor V, et al. Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PloS one 2012;7:e31524
- Steele C, Karim S, Leach J, Bailey P, Upstill-Goddard R, Rishi L, et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer cell 2016;29:832-45
- Nywening TM, Belt BA, Cullinan DR, Panni RZ, Han BJ, Sanford DE, et al. Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 2018;67:1112-23
- Hosoi F, Izumi H, Kawahara A, Murakami Y, Kinoshita H, Kage M, et al. N-myc downstream regulated gene 1/Cap43 suppresses tumor growth and angiogenesis of pancreatic cancer through attenuation of inhibitor of kappaB kinase beta expression. Cancer research 2009;69:4983-91
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annual review of immunology 1997;15:675-705
- Qiao B, Luo W, Liu Y, Wang J, Liu C, Liu Z, et al. The prognostic value of CXC chemokine receptor 2 (CXCR2) in cancers: a meta-analysis. Oncotarget 2018;9:15068-76
- Sano M, Ijichi H, Takahashi R, Miyabayashi K, Fujiwara H, Yamada T, et al. Blocking CXCLs-CXCR2 axis in tumor-stromal interactions contributes to survival in a mouse model of pancreatic ductal adenocarcinoma through reduced cell invasion/migration and a shift of immune-inflammatory microenvironment. Oncogenesis 2019;8:8
- Najjar YG, Rayman P, Jia X, Pavicic PG, Jr., Rini BI, Tannenbaum C, et al. Myeloid-Derived Suppressor Cell Subset Accumulation in Renal Cell Carcinoma Parenchyma Is Associated with Intratumoral Expression of IL1β, IL8, CXCL5, and Mip-1α. Clin Cancer Res 2017;23:2346-55
- Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, et al. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS biology 2011;9:e1001162
- Zhou Y, Guo F. A selective sphingosine-1-phosphate receptor 1 agonist SEW-2871 aggravates gastric cancer by recruiting myeloid-derived suppressor cells. Journal of biochemistry 2018;163:77-83
- Yu F, Shi Y, Wang J, Li J, Fan D, Ai W. Deficiency of Kruppel-like factor KLF4 in mammary tumor cells inhibits tumor growth and pulmonary metastasis and is accompanied by compromised recruitment of myeloid-derived suppressor cells. International journal of cancer 2013;133:2872-83
- Hoffman MT, Kemp SB, Salas-Escabillas DJ, Zhang Y, Steele NG, The S, et al. The Gustatory Sensory G-Protein GNAT3 Suppresses Pancreatic Cancer Progression in Mice. Cellular and molecular gastroenterology and hepatology 2021;11:349-69
- Iida-Norita R, Kawamura M, Suzuki Y, Hamada S, Masamune A, Furukawa T, et al. Vasohibin-2 plays an essential role in metastasis of pancreatic ductal adenocarcinoma. Cancer science 2019;110:2296-308
- Chao T, Furth E, Vonderheide R. CXCR2-Dependent Accumulation of Tumor-Associated Neutrophils Regulates T-cell Immunity in Pancreatic Ductal Adenocarcinoma. Cancer immunology research 2016;4:968-82
- Stromnes I, Brockenbrough J, Izeradjene K, Carlson M, Cuevas C, Simmons R, et al. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut 2014;63:1769-81
- Phan VT, Wu X, Cheng JH, Sheng RX, Chung AS, Zhuang G, et al. Oncogenic RAS pathway activation promotes resistance to anti-VEGF therapy through G-CSF-induced neutrophil recruitment. Proceedings of the National Academy of Sciences of the United States of America 2013;110:6079-84
- Liu J, Jiang W, Zhao K, Wang H, Zhou T, Bai W, et al. Tumoral EHF predicts the efficacy of anti-PD1 therapy in pancreatic ductal adenocarcinoma. The Journal of experimental medicine 2019;216:656-73
- Das S, Shapiro B, Vucic EA, Vogt S, Bar-Sagi D. Tumor Cell-Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer research 2020;80:1088-101
- Choueiry F, Torok M, Shakya R, Agrawal K, Deems A, Benner B, et al. CD200 promotes immunosuppression in the pancreatic tumor microenvironment. Journal for immunotherapy of cancer 2020;8
- Li J, Yuan S, Norgard RJ, Yan F, Yamazoe T, Blanco A, et al. Tumor Cell-Intrinsic USP22 Suppresses Antitumor Immunity in Pancreatic Cancer. Cancer immunology research 2020;8:282-91
- Liu X, Zhou Z, Cheng Q, Wang H, Cao H, Xu Q, et al. Acceleration of pancreatic tumorigenesis under immunosuppressive microenvironment induced by Reg3g overexpression. Cell Death Dis 2017;8:e3033
- Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47-52
- Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet 2015;47:1168-78
- Zhang Y, Chandra V, Riquelme Sanchez E, Dutta P, Quesada P, Rakoski A, et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. The Journal of experimental medicine 2020;217
- Jin W, Yin H, Li H, Yu XJ, Xu HX, Liu L. Neutrophil extracellular DNA traps promote pancreatic cancer cells migration and invasion by activating EGFR/ERK pathway. Journal of cellular and molecular medicine 2021
- McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell 2014;25:621-37
- Siret C, Collignon A, Silvy F, Robert S, Cheyrol T, André P, et al. Deciphering the Crosstalk Between Myeloid-Derived Suppressor Cells and Regulatory T Cells in Pancreatic Ductal Adenocarcinoma. Front Immunol 2019;10:3070
- Holokai L, Chakrabarti J, Lundy J, Croagh D, Adhikary P, Richards SS, et al. Murine- and Human-Derived Autologous Organoid/Immune Cell Co-Cultures as Pre-Clinical Models of Pancreatic Ductal Adenocarcinoma. Cancers (Basel) 2020;12
- Cartwright ANR, Suo S, Badrinath S, Kumar S, Melms J, Luoma A, et al. Immunosuppressive Myeloid Cells Induce Nitric Oxide-Dependent DNA Damage and p53 Pathway Activation in CD8(+) T Cells. Cancer immunology research 2021
- Li C, Cui L, Yang L, Wang B, Zhuo Y, Zhang L, et al. Pancreatic Stellate Cells Promote Tumor Progression by Promoting an Immunosuppressive Microenvironment in Murine Models of Pancreatic Cancer. Pancreas 2020;49:120-7
- Miller-Ocuin J, Liang X, Boone B, Doerfler W, Singhi A, Tang D, et al. DNA released from neutrophil extracellular traps (NETs) activates pancreatic stellate cells and enhances pancreatic tumor growth. Oncoimmunology 2019;8:e1605822
- Mayer P, Dinkic C, Jesenofsky R, Klauss M, Schirmacher P, Dapunt U, et al. Changes in the microarchitecture of the pancreatic cancer stroma are linked to neutrophil-dependent reprogramming of stellate cells and reflected by diffusion-weighted magnetic resonance imaging. Theranostics 2018;8:13-30
- Hu H, Hang JJ, Han T, Zhuo M, Jiao F, Wang LW. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2016;37:8657-64
- Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. Journal of immunology 2007;179:977-83
- Bausch D, Pausch T, Krauss T, Hopt UT, Fernandez-del-Castillo C, Warshaw AL, et al. Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis 2011;14:235-43
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532-5
- Berger-Achituv S, Brinkmann V, Abed UA, Kühn LI, Ben-Ezra J, Elhasid R, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol 2013;4:48
- Demers M, Wong SL, Martinod K, Gallant M, Cabral JE, Wang Y, et al. Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology 2016;5:e1134073
- Kanamaru R, Ohzawa H, Miyato H, Matsumoto S, Haruta H, Kurashina K, et al. Low density neutrophils (LDN) in postoperative abdominal cavity assist the peritoneal recurrence through the production of neutrophil extracellular traps (NETs). Sci Rep 2018;8:632
- Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018;361:eaao4227
- Jung HS, Gu J, Kim JE, Nam Y, Song JW, Kim HK. Cancer cell-induced neutrophil extracellular traps promote both hypercoagulability and cancer progression. PloS one 2019;14:e0216055
- Schoeps B, Eckfeld C, Prokopchuk O, Böttcher J, Häußler D, Steiger K, et al. TIMP-1 triggers neutrophil extracellular trap formation in pancreatic cancer. Cancer research 2021
- Kajioka H, Kagawa S, Ito A, Yoshimoto M, Sakamoto S, Kikuchi S, et al. Targeting neutrophil extracellular traps with thrombomodulin prevents pancreatic cancer metastasis. Cancer Lett 2021;497:1-13
- Cools-Lartigue J, Spicer J, Najmeh S, Ferri L. Neutrophil extracellular traps in cancer progression. Cellular and molecular life sciences : CMLS 2014;71:4179-94
- Munir H, Jones JO, Janowitz T, Hoffmann M, Euler M, Martins CP, et al. Stromal-driven and Amyloid β-dependent induction of neutrophil extracellular traps modulates tumor growth. Nature Communications 2021;12:683
- Chen C, Yang H, Cai D, Xiang L, Fang W, Wang R. Preoperative peripheral blood neutrophil-to-lymphocyte ratios (NLR) and platelet-to-lymphocyte ratio (PLR) related nomograms predict the survival of patients with limited-stage small-cell lung cancer. Translational lung cancer research 2021;10:866-77
- Orditura M, Galizia G, Diana A, Saccone C, Cobellis L, Ventriglia J, et al. Neutrophil to lymphocyte ratio (NLR) for prediction of distant metastasis-free survival (DMFS) in early breast cancer: a propensity score-matched analysis. ESMO open 2016;1:e000038
- Garcea G, Ladwa N, Neal CP, Metcalfe MS, Dennison AR, Berry DP. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World journal of surgery 2011;35:868-72
- Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. British journal of cancer 2013;109:416-21
- Suzuki R, Takagi T, Hikichi T, Konno N, Sugimoto M, Watanabe KO, et al. Derived neutrophil/lymphocyte ratio predicts gemcitabine therapy outcome in unresectable pancreatic cancer. Oncol Lett 2016;11:3441-5
- Toledano-Fonseca M, Cano M, Inga E, Gómez-España A, Guil-Luna S, García-Ortiz M, et al. The Combination of Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio with Liquid Biopsy Biomarkers Improves Prognosis Prediction in Metastatic Pancreatic Cancer. Cancers 2021;13
- Luo G, Guo M, Liu Z, Xiao Z, Jin K, Long J, et al. Blood Neutrophil–Lymphocyte Ratio Predicts Survival in Patients with Advanced Pancreatic Cancer Treated with Chemotherapy. Annals of surgical oncology 2015;22:670-6
- Shang J, Han X, Zha H, Tao H, Li X, Yuan F, et al. Systemic Immune-Inflammation Index and Changes of Neutrophil-Lymphocyte Ratio as Prognostic Biomarkers for Patients With Pancreatic Cancer Treated With Immune Checkpoint Blockade. Front Oncol 2021;11:585271
- Schernberg A, Vernerey D, Goldstein D, Van Laethem J, Glimelius B, van Houtte P, et al. Predictive Value of Neutrophils Count for Local Tumor Control After Chemoradiotherapy in Patients With Locally Advanced Pancreatic Carcinoma. International journal of radiation oncology, biology, physics 2021
- Deng G, Yan H, Guo Z, Dai G. Correlation Between Baseline Serum Tumor Markers and Clinical Characteristic Factors in Patients with Advanced Pancreatic Cancer. OncoTargets and therapy 2020;13:11151-63
- Wang Y, Fang T, Huang L, Wang H, Zhang L, Wang Z, et al. Neutrophils infiltrating pancreatic ductal adenocarcinoma indicate higher malignancy and worse prognosis. Biochemical and biophysical research communications 2018;501:313-9
- Jin W, Xu HX, Zhang SR, Li H, Wang WQ, Gao HL, et al. Tumor-Infiltrating NETs Predict Postsurgical Survival in Patients with Pancreatic Ductal Adenocarcinoma. Annals of surgical oncology 2019;26:635-43
- Attwood MM, Jonsson J, Rask-Andersen M, Schiöth HB. Soluble ligands as drug targets. Nature reviews Drug discovery 2020;19:695-710
- Schott AF, Goldstein LJ, Cristofanilli M, Ruffini PA, McCanna S, Reuben JM, et al. Phase Ib Pilot Study to Evaluate Reparixin in Combination with Weekly Paclitaxel in Patients with HER-2-Negative Metastatic Breast Cancer. Clin Cancer Res 2017;23:5358-65
- Zhang W, Wang H, Sun M, Deng X, Wu X, Ma Y, et al. CXCL5/CXCR2 axis in tumor microenvironment as potential diagnostic biomarker and therapeutic target. Cancer Commun (Lond) 2020;40:69-80
- Hsu YL, Hou MF, Kuo PL, Huang YF, Tsai EM. Breast tumor-associated osteoblast-derived CXCL5 increases cancer progression by ERK/MSK1/Elk-1/snail signaling pathway. Oncogene 2013;32:4436-47
- Kuo PL, Huang MS, Hung JY, Chou SH, Chiang SY, Huang YF, et al. Synergistic effect of lung tumor-associated dendritic cell-derived HB-EGF and CXCL5 on cancer progression. International journal of cancer 2014;135:96-108
- Wang X, Hu L-P, Qin W-T, Yang Q, Chen D-Y, Li Q, et al. Identification of a subset of immunosuppressive P2RX1-negative neutrophils in pancreatic cancer liver metastasis. Nature Communications 2021;12:174
