Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrea Persons | + 1461 word(s) | 1461 | 2021-07-27 03:42:25 | | | |
| 2 | Rita Xu | Meta information modification | 1461 | 2021-08-10 06:17:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Persons, A. Wearable Sensing Technologies. Encyclopedia. Available online: https://encyclopedia.pub/entry/12967 (accessed on 07 February 2026).
Persons A. Wearable Sensing Technologies. Encyclopedia. Available at: https://encyclopedia.pub/entry/12967. Accessed February 07, 2026.
Persons, Andrea. "Wearable Sensing Technologies" Encyclopedia, https://encyclopedia.pub/entry/12967 (accessed February 07, 2026).
Persons, A. (2021, August 09). Wearable Sensing Technologies. In Encyclopedia. https://encyclopedia.pub/entry/12967
Persons, Andrea. "Wearable Sensing Technologies." Encyclopedia. Web. 09 August, 2021.
Copy Citation
Standards for the fatigue testing of wearable sensing technologies are lacking. The majority of published fatigue tests for wearable sensors are performed on proof-of-concept stretch sensors fabricated from a variety of materials. Due to their flexibility and stretchability, polymers are often used in the fabrication of wearable sensors. Other materials, including textiles, carbon nanotubes, graphene, and conductive metals or inks, may be used in conjunction with polymers to fabricate wearable sensors.
fatigue testing
cyclic testing
low-cycle fatigue
high-cycle fatigue
wearables
lead failure
stretch sensor
hysteresis
cyclic softening
1. Introduction
Interest in wearable stretch sensors has increased due to their potential uses in medical applications to monitor the health of a patient [1][2][3][4][5][6][7][8][9][10], to assess biomechanics, [11][12][13][14][15][16][17][18][19][20][21], and as drug delivery systems in pharmaceutical applications [22][23]. Wearable sensors may also have applications in athletics. [11][18][21][24][25][26][27][28], soft robotics [21][29][30], ergonomic assessments [19] and deep space exploration [31]. This interest is especially timely as the SARS-CoV-2 (COVID-19) epidemic has led to decreased in-person office visits to medical professionals while concomitantly increasing the number of virtual visits via telemedical platforms [32][33][34]. The increased use of telemedicine has led to an increased interest in the use of wearable sensors to monitor the health of patients outside of the clinical setting [18][35][36]. By 2022, over 1,000,000,000 wearables are expected to be in use globally [37], and although the opportunities provided by wearable sensors are recognized, few sensors have been formally validated [38], and research is still needed to determine the accuracy, interpretation, and applicability of the data provided by wearable sensors [38][39]. While many wearable sensor prototypes have been described for the previously mentioned applications, for these sensors to move from “bench to bedside”, standardized testing methods, including those that focus on the fatigue life of a wearable sensor, are needed [40].
Wearable sensing technology may be broadly defined as electronic devices embedded within or worn upon the body that rely on sensors to capture and transmit data to an integrated display unit, a computer, or a smartphone [39][41][42][43][44]. Based on this definition, currently marketed wearable sensor technologies can be divided into internal sensors that are subcutaneously implanted in the body and external sensors that are worn on the body. Examples of the former include implantable cardioverter defibrillators (ICD), which sense cardiac depolarization [45][46][47][48][49][50][51][52][53][54][55][56][57]; implantable loop recorders, which are implantable electrocardiograms (EKG or ECG) [22][23]; and invasive continuous glucose monitors (CGM), which use a sensor embedded in the upper arm, abdomen, or gluteus to measure glucose levels from interstitial fluid [58][59], while examples of wearables that fall into the latter include smart watches, sleep and fitness trackers, or ECG sensors (Figure 1). Fatigue failures of wearables, such as ICDs, are widely recognized and result in significant morbidity and mortality [45][46][47][48][49][50][51][52][53][54][55][56][57]; therefore, understanding the electromechanical fatigue and failure properties of proposed wearable sensors is paramount in insuring patient safety. Although such failures are recognized with internally implanted sensors, a lack of standardized fatigue testing methods and validation studies [38][40], coupled with sensors fabricated from varying materials, has led to a paucity of comparable data regarding the durability and accuracy of wearable sensors. Further, the fatigue testing of wearable sensors is confounded due to the need to predict not only the fatigue life of the materials comprising the sensor but also the fatigue life (stability) of the signal produced by the sensor.
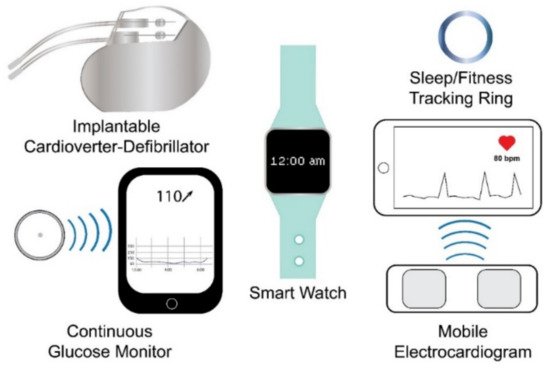
Figure 1. Examples of wearable sensing technologies. Wearable sensing technologies include internal (e.g., internal cardioverter-defibrillator and continuous glucose monitor) and external (e.g., smart watch, sleep/fitness tracking ring, and mobile electrocardiogram) wearable sensing technologies.
2. Internal Wearable Sensing Technologies
Internal wearable sensing technologies are fabricated from inert materials that do not elicit a bioreactive response upon implantation. Implantable materials include metals such as stainless steel and titanium, polymers such as polyethylene (PE) and polymethylmethacrylate (PMMA), and ceramics such as hydroxyapatite [60]. Within the United States, implanted medical devices, including ICDs, internal loop recorders, and CGMs, which may also be considered wearable technologies, are regulated by the Food and Drug Administration (FDA) as Class III medical devices [61][62][63]. For internal wearable sensing technologies to receive FDA approval to enter the marketplace, the sensor must either receive Premarket Approval (PMA) by undergoing clinical trials to demonstrate its safety and efficacy, or the sensor may be cleared to enter the market by the Premarket Notification 510(k) process which involves submitting premarket data to the FDA to show that the proposed internal sensor is “substantially equivalent” in both safety and efficiency to a sensor that did not require PMA approval and is already on the market [61][62][63][64][65]; however, “substantially equivalent” devices may be fabricated using both differing materials and differing mechanisms of action providing the safety profiles of the two devices are similar [61], but because of the variation in materials and mechanisms of action, testing standards that apply to one device do not necessarily correlate with the comparative device [61]. Further, incremental changes to medical devices initially approved by the PMA process may be submitted to the FDA for market clearance via a supplement that may not require clinical data [48][49], and underlying issues with the updated device may not be recognized until the device is in widespread use [61]. In contrast, the European Union has recently implemented regulations that require clinical evaluations for implantable medical devices throughout the lifespan of the device; therefore, clinical data is required from pre-market evaluations to post-market evaluations. Further, clinical data is required even in the case of incremental changes [66][67]. For example, in the United States, updated ICD leads can enter the marketplace through the use of a PMA supplement that does not include clinical data demonstrating the safety and efficacy of the updated lead; whereas, under the new regulations adopted by the European Union, the introduction of updated pacemaker leads requires clinical data that address the safety and efficacy of the lead [48][49][66][67]. Fatigue failures of ICD leads approved via PMA supplementation in the United States are common [45][46][47][48][49][50][51][52][53][54][55][56][57], but whether the new European Union regulations either prevent the entry of such leads into the market or prevent adverse outcomes by the timely recognition of issues with the leads during post-market surveillance remains unclear [68].
ICD leads are typically comprised of either a low-voltage, nickel–cobalt–chromium–molybdenum alloy coil conductor or a high-voltage, silver or platinum coil conductor that is coated with ethylene tetrafluoroethylene and poly-tetrafluoroethylene and housed within a silicone cylinder that also acts as insulation to separate the conductive cables from the electrode tips [53][69][70] (Figure 2). Leads are thin and flexible, ranging in diameter from 2.1 to 2.87 mm, to navigate the vasculature and are inserted into the myocardium [69][71]. Electrodes at the tips of ICD leads act as sensors to recognize atrial and ventricular depolarizations [71]. Upon sensing a depolarization event, a signal is sent to the pulse generator, which contains a battery and a circuit board, where the signal is processed, allowing for both the detection and correction of abnormal heart rates and rhythms based on programmable thresholds [71]; therefore, ICD leads are critical sensing mechanisms, and preventing fatigue failures of the leads will prevent adverse patient outcomes.

Figure 2. Simplified cross-section of an implantable cardioverter-defibrillator lead.
ICD leads are subjected to more than 100,000 cycles of flexure per day [53], and fatigue failures of ICD leads may result in high impedance if the lead is fractured or low impedance if the insulation fails, causing the lead to short circuit [70]. Additionally, fatigue failures of ICD leads may result in noise in the signal [54][55][57][70], inappropriate pacing [45][69], inappropriate defibrillation [45], the delivery of unnecessary shocks resulting from oversensing [45][46][50], or mortality [46][47], especially in a failure to detect ventricular fibrillation [71].
Few published studies have assessed the fatigue life of ICD leads [72]; however, Altman et al. [72] have noted that how the interaction between the coil and the individual wires comprising the coil affect the fatigue life of the lead is unknown and may require special consideration when evaluating fatiguing methods. Liu et al. [73] found that the stresses placed on the lead in vivo could be determined by applying classic mechanical principles to a model created from 3D images rendered from angiograms and argue that this method can facilitate fatigue-life predictions of the leads. Recently, due to the ongoing high incidence of failure, standard protocols for the fatigue testing of ICD leads have been proposed [74]. The proposed method involves the application of a buckling or a bending force at a rate of 5 Hz to 12 samples per four curvature amplitudes of 0.78 cm−1, 1.11 cm−1, 2.12 cm−1, and 2.45 cm−1 (n = 48). All tests are performed at a temperature of 23 ± 5 °C. The first 1000 cycles are considered run-in, and testing is terminated upon failure of the lead or after the completion of 5,000,000 cycles. Disruption of electrical continuity or a 150% rise in the resistance over the initial resistance value constitutes failure [74]. While researchers are working to standardize testing methodologies for some internal wearable sensors [74], fatigue testing methods for external wearable sensors are not standardized [40], and a variety of testing methodologies, rates, and cycling regimes have been reported in the fatigue testing of proposed wearable sensors [6][11][12][13][20][21][30][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100].
References
- Wang, F.; Liu, S.; Shu, L.; Tao, X.-M. Low-Dimensional Carbon Based Sensors and Sensing Network for Wearable Health and Environmental Monitoring. Carbon 2017, 121, 353–367.
- Yeo, J.C.; Lim, C.T. Emerging Flexible and Wearable Physical Sensing Platforms for Healthcare and Biomedical Applications. Microsyst. Nanoeng. 2016, 2, 1–19.
- Carvalho, H.; Catarino, A.P.; Rocha, A.; Postolache, O. Health Monitoring Using Textile Sensors and Electrodes: An Overview and Integration of Technologies. In Proceedings of the 2014 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Lisbon, Portugal, 11 June 2014; pp. 1–6.
- Hehr, A.; Song, Y.; Suberu, B.; Sullivan, J.; Shanov, V.; Schulz, M. Chapter 24—Embedded Carbon Nanotube Sensor Thread for Structural Health Monitoring and Strain Sensing of Composite Materials. In Nanotube Superfiber Materials; Schulz, M.J., Shanov, V.N., Yin, Z., Eds.; William Andrew Publishing: Boston, MA, USA, 2014; pp. 671–712. ISBN 978-1-4557-7863-8.
- Li, R.; Nie, B.; Zhai, C.; Cao, J.; Pan, J.; Chi, Y.-W.; Pan, T. Telemedical Wearable Sensing Platform for Management of Chronic Venous Disorder. Ann. Biomed. Eng. 2016, 44, 2282–2291.
- Pegan, J.D.; Zhang, J.; Chu, M.; Nguyen, T.; Park, S.-J.; Paul, A.; Kim, J.; Bachman, M.; Khine, M. Skin-Mountable Stretch Sensor for Wearable Health Monitoring. Nanoscale 2016, 8, 17295–17303.
- Byrom, B.; McCarthy, M.; Schueler, P.; Muehlhausen, W. Brain Monitoring Devices in Neuroscience Clinical Research: The Potential of Remote Monitoring Using Sensors, Wearables, and Mobile Devices. Clin. Pharmacol. Ther. 2018, 104, 59–71.
- Takei, K. Flexible and Stretchable Medical Devices; Wiley-VCH: Weinheim, Germany, 2018.
- Bernstein, R.A.; Kamel, H.; Granger, C.B.; Piccini, J.P.; Sethi, P.P.; Katz, J.M.; Vives, C.A.; Ziegler, P.D.; Franco, N.C.; Schwamm, L.H.; et al. Effect of Long-Term Continuous Cardiac Monitoring vs Usual Care on Detection of Atrial Fibrillation in Patients With Stroke Attributed to Large- or Small-Vessel Disease: The STROKE-AF Randomized Clinical Trial. JAMA 2021, 325, 2169.
- Buck, B.H.; Hill, M.D.; Quinn, F.R.; Butcher, K.S.; Menon, B.K.; Gulamhusein, S.; Siddiqui, M.; Coutts, S.B.; Jeerakathil, T.; Smith, E.E.; et al. Effect of Implantable vs Prolonged External Electrocardiographic Monitoring on Atrial Fibrillation Detection in Patients with Ischemic Stroke: The PER DIEM Randomized Clinical Trial. JAMA 2021, 325, 2160.
- Lee, J.; Kim, S.; Lee, J.; Yang, D.; Park, B.C.; Ryu, S.; Park, I. A Stretchable Strain Sensor Based on a Metal Nanoparticle Thin Film for Human Motion Detection. Nanoscale 2014, 6, 11932–11939.
- O’Quigley, C.; Sabourin, M.; Coyle, S.; Connolly, J.; Condall, J.; Curran, K.; Corcoran, B.; Diamond, D. Characteristics of a Piezo-Resistive Fabric Stretch Sensor Glove for Home-Monitoring of Rheumatoid Arthritis. In Proceedings of the 2014 11th International Conference on Wearable and Implantable Body Sensor Networks Workshops, Zurich, Sweden, 16–19 June 2014; pp. 23–26.
- Choi, D.Y.; Kim, M.H.; Oh, Y.S.; Jung, S.-H.; Jung, J.H.; Sung, H.J.; Lee, H.W. Highly Stretchable, Hysteresis-Free Ionic Liquid-Based Strain Sensor for Precise Human Motion Monitoring. ACS Appl. Mater. Interfaces 2017, 9, 1770–1780.
- Lorussi, F.; Rocchia, W.; Scilingo, E.P.; Tognetti, A.; De Rossi, D. Wearable, Redundant Fabric-Based Sensor Arrays for Reconstruction of Body Segment Posture. IEEE Sens. J. 2004, 4, 807–818.
- Scilingo, E.P.; Gemignani, A.; Paradiso, R.; Taccini, N.; Ghelarducci, B.; De Rossi, D. Performance Evaluation of Sensing Fabrics for Monitoring Physiological and Biomechanical Variables. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 345–352.
- Wang, Y.; Wang, L.; Yang, T.; Li, X.; Zang, X.; Zhu, M.; Wang, K.; Wu, D.; Zhu, H. Wearable and Highly Sensitive Graphene Strain Sensors for Human Motion Monitoring. Adv. Funct. Mater. 2014, 24, 4666–4670.
- Yoon, S.G.; Koo, H.-J.; Chang, S.T. Highly Stretchable and Transparent Microfluidic Strain Sensors for Monitoring Human Body Motions. ACS Appl. Mater. Interfaces 2015, 7, 27562–27570.
- Amjadi, M.; Kyung, K.-U.; Park, I.; Sitti, M. Stretchable, Skin-Mountable, and Wearable Strain Sensors and Their Potential Applications: A Review. Adv. Funct. Mater. 2016, 26, 1678–1698.
- Rose, M.; Curtze, C.; O’Sullivan, J.; El-Gohary, M.; Crawford, D.; Friess, D.; Brady, J.M. Wearable Inertial Sensors Allow for Quantitative Assessment of Shoulder and Elbow Kinematics in a Cadaveric Knee Arthroscopy Model. Arthroscopy 2017, 33, 2110–2116.
- Wang, Y.; Jia, Y.; Zhou, Y.; Wang, Y.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. Ultra-Stretchable, Sensitive and Durable Strain Sensors Based on Polydopamine Encapsulated Carbon Nanotubes/Elastic Bands. J. Mater. Chem. C 2018, 6, 8160–8170.
- Xia, S.; Song, S.; Gao, G. Robust and Flexible Strain Sensors Based on Dual Physically Cross-Linked Double Network Hydrogels for Monitoring Human-Motion. Chem. Eng. J. 2018, 354, 817–824.
- Lee, K.Y.; Peters, M.C.; Mooney, D.J. Controlled Drug Delivery from Polymers by Mechanical Signals. Adv. Mater. 2001, 13, 837–839.
- Di, J.; Yao, S.; Ye, Y.; Cui, Z.; Yu, J.; Ghosh, T.K.; Zhu, Y.; Gu, Z. Stretch-Triggered Drug Delivery from Wearable Elastomer Films Containing Therapeutic Depots. ACS Nano 2015, 9, 9407–9415.
- Barton, C.J.; Kappel, S.L.; Ahrendt, P.; Simonsen, O.; Rathleff, M.S. Dynamic Navicular Motion Measured Using a Stretch Sensor Is Different between Walking and Running, and between over-Ground and Treadmill Conditions. J. Foot Ankle Res. 2015, 8, 5.
- Luczak, T.; Saucier, D.; Burch, V.R.F.; Ball, J.E.; Chander, H.; Knight, A.; Wei, P.; Iftekhar, T. Closing the Wearable Gap: Mobile Systems for Kinematic Signal Monitoring of the Foot and Ankle. Electronics 2018, 7, 117.
- Saucier, D.; Luczak, T.; Nguyen, P.; Davarzani, S.; Peranich, P.; Ball, J.E.; Burch, R.F.; Smith, B.K.; Chander, H.; Knight, A.; et al. Closing the Wearable Gap—Part II: Sensor Orientation and Placement for Foot and Ankle Joint Kinematic Measurements. Sensors 2019, 19, 3509.
- Chander, H.; Stewart, E.; Saucier, D.; Nguyen, P.; Luczak, T.; Ball, J.E.; Knight, A.C.; Smith, B.K.; Prabhu, R.K. Closing the Wearable Gap—Part III: Use of Stretch Sensors in Detecting Ankle Joint Kinematics During Unexpected and Expected Slip and Trip Perturbations. Electronics 2019, 8, 1083.
- Saucier, D.; Davarzani, S.; Turner, A.; Luczak, T.; Nguyen, P.; Carroll, W.; Burch, V.R.F.; Ball, J.E.; Smith, B.K.; Chander, H.; et al. Closing the Wearable Gap—Part IV: 3D Motion Capture Cameras Versus Soft Robotic Sensors Comparison of Gait Movement Assessment. Electronics 2019, 8, 1382.
- Kumbay Yildiz, S.; Mutlu, R.; Alici, G. Fabrication and Characterisation of Highly Stretchable Elastomeric Strain Sensors for Prosthetic Hand Applications. Sens. Actuators A Phys. 2016, 247, 514–521.
- White, E.L.; Yuen, M.C.; Case, J.C.; Kramer, R.K. Low-Cost, Facile, and Scalable Manufacturing of Capacitive Sensors for Soft Systems. Adv. Mater. Technol. 2017, 2, 1700072.
- Litteken, D. Evaluation of Strain Measurement Devices for Inflatable Structures. In Proceedings of the 58th AIAA/ASCE/AHS/ASC Structures, Structural Dynamics, and Materials Conference, AIAA SciTech Forum, Grapevine, TX, USA, 9–13 January 2017.
- Mehrotra, A.; Nimgaonkar, A.; Richman, B. Telemedicine and Medical Licensure—Potential Paths for Reform. N. Engl. J. Med. 2021, 384, 687–690.
- Patel, S.Y.; Mehrotra, A.; Huskamp, H.A.; Uscher-Pines, L.; Ganguli, I.; Barnett, M.L. Trends in Outpatient Care Delivery and Telemedicine During the COVID-19 Pandemic in the US. JAMA Intern. Med. 2021, 181, 388.
- Shachar, C.; Gupta, A.; Katznelson, G. Modernizing Medical Licensure to Facilitate Telemedicine Delivery After the COVID-19 Pandemic. JAMA Health Forum 2021, 2, e210405.
- DeVore, A.D.; Wosik, J.; Hernandez, A.F. The Future of Wearables in Heart Failure Patients. JACC Heart Fail. 2019, 7, 922–932.
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent Developments in Biosensors for Healthcare and Biomedical Applications: A Review. Measurement 2021, 167, 108293.
- Vailshery, L.S. Global Connected Wearable Devices 2016–2022. Available online: https://www.statista.com/statistics/487291/global-connected-wearable-devices/ (accessed on 29 May 2021).
- Peake, J.M.; Kerr, G.; Sullivan, J.P. A Critical Review of Consumer Wearables, Mobile Applications, and Equipment for Providing Biofeedback, Monitoring Stress, and Sleep in Physically Active Populations. Front. Physiol. 2018, 9.
- Dunn, J.; Runge, R.; Snyder, M. Wearables and the Medical Revolution. Pers. Med. 2018, 15, 429–448.
- Lall, P.; Narangaparambil, J.; Abrol, A.; Leever, B.; Marsh, J. Development of Test Protocols for the Flexible Substrates in Wearable Applications. In Proceedings of the 2018 17th IEEE Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (ITherm), San Diego, CA, USA, 29 May–1 June 2018; pp. 1120–1127.
- Salah, H.; MacIntosh, E.; Rajakulendran, N. Wearable Tech: Leveraging Canadian Innovation to Improve Health; MaRS Discovery District: Toronto, ON, Canada, 2014.
- Slade Shantz, J.A.; Veillette, C.J.H. The Application of Wearable Technology in Surgery: Ensuring the Positive Impact of the Wearable Revolution on Surgical Patients. Front. Surg. 2014, 1.
- Kolodzey, L.; Grantcharov, P.D.; Rivas, H.; Schijven, M.P.; Grantcharov, T.P. Wearable Technology in the Operating Room: A Systematic Review. BMJ Innov. 2017, 3.
- Yetisen, A.K.; Martinez-Hurtado, J.L.; Ünal, B.; Khademhosseini, A.; Butt, H. Wearables in Medicine. Adv. Mater. 2018, 30, 1706910.
- Maisel, W.H. Semper Fidelis—Consumer Protection for Patients with Implanted Medical Devices. N. Eng. J. Med. 2008, 358, 985–987.
- Hauser, R.G.; Maisel, W.H.; Friedman, P.A.; Kallinen, L.M.; Mugglin, A.S.; Kumar, K.; Hodge, D.O.; Morrison, T.B.; Hayes, D.L. Longevity of Sprint Fidelis Implantable Cardioverter-Defibrillator Leads and Risk Factors for Failure. Circulation 2011, 123, 358–363.
- Hauser, R.G.; Abdelhadi, R.; McGriff, D.; Retel, L.K. Deaths Caused by the Failure of Riata and Riata ST Implantable Cardioverter-Defibrillator Leads. Heart Rhythm 2012, 9, 1227–1235.
- Rome, B.N.; Kramer, D.B.; Kesselheim, A.S. FDA Approval of Cardiac Implantable Electronic Devices via Original and Supplement Premarket Approval Pathways, 1979–2012. JAMA 2014, 311, 385–391.
- Rome, B.N.; Kramer, D.B.; Kesselheim, A.S. Approval of High-Risk Medical Devices in the US: Implications for Clinical Cardiology. Curr. Cardiol. Rep. 2014, 16, 489.
- Swerdlow, C.D.; Asirvatham, S.J.; Ellenbogen, K.A.; Friedman, P.A. Troubleshooting Implanted Cardioverter Defibrillator Sensing Problems I. Circ. Arrhythmia Electrophysiol. 2014, 7, 1237–1261.
- Cingolani, E.; Goldhaber, J.I.; Marbán, E. Next-Generation Pacemakers: From Small Devices to Biological Pacemakers. Nat. Rev. Cardiol. 2018, 15, 139–150.
- Koneru, J.N.; Jones, P.W.; Hammill, E.F.; Wold, N.; Ellenbogen, K.A. Risk Factors and Temporal Trends of Complications Associated With Transvenous Implantable Cardiac Defibrillator Leads. J. Am. Heart Assoc. 2018, 7, e007691.
- DeForge, W.F. Cardiac Pacemakers: A Basic Review of the History and Current Technology. J. Vet. Cardiol. 2019, 22, 40–50.
- El-Chami, M.F.; Rao, B.; Shah, A.D.; Wood, C.; Sayegh, M.; Zakka, P.; Ginn, K.; Pallotta, L.; Evans, B.; Hoskins, M.H.; et al. Long-Term Performance of a Pacing Lead Family: A Single-Center Experience. Heart Rhythm 2019, 16, 572–578.
- Segan, L.; Samuel, R.; Lim, M.; Ridley, D.; Sen, J.; Perrin, M. Incidence of Premature Lead Failure in 2088 TendrilTM Pacing Leads: A Single Centre Experience. Heart Lung Circ. 2020.
- Sengupta, J.; Storey, K.; Casey, S.; Trager, L.; Buescher, M.; Horning, M.; Gornick, C.; Abdelhadi, R.; Tang, C.; Brill, S.; et al. Outcomes Before and After the Recall of a Heart Failure Pacemaker. JAMA Intern. Med. 2020, 180, 198.
- Adelstein, E.; Zhang, L.; Nazeer, H.; Loka, A.; Steckman, D. Increased Incidence of Electrical Abnormalities in a Pacemaker Lead Family. J. Cardiovasc. Electrophysiol. 2021, 32, 1111–1121.
- Faccioli, S.; Del Favero, S.; Visentin, R.; Bonfanti, R.; Iafusco, D.; Rabbone, I.; Marigliano, M.; Schiaffini, R.; Bruttomesso, D.; Cobelli, C. Accuracy of a CGM Sensor in Pediatric Subjects with Type 1 Diabetes. Comparison of Three Insertion Sites: Arm, Abdomen, and Gluteus. J. Diabetes Sci. Technol. 2017, 11, 1147–1154.
- Klonoff, D.C.; Ahn, D.; Drincic, A. Continuous Glucose Monitoring: A Review of the Technology and Clinical Use. Diabetes Res. Clin. Pract. 2017, 133, 178–192.
- Teoh, S.H. Fatigue of Biomaterials: A Review. Int. J. Fatigue 2000, 22, 825–837.
- Zuckerman, D.M.; Brown, P.; Nissen, S.E. Medical Device Recalls and the FDA Approval Process. Arch. Intern. Med. 2011, 171, 1006–1011.
- Jones, A.-A.D.; Mi, G.; Webster, T.J. A Status Report on FDA Approval of Medical Devices Containing Nanostructured Materials. Trends Biotechnol. 2019, 37, 117–120.
- United States Food and Drug Administration. Medical Device Overview. Available online: https://www.fda.gov/industry/regulated-products/medical-device-overview (accessed on 30 May 2021).
- United States Food and Drug Administration. Premarket Approval (PMA). Available online: https://www.fda.gov/medical-devices/premarket-submissions/premarket-approval-pma (accessed on 30 May 2021).
- United States Food and Drug Administration. Premarket Notification 510(k). Available online: https://www.fda.gov/medical-devices/premarket-submissions/premarket-notification-510k (accessed on 30 May 2021).
- Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC (Text with EEA Relevance) Text with EEA RelevanceLex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02017R0745-20170505 (accessed on 7 July 2021).
- European Commission, Health Technology and Cosmetics. Guidelines for Medical Devices. In Medical Devices Directives, Clinical Investigation; Clinical Evaluation: A Guide for Manufacturers and Notified Bodies under Directives 93/42/EEC and 90/385/EEC, MEDDEV 2.7/1, Revision 4. 2016. Available online: https://ec.europa.eu/docsroom/documents/17522/attachments/1/translations/en/renditions/native (accessed on 19 July 2021).
- Auricchio, A.; Gropp, M.; Ludgate, S.; Vardas, P.; Brugada, J.; Priori, S.G. Writing Committee for the European Heart Rhythm Association Guidance Document on Cardiac Rhythm Management Product Performance European Heart Rhythm Association Guidance Document on Cardiac Rhythm Management Product Performance. EP Europace 2006, 8, 313–322.
- Swerdlow, C.D.; Kalahasty, G.; Ellenbogen, K.A. Implantable Cardiac Defibrillator Lead Failure and Management. J. Am. Coll. Cardiol. 2016, 67, 1358–1368.
- Mulpuru, S.K.; Madhavan, M.; McLeod, C.J.; Cha, Y.-M.; Friedman, P.A. Cardiac Pacemakers: Function, Troubleshooting, and Management: Part 1 of a 2-Part Series. J. Am. Coll. Cardiol. 2017, 69, 189–210.
- Swerdlow, C.D.; Hayes, D.L.; Zipes, D.P. Pacemakers and Implantable Cardioverter-Defibrillators. In Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine; Bonow, R.O., Mann, D.L., Zipes, D.P., Libby, P., Braunwald, E., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2012; pp. 745–768.
- Altman, P.A.; Meagher, J.M.; Walsh, D.W.; Hoffmann, D.A. Rotary Bending Fatigue of Coils and Wires Used in Cardiac Lead Design. J. Biomed. Mater. Res. 1998, 43, 21–37.
- Liu, L.; Wang, J.; Yang, W.; Chen, S.J. In Vivo Stress Analysis of a Pacing Lead From an Angiographic Sequence. J. Biomech. Eng. 2011, 133.
- Quinn, T.; Splett, J.; McColskey, J.; Dawson, J.; Smith, D.; Himes, A.; Cooke, D. The Reproducibility of a Proposed Standard Fatigue Test for Cardiac Device Leads. Fourth Symp. Fatigue Fract. Met. Med. Mater. Devices 2019.
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A Stretchable Carbon Nanotube Strain Sensor for Human-Motion Detection. Nat. Nanotechnol. 2011, 6, 296–301.
- Atalay, O.; Kennon, W.R.; Husain, M.D. Textile-Based Weft Knitted Strain Sensors: Effect of Fabric Parameters on Sensor Properties. Sensors 2013, 13, 11114–11127.
- Cai, L.; Song, L.; Luan, P.; Zhang, Q.; Zhang, N.; Gao, Q.; Zhao, D.; Zhang, X.; Tu, M.; Yang, F.; et al. Super-Stretchable, Transparent Carbon Nanotube-Based Capacitive Strain Sensors for Human Motion Detection. Sci. Rep. 2013, 3, 1–9.
- Gioberto, G.; Dunne, L.E. Overlock-Stitched Stretch Sensors: Characterization and Effect of Fabric Property. J. Text. Appar. Technol. Manag. 2013, 8, 1–14. Available online: https://ojs.cnr.ncsu.edu/index.php/JTATM/article/view/4417 (accessed on 20 July 2021).
- Zhang, R.; Deng, H.; Valenca, R.; Jin, J.; Fu, Q.; Bilotti, E.; Peijs, T. Strain Sensing Behaviour of Elastomeric Composite Films Containing Carbon Nanotubes under Cyclic Loading. Compos. Sci. Technol. 2013, 74, 1–5.
- Kang, D.; Pikhitsa, P.V.; Choi, Y.W.; Lee, C.; Shin, S.S.; Piao, L.; Park, B.; Suh, K.-Y.; Kim, T.; Choi, M. Ultrasensitive Mechanical Crack-Based Sensor Inspired by the Spider Sensory System. Nature 2014, 516, 222–226.
- Matsuzaki, R.; Tabayashi, K. Highly Stretchable, Global, and Distributed Local Strain Sensing Line Using GaInSn Electrodes for Wearable Electronics. Adv. Funct. Mater. 2015, 25, 3806–3813.
- Borghetti, M.; Serpelloni, M.; Sardini, E.; Pandini, S. Mechanical Behavior of Strain Sensors Based on PEDOT:PSS and Silver Nanoparticles Inks Deposited on Polymer Substrate by Inkjet Printing. Sens. Actuators A Phys. 2016, 243, 71–80.
- Kim, D.-G.; Kim, J.; Jung, S.-B.; Kim, Y.-S.; Kim, J.-W. Electrically and Mechanically Enhanced Ag Nanowires-Colorless Polyimide Composite Electrode for Flexible Capacitive Sensor. Appl. Surface Sci. 2016, 380, 223–228.
- Liu, H.; Li, Y.; Dai, K.; Zheng, G.; Liu, C.; Shen, C.; Yan, X.; Guo, J.; Guo, Z. Electrically Conductive Thermoplastic Elastomer Nanocomposites at Ultralow Graphene Loading Levels for Strain Sensor Applications. J. Mater. Chem. C 2016, 4, 157–166.
- Yang, T.; Li, X.; Jiang, X.; Lin, S.; Lao, J.; Shi, J.; Zhen, Z.; Li, Z.; Zhu, H. Structural Engineering of Gold Thin Films with Channel Cracks for Ultrasensitive Strain Sensing. Mater. Horiz. 2016, 3, 248–255.
- Yokus, M.A.; Foote, R.; Jur, J.S. Printed Stretchable Interconnects for Smart Garments: Design, Fabrication, and Characterization. IEEE Sens. J. 2016, 16, 7967–7976.
- Cao, X.; Wei, X.; Li, G.; Hu, C.; Dai, K.; Guo, J.; Zheng, G.; Liu, C.; Shen, C.; Guo, Z. Strain Sensing Behaviors of Epoxy Nanocomposites with Carbon Nanotubes under Cyclic Deformation. Polymer 2017, 112, 1–9.
- Keulemans, G.; Ceyssens, F.; Puers, R. An Ionic Liquid Based Strain Sensor for Large Displacement Measurement. Biomed. Microdevices 2017, 19, 1.
- Zheng, Y.; Li, Y.; Li, Z.; Wang, Y.; Dai, K.; Zheng, G.; Liu, C.; Shen, C. The Effect of Filler Dimensionality on the Electromechanical Performance of Polydimethylsiloxane Based Conductive Nanocomposites for Flexible Strain Sensors. Compos. Sci. Technol. 2017, 139, 64–73.
- Atalay, O. Textile-Based, Interdigital, Capacitive, Soft-Strain Sensor for Wearable Applications. Materials 2018, 11, 768.
- Chen, R.; Xu, X.; Yu, D.; Xiao, C.; Liu, M.; Huang, J.; Mao, T.; Zheng, C.; Wang, Z.; Wu, X. Highly Stretchable and Fatigue Resistant Hydrogels with Low Young’s Modulus as Transparent and Flexible Strain Sensors. J. Mater. Chem. C 2018, 6, 11193–11201.
- Seyedin, S.; Moradi, S.; Singh, C.; Razal, J.M. Continuous Production of Stretchable Conductive Multifilaments in Kilometer Scale Enables Facile Knitting of Wearable Strain Sensing Textiles. Appl. Mater. Today 2018, 11, 255–263.
- Zhou, J.; Xu, X.; Xin, Y.; Lubineau, G. Coaxial Thermoplastic Elastomer-Wrapped Carbon Nanotube Fibers for Deformable and Wearable Strain Sensors. Adv. Funct. Mater. 2018, 28, 1705591.
- Gao, J.; Li, B.; Huang, X.; Wang, L.; Lin, L.; Wang, H.; Xue, H. Electrically Conductive and Fluorine Free Superhydrophobic Strain Sensors Based on SiO2/Graphene-Decorated Electrospun Nanofibers for Human Motion Monitoring. Chem. Eng. J. 2019, 373, 298–306.
- Isaia, C.; McNally, D.S.; McMaster, S.A.; Branson, D.T. Effect of Mechanical Preconditioning on the Electrical Properties of Knitted Conductive Textiles during Cyclic Loading. Text. Res. J. 2019, 89, 445–460.
- Jia, Y.; Shen, L.; Liu, J.; Zhou, W.; Du, Y.; Xu, J.; Liu, C.; Zhang, G.; Zhang, Z.; Jiang, F. An Efficient PEDOT-Coated Textile for Wearable Thermoelectric Generators and Strain Sensors. J. Mater. Chem. C 2019, 7, 3496–3502.
- Lai, J.; Zhou, H.; Jin, Z.; Li, S.; Liu, H.; Jin, X.; Luo, C.; Ma, A.; Chen, W. Highly Stretchable, Fatigue-Resistant, Electrically Conductive, and Temperature-Tolerant Ionogels for High-Performance Flexible Sensors. ACS Appl. Mater. Interfaces 2019, 11, 26412–26420.
- Liang, A.; Stewart, R.; Bryan-Kinns, N. Analysis of Sensitivity, Linearity, Hysteresis, Responsiveness, and Fatigue of Textile Knit Stretch Sensors. Sensors 2019, 19, 3618.
- Losaria, P.M.; Yim, J.-H. A Highly Stretchable Large Strain Sensor Based on PEDOT—Thermoplastic Polyurethane Hybrid Prepared via in Situ Vapor Phase Polymerization. J. Ind. Eng. Chem. 2019, 74, 108–117.
- Zou, Q.; Zheng, J.; Su, Q.; Wang, W.; Gao, W.; Ma, Z. A Wave-Inspired Ultrastretchable Strain Sensor with Predictable Cracks. Sens. Actuators A Phys. 2019, 300, 111658.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
10 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




