
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Helena Neves Lobo Silva-Filha | + 1264 word(s) | 1264 | 2021-08-04 10:53:49 | | | |
| 2 | Enzi Gong | Meta information modification | 1264 | 2021-08-10 04:33:21 | | |
Video Upload Options
Insects can act as vectors of etiological agents of different diseases and can be a nuisance to humans, being responsible for health burdens worldwide. Re-emergent and emergent diseases, in particular arboviruses, remain a global challenge as recently shown for the epidemic problems caused by the Zika virus. Microbial larvicides based on entomopathogen bacteria have been successfully used for controlling mosquito and black-fly populations, as an alternative to the conventional classes of chemical insecticides, due to their high effectiveness and environmental safety. Bacillus thuringiensis serovariety (svar.) israelensis (Bti) de Barjac was the first B. thuringiensis (Bt) bacterial serotype identified as active against some Diptera larvae.
1. Overview
Larvicides based on the bacteria Bacillus thuringiensis svar. israelensis (Bti) and Lysinibacillus sphaericus are effective and environmentally safe compounds for the control of dipteran insects of medical importance. They produce crystals that display specific and potent insecticidal activity against larvae. Bti crystals are composed of multiple protoxins: three from the three-domain Cry type family, which bind to different cell receptors in the midgut, and one cytolytic (Cyt1Aa) protoxin that can insert itself into the cell membrane and act as surrogate receptor of the Cry toxins. Together, those toxins display a complex mode of action that shows a low risk of resistance selection. L. sphaericus crystals contain one major binary toxin that display an outstanding persistence in field conditions, which is superior to Bti. However, the action of the Bin toxin based on its interaction with a single receptor is vulnerable for resistance selection in insects. In this review we present the most recent data on the mode of action and synergism of these toxins, resistance issues, and examples of their use worldwide. Data reported in recent years improved our understanding of the mechanism of action of these toxins, showed that their combined use can enhance their activity and counteract resistance, and reinforced their relevance for mosquito control programs in the future years.
2. Larvicidal Activity
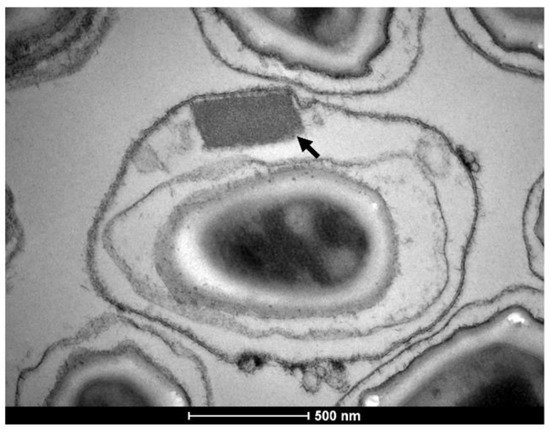
3. Conclusions
References
- Ferguson, N.M. Challenges and opportunities in controlling mosquito-borne infections. Nature 2018, 559, 490–497.
- Baud, D.; Gubler, D.J.; Schaub, B.; Lanteri, M.C.; Musso, D. An update on Zika virus infection. Lancet 2017, 390, 2099–2109.
- Becker, N.; Ludwig, M.; Su, T. Lack of Resistance in Aedes vexans Field Populations after 36 Years of Bacillus thuringiensis subsp. israelensis Applications in the Upper Rhine Valley, Germany. J. Am. Mosq. Control Assoc. 2018, 34, 154–157.
- Derua, Y.A.; Kweka, E.J.; Kisinza, W.N.; Githeko, A.K.; Mosha, F.W. Bacterial larvicides used for malaria vector control in sub-Saharan Africa: Review of their effectiveness and operational feasibility. Parasites Vectors 2019, 12, 426.
- Lacey, L.A. Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J. Am. Mosq. Control Assoc. 2007, 23, 133–163.
- De Barjac, H. A new variety of Bacillus thuringiensis very toxic to mosquitoes: B. thuringiensis var. israelensis serotype 14. Comptes Rendus Seances Hebd. l’Academ. Sci. Ser. D 1978, 286, 797–800.
- Kellen, W.R.; Clark, T.B.; Lindegren, J.E.; Ho, B.C.; Rogoff, M.H.; Singer, S. Bacillus sphaericus Neide as a pathogen of mosquitoes. J. Invertebr. Pathol. 1965, 7, 442–448.
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41.
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberón, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431.
- Ben-Dov, E. Bacillus thuringiensis subsp. israelensis and its dipteran-specific toxins. Toxins 2014, 6, 1222–1243.
- Valtierra-de-Luis, D.; Villanueva, M.; Berry, C.; Caballero, P. Potential for Bacillus thuringiensis and Other Bacterial Toxins as Biological Control Agents to Combat Dipteran Pests of Medical and Agronomic Importance. Toxins 2020, 12, 773.
- Soberón, M.; Fernández, L.E.; Pérez, C.; Gill, S.S.; Bravo, A. Mode of action of mosquitocidal Bacillus thuringiensis toxins. Toxicon 2007, 49, 597–600.
- Charles, J.F.; Nielsen-LeRoux, C.; Delecluse, A. Bacillus sphaericus toxins: Molecular biology and mode of action. Ann. Rev. Entomol. 1996, 41, 451–472.
- Berry, C. The bacterium, Lysinibacillus sphaericus, as an insect pathogen. J. Invertebr. Pathol. 2012, 109, 1–10.
- Goldberg, L.H.; Margalit, J. A bacterial spore demostrating rapid larvicidal activity against Anopheles segentii, Uranotaenia unguiculata, Culex univitatus, Aedes aegypti and Culex pipiens. Mosq. News 1978, 37, 355–358.
- Crickmore, N.; Berry, C.; Panneerselvam, S.; Mishra, R.; Connor, T.R.; Bonning, B.C. A structure-based nomenclature for Bacillus thuringiensis and other bacteria-derived pesticidal proteins. J. Invertebr. Pathol. 2020, 107438.
- Berry, C.; O’Neil, S.; Ben-Dov, E.; Jones, A.F.; Murphy, L.; Quail, M.A.; Holden, M.T.; Harris, D.; Zaritsky, A.; Parkhill, J. Complete sequence and organization of pBtoxis, the toxin-coding plasmid of Bacillus thuringiensis subsp. israelensis. App. Environ. Microbiol. 2002, 68, 5082–5095.
- Becker, N. Bacterial control of vector mosquitoes and black flies. In Entomopathogenic Bacteria: From Laboratory to Filed Application; Charles, J.F., Delecluse, A., Nielsen-LeRoux, C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 384–398.
- Ahmed, I.; Yokota, A.; Yamazoe, A.; Fujiwara, T. Proposal of Lysinibacillus boronitolerans gen. nov. sp. nov., and transfer of Bacillus fusiformis to Lysinibacillus fusiformis comb. nov. and Bacillus sphaericus to Lysinibacillus sphaericus comb. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 1117–1125.
- Singer, S. Isolation and development of bacterial pathogens in vectors. In Biological Regulationof Vectors; No. (NIH) 77-1180; DHEW Publication: Bethesda, MD, USA, 1977; pp. 3–18.
- Yuan, Z.; Neilsen-LeRoux, C.; Pasteur, N.; Delecluse, A.; Charles, J.F.; Frutos, R. Cloning and expression of the binary toxin genes of Bacillus sphaericus C3-41 in a crystal minus B. thuringiensis subsp. israelensis. Acta Microbiol. Sin. 1999, 39, 29–35.
- Weiser, J. A mosquito-virulent Bacillus sphaericus in adult Simulium damnosum from northern Nigeria. Zent. Mikrobiol. 1984, 139, 57–60.
- Krych, V.K.; Johnson, J.L.; Yousten, A.A. Deoxyribonucleic acid homologies among strans of Bacillus sphaericus. Int. J. Syst. Bacteriol. 1980, 30, 253–256.
- Xu, K.; Yuan, Z.; Rayner, S.; Hu, X. Genome comparison provides molecular insights into the phylogeny of the reassigned new genus Lysinibacillus. BMC Genom. 2015, 16, 140.
- Wirth, M.C. Mosquito resistance to bacterial larvicidal proteins. Open J. Toxicol. 2010, 3, 101–115.
- Silva Filha, M.H.N.L.; Berry, C.; Regis, L.N. Lysinibacillus sphaericus: Toxins and mode of action, applications for mosquito control and resistance management. In Insect Midgut and Insecticidal Proteins; Dhadialla, T.S., Gill, S.S., Eds.; Academic Press: Oxford, UK, 2014; Volume 47, pp. 89–176.
- Ferreira, L.M.; Silva-Filha, M.H.N.L. Bacterial larvicides for vector control: Mode of action of toxins and implications for resistance. Biocontrol Sci. Technol. 2013, 23, 1137–1168.




