
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paula De Luca | + 1424 word(s) | 1424 | 2021-08-05 04:52:58 | | | |
| 2 | Paula De Luca | Meta information modification | 1424 | 2021-08-09 21:29:07 | | |
Video Upload Options
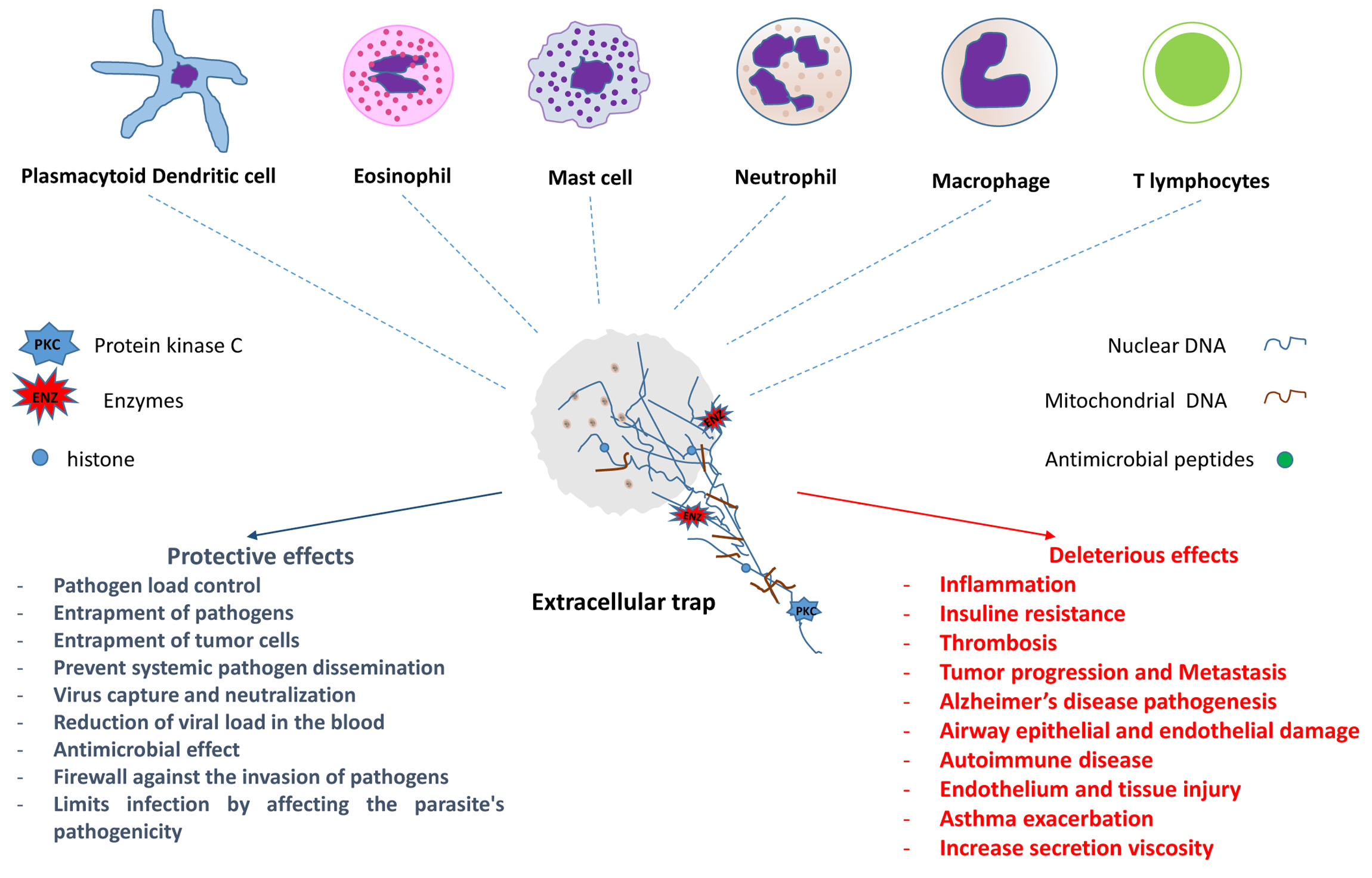
The first formal description of the microbicidal activity of extracellular traps (ETs) containing DNA occurred in neutrophils in 2004. Since then, ETs have been identified in different populations of cells involved in both innate and adaptive immune responses. Much of the knowledge has been obtained from in vitro or ex vivo studies; however, in vivo evaluations in experimental models and human biological materials have corroborated some of the results obtained. Two types of ETs have been described—suicidal and vital ETs, with or without the death of the producer cell. The studies showed that the same cell type may have more than one ETs formation mechanism and that different cells may have similar ETs formation mechanisms. ETs can act by controlling or promoting the mechanisms involved in the development and evolution of various infectious and non-infectious diseases, such as autoimmune, cardiovascular, thrombotic, and neoplastic diseases, among others.
1- Introduction
With the great impetus given to the understanding of cellular functions in the immune system in the early 1950s, much information has been obtained; nevertheless, some mechanisms have not yet been fully elucidated. This knowledge is crucial to understand the mechanisms of disease and/or protection, since knowing how to recognize in what way diseases occur develop new treatments and effective vaccines with fewer adverse effects. Thus, paraphrasing Stephen Hawking [1], cells are a universe involved in a plasma membrane, and new components and functions are described every day [2][3][4][5].
The ETs formation seems to be a fast event, and perhaps, for this reason, it has not been easily observed in vivo until now. In addition, some cells in which their formation has been described are difficult to handle. Thus, much of the knowledge was obtained from in vitro or ex vivo studies with different protocols (reviewed in [2]). However, evaluations in experimental models and human samples have corroborated many of these results [6]. Although some mechanisms and effects of ETs release still need further elucidation, what is already known shows the importance of ETs in the control and/or development of the immune response.
2 - Types of Extracellular Traps
3 - ETs in Health and Disease
References
- Hawking, S. The Universe in a Nutshell, 1st ed.; Transworld Publishers Ltd: London, UK, 2001; ISBN 978-0-593-04815-3.
- Tan, C.; Aziz, M.; Wang, P. The Vitals of NETs. J. Leukoc. Biol. 2020, 1–12, doi:10.1002/JLB.3RU0620-375R.
- Brinkmann, V. Neutrophil Extracellular Traps in the Second Decade. J. Innate Immun 2018, 10, 414–421, doi:10.1159/000489829.
- Kenny, E.F.; Herzig, A.; Krüger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; von Bernuth, H.; Zychlinsky, A. Diverse Stimuli Engage Different Neutrophil Extracellular Trap Pathways. eLife 2017, 6, e24437, doi:10.7554/eLife.24437.
- Burgener, S.S.; Schroder, K. Neutrophil Extracellular Traps in Host Defense. Cold Spring Harb. Perspect. Biol. 2020, 12, a037028, doi:10.1101/cshperspect.a037028.
- Yousefi, S.; Simon, D.; Stojkov, D.; Karsonova, A.; Karaulov, A.; Simon, H.-U. In Vivo Evidence for Extracellular DNA Trap Formation. Cell Death Dis. 2020, 11, 300, doi:10.1038/s41419-020-2497-x.
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schuize, I.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel Cell Death Program Leads to Neutrophi Extracellular Traps. J. Cell Biol. 2007, 176, 231–241, doi:10.1083/jcb.200606027.
- Wartha, F.; Henriques-Normark, B. ETosis: A Novel Cell Death Pathway. Sci. Signal. 2008, 1, pe25, doi:10.1126/stke.121pe25.
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535, doi:10.1126/science.1092385.
- Papayannopoulos, V. Neutrophil Extracellular Traps in Immunity and Disease. Nat. Rev. Immunol. 2018, 18, 134–147, doi:10.1038/nri.2017.105.
- Rawat, K.; Syeda, S.; Shrivastava, A. Neutrophil-Derived Granule Cargoes: Paving the Way for Tumor Growth and Pro-gression. Cancer Metastasis Rev. 2021, 40, 221–244, doi:10.1007/s10555-020-09951-1.
- Wu, M.; Ma, M.; Tan, Z.; Zheng, H.; Liu, X. Neutrophil: A New Player in Metastatic Cancers. Front. Immunol. 2020, 11, 565165, doi:10.3389/fimmu.2020.565165.
- Goldmann, O.; Medina, E. The Expanding World of Extracellular Traps: Not Only Neutrophils but Much More. Front. Im-munol. 2012, 3, 420, doi:10.3389/fimmu.2012.00420.
- Ingelsson, B.; Söderberg, D.; Strid, T.; Söderberg, A.; Bergh, A.-C.; Loitto, V.; Lotfi, K.; Segelmark, M.; Spyrou, G.; Rosén, A. Lymphocytes Eject Interferogenic Mitochondrial DNA Webs in Response to CpG and Non-CpG Oligodeoxynucleotides of Class C. Proc. Natl. Acad. Sci. USA 2018, 115, E478–E487, doi:10.1073/pnas.1711950115.
- de Bont, C.M.; Stokman, M.E.M.; Faas, P.; Thurlings, R.M.; Boelens, W.C.; Wright, H.L.; Pruijn, G.J.M. Autoantibodies to Neutrophil Extracellular Traps Represent a Potential Serological Biomarker in Rheumatoid Arthritis. J. Autoimmun. 2020, 113, 102484, doi:10.1016/j.jaut.2020.102484.
- Hofbauer, T.M.; Ondracek, A.S.; Mangold, A.; Scherz, T.; Nechvile, J.; Seidl, V.; Brostjan, C.; Lang, I.M. Neutrophil Extra-cellular Traps Induce MCP-1 at the Culprit Site in ST-Segment Elevation Myocardial Infarction. Front. Cell Dev. Biol. 2020, 8, 564169, doi:10.3389/fcell.2020.564169.
- Yang, L.; Liu, Q.; Zhang, X.; Liu, X.; Zhou, B.; Chen, J.; Huang, D.; Li, J.; Li, H.; Chen, F.; et al. DNA of Neutrophil Extra-cellular Traps Promotes Cancer Metastasis via CCDC25. Nature 2020, 583, 133–138, doi:10.1038/s41586-020-2394-6.
- Pérez-Figueroa, E.; Álvarez-Carrasco, P.; Ortega, E.; Maldonado-Bernal, C. Neutrophils: Many Ways to Die. Front. Immunol. 2021, 12, 631821, doi:10.3389/fimmu.2021.631821.
- Pilsczek, F.H.; Salina, D.; Poon, K.K.H.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.Y.; Surette, M.G.; Sugai, M.; et al. A Novel Mechanism of Rapid Nuclear Neutrophil Extracellular Trap Formation in Response to Staphylococcus Aureus. J. Immunol. 2010, 185, 7413–7425, doi:10.4049/jimmunol.1000675.
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.V.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-Induced NETosis Is a Dynamic Process Involving Neutrophil Multitasking in Vivo. Nat. Med. 2012, 18, 1386–1393, doi:10.1038/nm.2847.
- Yipp, B.G.; Kubes, P. NETosis: How Vital Is It? Blood 2013, 122, 2784–2794, doi:10.1182/blood-2013-04-457671.
- Ravindran, M.; Khan, M.A.; Palaniyar, N. Neutrophil Extracellular Trap Formation: Physiology, Pathology, and Pharma-cology. Biomolecules 2019, 9, 365, doi:10.3390/biom9080365.
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochem. (Mosc.) 2020, 85, 1178–1190, doi:10.1134/S0006297920100065.
- Daniel, C.; Leppkes, M.; Muñoz, L.E.; Schley, G.; Schett, G.; Herrmann, M. Extracellular DNA Traps in Inflammation, Injury and Healing. Nat. Rev. Nephrol. 2019, 15, 559–575, doi:10.1038/s41581-019-0163-2.
- Baker, V.S.; Imade, G.E.; Molta, N.B.; Tawde, P.; Pam, S.D.; Obadofin, M.O.; Sagay, S.A.; Egah, D.Z.; Iya, D.; Afolabi, B.B.; et al. Cytokine-Associated Neutrophil Extracellular Traps and Antinuclear Antibodies in Plasmodium Falciparum Infected Children under Six Years of Age. Malar. J. 2008, 7, 41, doi:10.1186/1475-2875-7-41.
- Abi Abdallah, D.S.; Lin, C.; Ball, C.J.; King, M.R.; Duhamel, G.E.; Denkers, E.Y. Toxoplasma Gondii Triggers Release of Human and Mouse Neutrophil Extracellular Traps. Infect. Immun. 2012, 80, 768–777, doi:10.1128/IAI.05730-11.
- Morgado, F.N.; Nascimento, M.T.C.; Saraiva, E.M.; de Oliveira-Ribeiro, C.; de Fátima Madeira, M.; da Costa-Santos, M.; Vasconcellos, E.C.F.; Pimentel, M.I.F.; Rosandiski Lyra, M.; Schubach, A. de O.; et al. Are Neutrophil Extracellular Traps Playing a Role in the Parasite Control in Active American Tegumentary Leishmaniasis Lesions? PLoS ONE 2015, 10, e0133063, doi:10.1371/journal.pone.0133063.
- Morgado, F.N.; Schubach, A.O.; Barros, M.B.L.; Conceição-Silva, F. The in Situ Inflammatory Profile of Lymphocutaneous and Fixed Forms of Human Sporotrichosis. Med. Mycol. 2011, 49, 612–620, doi:10.3109/13693786.2011.552532.
- Saitoh, T.; Komano, J.; Saitoh, Y.; Misawa, T.; Takahama, M.; Kozaki, T.; Uehata, T.; Iwasaki, H.; Omori, H.; Yamaoka, S.; et al. Neutrophil Extracellular Traps Mediate a Host Defense Response to Human Immunodeficiency Virus-1. Cell Host Microbe 2012, 12, 109–116, doi:10.1016/j.chom.2012.05.015.
- Koh, C.C.; Wardini, A.B.; Vieira, M.; Passos, L.S.A.; Martinelli, P.M.; Neves, E.G.A.; do Vale Antonelli, L.R.; Barbosa, D.F.; Velikkakam, T.; Gutseit, E.; et al. Human CD8+ T Cells Release Extracellular Traps Co-Localized with Cytotoxic Vesicles That Are Associated With Lesion Progression and Severity in Human Leishmaniasis. Front. Immunol. 2020, 11, 594581, doi:10.3389/fimmu.2020.594581.
- von Köckritz-Blickwede, M.; Goldmann, O.; Thulin, P.; Heinemann, K.; Norrby-Teglund, A.; Rohde, M.; Medina, E. Phag-ocytosis-Independent Antimicrobial Activity of Mast Cells by Means of Extracellular Trap Formation. Blood 2008, 111, 3070–3080, doi:10.1182/blood-2007-07-104018.




