Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Benjamin Pradere | + 1102 word(s) | 1102 | 2021-07-08 11:51:09 | | | |
| 2 | Lily Guo | + 105 word(s) | 1207 | 2021-08-10 02:51:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pradere, B. Endoscopic Papillary Abnormalities and EPSR. Encyclopedia. Available online: https://encyclopedia.pub/entry/12944 (accessed on 08 February 2026).
Pradere B. Endoscopic Papillary Abnormalities and EPSR. Encyclopedia. Available at: https://encyclopedia.pub/entry/12944. Accessed February 08, 2026.
Pradere, Benjamin. "Endoscopic Papillary Abnormalities and EPSR" Encyclopedia, https://encyclopedia.pub/entry/12944 (accessed February 08, 2026).
Pradere, B. (2021, August 09). Endoscopic Papillary Abnormalities and EPSR. In Encyclopedia. https://encyclopedia.pub/entry/12944
Pradere, Benjamin. "Endoscopic Papillary Abnormalities and EPSR." Encyclopedia. Web. 09 August, 2021.
Copy Citation
The increasing efficiency of the different lasers and the improved performance of endoscopic devices have led to smaller stone fragments that impact the accuracy of microscopic evaluation (morphological and infrared). Before the stone destruction, the urologist has the opportunity to analyze the stone and the papillary abnormalities endoscopically (endoscopic papillary recognition (EPR) and endoscopic stone recognition (ESR)). Our objective was to evaluate the value for those endoscopic descriptions.
papilla abnormalities
endoscopy
stone
kidney
1. Introduction
The number of endoscopic treatments of urinary stones increases all over the world.
As previously demonstrated by Daudon et al. [1][2][3][4][5], the morpho-constitutional stone analysis plays a major role in identifying its etiology and thus consider its risk of recurrence. The increasing efficiency of lasers in “dusting” and “popcorning” modes [6][7][8][9] and the improved performance of endoscopic devices led to smaller stone fragments, which reduce the accuracy of the microscopic study (morphological and infrared) by the lack of components representativeness (48.6% of the stones have a mixed composition [10])). Moreover, Keller et al. [8][9] have demonstrated that laser-based Thulium fiber changed in stone composition in the infrared spectra that resulted in insufficient information of stone powder examination (Figure 1).
Since Randall’s works [11] in the 1930s, it is known that papillary calculi resulted from subepithelial lesions [12][13][14][15][16][17]. The advent quality of images with flexible retrograde ureteroscopy has allowed the in vivo description of papillary abnormalities [18][19][20][21] that can be related to various lithogenesis mechanisms [22][23][24][25][26][27][28].
Before the destruction of the stone, the urologist has the opportunity to hold a key role in stone prevention by recognizing the papillary abnormalities (endoscopic papillary recognition (EPR)) and the stone’s type (endoscopic stone recognition (ESR)).
2. Study Selection and Characteristics
After a bibliographic search and the removal of duplicates, a total of 54 articles were screened.
After full text assessment, a total of 17 publications met the inclusion criteria (Figure 1 and Table 1).
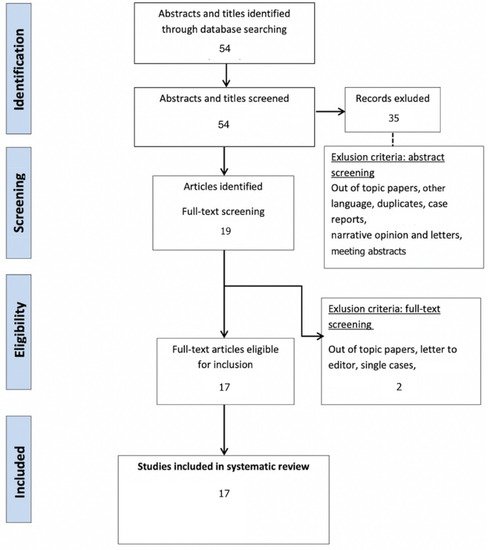
Figure 1. Systematic review PRISMA flow diagram.
Table 1. Identified and selected publications on ESR and EPR
| Type | Subject | Number | Year | |
|---|---|---|---|---|
| Low [18] | EPR | RP | 57 | 1997 |
| Darves-Bornoz [27] | EPR | RP in pediatric stone formers | 8 | 2019 |
| Strohmaier [29] | EPR | RP and number of stone episodes | 100 | 2013 |
| Kim [30] | EPR | RP and number of stones | 17 | 2005 |
| Wang [31] | EPR | Low RP and CaOx stone formers | 42 | 2014 |
| Matlaga [19] | EPR | Anchored stone and RP | 23 | 2006 |
| Borofsky [20] | EPR | Grading Score | 342 | 2016 |
| Almeras [21] | EPR | Classification | 164 | 2016 |
| Jaeger [22] | EPR | Struvite | 119 | 2016 |
| Cohen [23] | EPR | Score use and correlation RP/pitting | 76 | 2019 |
| Borofsky [24] | EPR | Anchored stone/pitting | 28 | 2019 |
| Almeras [25] | EPR | Classification use and correlations RP, stones, … | 88 | 2021 |
| Pless [26] | EPR | Score use | 46 | 2019 |
| Sabaté [28] | EPR | Description | 41 | 2020 |
| Fernandez [32] | EPR | CP plugs detection by AI | 200 | 2019 |
| Estrade [10] | ESR | Correlation endoscopy/microscopy | 399 | 2020 |
| Marien [33] | EPR | Review | 13 | 2016 |
3. Endoscopic Papillary Abnormalities and Stone Recognition (EPSR) during Flexible Ureteroscopy
In the last century, eating habits have changed with an increased intake of salt, animal proteins, and refined sugar and a decreased intake of vegetables [34]. That consequently implied a change in stone composition and a prevalence increased [35]. These changes are especially concerning COM (subtype Ia [1]) that are mostly correlated with the prevalence of RP [19][25], low fluid intake [36], and the evolution of dietary habits [35][37][38].
As previously demonstrated by Daudon et al. [1][2][3][4][5], the morpho-constitutional stone analysis plays a major role in identifying the etiology of the stone disease and thus in stone recurrence. The increasing efficiency of lasers in “dusting” and “popcorning” modes [6][7][8][9] decreases the size of stone fragments and the accuracy of the microscopic study (morphological and infrared), thus impairing the etiologic investigation’s results. This lack of data may be balanced by EPR-ESR [10][18][20][25] and the papillary anchored stones analysis.
However, some limitations are still debated. First, the literature addressing the endoscopic papilla and stone recognition is poor and most of the published studies were from a single institution and had a small cohort.
As the endoscopic interpretations of the papillary abnormalities are only based on endourologist descriptions, their reliability, especially concerning the type of deposits (RP, plugs) and the origin of the crystallization (RP anchored, intraductal origin), remain a potential limitation and a potential interpretation bias [10][18][19][20][21][25][28].
The main problem in recognizing papillary abnormalities and stones composition is the very large array of descriptions and entities [20][21][25]. Thus, the learning curve for EPR and ESR is long and difficult, it has been shown that a perfect recognition of the stone was obtained in only 40.7% of the cases for urologist in training who benefited from nine specific teaching classes [39]. Nevertheless, the concordance between expert endoscopic description and microscopic analysis was much better with 86.1% (COM), 85% (COD), 91% (UA), 79% (CP), 65% (Brushite), 75% (Struvite), and 100% (Cystine) [10]. Although learning this specific skill might be time-consuming, training is certainly the key until the development of recognition models created by artificial intelligence (AI). In vitro, automatic detection of kidney stones composition from digital stone pictures has been described with a prediction of 94% (UA), 90% (COM), 86% (Struvite), 75% (Cystine), and 71% (Brushite) [40]. AI is about to be applied to in vivo validated endoscopic pictures, but stone morphological laser changes and heterogenous vision quality may hamper its development. AI will also be used to simplify EPR. Indeed, the efficacy of deep learning to segment the renal papilla, plaque, and plugs has already been described 46].
The backbone of ESR and EPR remains the recognition, which is based on a good intraoperative vision. Therefore, some variables have to be considered, such as fiberoptic devices that do not have high-definition vision quality [41], single-use and reusable digital ureteroscopes that do not seem to be equivalent in term of color, brightness, and definition [42][43][44]; and PCNL that cannot allow a complete exploration of the papillae. Today and for those reasons, the best way to proceed EPR and ESR is the use of digital flexible ureteroscopes.
Recently, it has been shown that lasers impacted the infrared analysis regarding stone composition [8][9]. Moreover, recognition could be biased by the dusting settings (high frequency and long pulse) that might change the surface appearance (Figure 2) especially due to a carbonization effect (Figure 3) (mainly described with Thulium Fiber Laser).
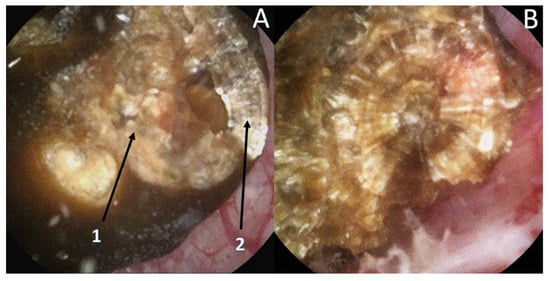
Figure 2. Stone morphological laser-induced changes. Example of a pure COM stone. (A1) After dusting settings use: Loss of the typical radiating organization of the internal layers aspect. (A2) After fragmentation settings use: visualization of the typical radiating organization of the internal layers. (B) Pure fragmentation in progress aspect: respect of the internal structure.
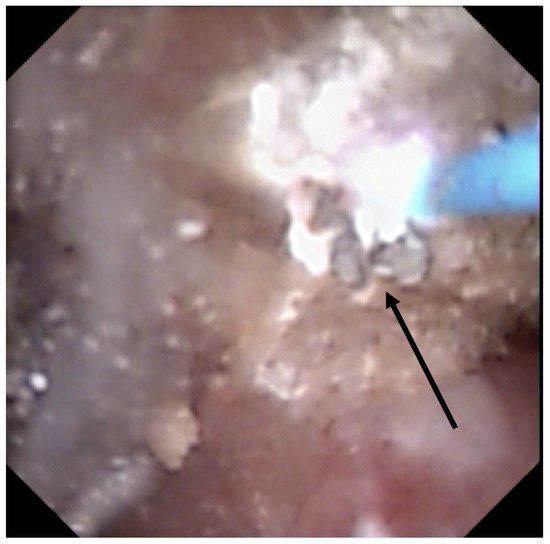
Figure 3. Stone morphological laser-induced changes. Carbonization (shown by the black arrow) during TFL treatment, which can be misleading for ESR.
To limit these biases, an initial transection of the stone has been proposed but remains difficult, time consuming, and provides more fragments to treat. Therefore, the use of the fragmentation setting might help to properly assess the internal layers and the use of dusting should be used only after the complete description [8][9].
Although it represents the origin of crystallization, stone analysis and ESR often miss the nucleus structure analysis due to stone destruction. Hence, the additional EPR analysis could provide essential information regarding the lithogenesis mechanism and avoid misdiagnosis of high-risk diseases like distal tubular acidosis. Although it is still under evaluation, the intensity and the amount of the papillary abnormalities may also have a prognostic value regarding stone recurrence.
Combining these complementary methods should be gathered in a single process of endoscopic papilla and stone recognition (EPSR). It could support the preventive care of the stone formers in improving the diagnosis of the lithogenesis mechanism and in identifying the high-risk stone formers.
In this way, the urologist should play a key role in lithiasis prevention and stone formers’ care improvement.
References
- Corrales, M.; Doizi, S.; Barghouthy, Y.; Traxer, O.; Daudon, M. Classification of Stones According to Michel Daudon: A Narrative Review. Eur. Urol. Focus 2021, 7, 13–21.
- Daudon, M.; Jungers, P.; Bazin, D.; Williams, J.C., Jr. Recurrence rates of urinary calculi according to stone composition and morphology. Urolithiasis 2018, 46, 459–470.
- Cloutier, J.; Villa, L.; Traxer, O.; Daudon, M. Kidney stone analysis: “Give me your stone, I will tell you who you are!”. World J. Urol. 2015, 33, 157–169.
- Daudon, M.; Dessombz, A.; Frochot, V.; Letavernier, E.; Haymann, J.-P.; Jungers, P.; Bazin, D. Comprehensive morpho-constitutional analysis of urinary stones improves etiological diagnosis and therapeutic strategy of nephrolithiasis. Comptes Rendus Chim. 2016, 19, 1470–1491.
- Dessombz, A.; Letavernier, E.; Haymann, J.-P.; Bazin, D.; Daudon, M. Calcium Phosphate Stone Morphology Can Reliably Predict Distal Renal Tubular Acidosis. J. Urol. 2015, 193, 1564–1569.
- Emiliani, E.; Talso, M.; Cho, S.-Y.; Baghdadi, M.; Mahmoud, S.; Pinheiro, H.; Traxer, O. Optimal Settings for the Noncontact Holmium: YAG Stone Fragmentation Popcorn Technique. J. Urol. 2017, 198, 702–706.
- Doizi, S.; Keller, E.X.; De Coninck, V.; Traxer, O. Dusting technique for lithotripsy: What does it mean? Nat. Rev. Urol. 2018, 15, 653–654.
- Keller, E.X.; De Coninck, V.; Audouin, M.; Doizi, S.; Bazin, D.; Daudon, M.; Traxer, O. Fragments and dust after Holmium laser lithotripsy with or without “Moses technology”: How are they different? J. Biophotonics 2018, 12, e201800227.
- Keller, E.X.; De Coninck, V.; Doizi, S.; Daudon, M.; Traxer, O. Thulium fiber laser: Ready to dust all urinary stone composition types? World J. Urol. 2021, 39, 1693–1698.
- Estrade, V.; De Senneville, B.D.; Meria, P.; Almeras, C.; Bladou, F.; Bernhard, J.; Robert, G.; Traxer, O.; Daudon, M. Toward improved endoscopic examination of urinary stones: A concordance study between endoscopic digital pictures vs microscopy. BJU Int. 2020.
- Randall, A. The origin and growth of renal calculi. Ann. Surg. 1937, 105, 1009–1027.
- Daudon, M.; Bazin, D.; Letavernier, E. Randall’s plaque as the origin of calcium oxalate kidney stones. Urolithiasis 2014, 43 (Suppl. 1), 5–11.
- Williams, J.C., Jr.; Borofsky, M.S.; Bledsoe, S.B.; Evan, A.P.; Coe, F.L.; Worcester, E.M.; Lingeman, J.E. Papillary ductal plugging is a mechanism for early stone retention in brushite stone disease. J. Urol. 2018, 199, 186–192.
- Coe, F.L.; Evan, A.P.; Worcester, E.M.; Lingeman, J.E. Three pathways for human kidney stone formation. Urol. Res. 2010, 38, 147–160.
- Evan, A.; Lingeman, J.; Coe, F.L.; Worcester, E. Randall’s plaque: Pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int. 2006, 69, 1313–1318.
- Evan, A.P.; Lingeman, J.E.; Coe, F.L.; Parks, J.H.; Bledsoe, S.B.; Shao, Y.; Sommer, A.J.; Paterson, R.F.; Kuo, R.L.; Grynpas, M. Randall’s plaque of patients with nephrolithiasis begins in basement mem-branes of thin loops of Henle. J. Clin. Investig. 2003, 111, 607–616.
- Evan, A.; Lingeman, J.; Coe, F.; Shao, Y.; Miller, N.; Matlaga, B.; Phillips, C.; Sommer, A.; Worcester, E. Renal histopathology of stone-forming patients with distal renal tubular acidosis. Kidney Int. 2007, 71, 795–801.
- Low, R.K.; Stoller, M.L. Endoscopic mapping of renal papillae for Randall’s plaques in patients with urinary stone dis-ease. J. Urol. 1997, 158, 2062–2064.
- Matlaga, B.R.; Williams, J.; Kim, S.C.; Kuo, R.L.; Evan, A.P.; Bledsoe, S.B.; Coe, F.L.; Worcester, E.M.; Munch, L.C.; Lingeman, J.E. Endoscopic Evidence of Calculus Attachment to Randall’s Plaque. J. Urol. 2006, 175, 1720–1724.
- Borofsky, M.S.; Paonessa, J.E.; Evan, A.P.; Williams, J.C.; Coe, F.L.; Worcester, E.M.; Lingeman, J.E. A proposed grading system to standardize the description of renal papil-lary appearance at the time of endoscopy in patients with nephrolithiasis. J. Endourol. 2016, 30, 122–127.
- Almeras, C.; Daudon, M.; Ploussard, G.; Gautier, J.R.; Traxer, O.; Meria, P. Endoscopic description of renal papillary abnormalities in stone disease by flexible ureteroscopy: A proposed classification of severity and type. World J. Urol. 2016, 34, 1575–1582.
- Jaeger, C.D.; Rule, A.D.; Mehta, R.A.; Vaughan, L.E.; Vrtiska, T.J.; Holmes, D.R.; McCollough, C.M.; Ziegelmann, M.J.; Hernandez, L.P.H.; Lieske, J.C.; et al. Endoscopic and Pathologic Characterization of Papillary Architecture in Struvite Stone Formers. Urology 2016, 90, 39–44.
- Cohen, A.J.; Borofsky, M.S.; Anderson, B.B.; Dauw, C.A.; Gillen, D.L.; Gerber, G.S.; Worcester, E.M.; Coe, F.L.; Lingeman, J.E. Endoscopic Evidence That Randall’s Plaque is Associated with Surface Erosion of the Renal Papilla. J. Endourol. 2017, 31, 85–90.
- Borofsky, M.S.; Williams, J.C., Jr.; Dauw, C.A.; Cohen, A.; Evan, A.C.; Coe, F.L.; Worcester, E.M.; Lingeman, J.E.; Coe, F. Association between Randall’s Plaque Stone Anchors and Renal Papillary Pits. J. Endourol. 2019, 33, 337–342.
- Almeras, C.; Daudon, M.; Estrade, V.; Gautier, J.R.; Traxer, O.; Meria, P. Classification of the renal papillary abnormalities by flexible ureteroscopy: Evaluation of the 2016 version and update. World J. Urol. 2021, 39, 177–185.
- Pless, M.S.; Williams, J.C.; Andreassen, K.H.; Jung, H.; Osther, S.S.; Christensen, D.R.; Osther, P.J.S. Endoscopic observations as a tool to define underlying pathology in kidney stone formers. World J. Urol. 2019, 37, 2207–2215.
- Darves-Bornoz, A.L.; Marien, T.; Thomas, J.; Fiscus, G.; Brock, J.; Clayton, D.B.; Miller, N.L. Renal Papillary Mapping and Quantification of Randall’s Plaque in Pediatric Calcium Oxalate Stone Formers. J. Endourol. 2019, 33, 863–867.
- Arroyo, X.S.; Freixedas, F.G.; Quetglas, J.L.B.; Garcia, J.G.; Ayala, E.P. Relationship of endoscopic lesions of the renal papilla with type of renal stone and 24 h urine analysis. BMC Urol. 2020, 20, 1–6.
- Strohmaier, W.L.; Hörmann, M.; Schubert, G. Papillary calcifications: A new prognostic factor in idiopathic calcium oxalate urolithiasis. Urolithiasis 2013, 41, 475–479.
- Kim, S.C.; Coe, F.L.; Tinmouth, W.W.; Kuo, R.L.; Paterson, R.F.; Parks, J.H.; Munch, L.C.; Evan, A.P.; Lingeman, J.E. Stone formation is proportional to papillary surface coverage by randall’s plaque. J. Urol. 2005, 173, 117–119.
- Wang, X.; Krambeck, A.E.; Williams, J.C.; Tang, X.; Rule, A.D.; Zhao, F.; Bergstralh, E.; Haskic, Z.; Edeh, S.; Holmes, D.R.; et al. Distinguishing Characteristics of Idiopathic Calcium Oxalate Kidney Stone Formers with Low Amounts of Randall’s Plaque. Clin. J. Am. Soc. Nephrol. 2014, 9, 1757–1763.
- Fernandez, K.; Korinek, M.; Camp, J.; Lieske, J.; Holmes, D. Automatic detection of calcium phosphate deposit plugs at the terminal ends of kidney tubules. Healthc. Technol. Lett. 2019, 6, 271–274.
- Marien, T.P.; Miller, N.L. Advanced ureteroscopy for stone disease: Characteristics of renal papillae in kidney stone formers. Minerva Urol. Nefrol. 2016, 68, 496–515.
- Siener, R.; Hesse, A. Fluid intake and epidemiology of urolithiasis. Eur. J. Clin. Nutr. 2003, 57, S47–S51.
- Kittanamongkolchai, W.; Vaughan, L.E.; Enders, F.T.; Dhondup, T.; Mehta, R.A.; Krambeck, A.E.; McCollough, C.H.; Vrtiska, T.J.; Lieske, J.C.; Rule, A.D. The Changing Incidence and Presentation of Urinary Stones Over 3 Decades. Mayo Clin. Proc. 2018, 93, 291–299.
- Coe, F.L.; Worcester, F.L.C.E.M.; Evan, A.P. Idiopathic hypercalciuria and formation of calcium renal stones. Nat. Rev. Nephrol. 2016, 12, 519–533.
- Robertson, W.; Peacock, M. The Pattern of Urinary Stone Disease in Leeds and in the United Kingdom in Relation to Animal Protein Intake during the Period 1960–1980. Urol. Int. 1982, 37, 394–399.
- Siener, R. The Effect of Different Diets on Urine Composition and the Risk of Calcium Oxalate Crystallisation in Healthy Subjects. Eur. Urol. 2002, 42, 289–296.
- Bergot, C.; Robert, G.; Bernhard, J.C.; Ferrière, J.-M.; Bensadoun, H.; Capon, G.; Estrade, V. The basis of endoscopic stones recognition, a prospective monocentric study [Article in French]. Prog. Urol. 2019, 29, 312–317.
- Black, K.M.; Law, H.; Aldoukhi, A.; Deng, J.; Ghani, K.R. Deep learning computer vision algorithm for detecting kidney stone composition. BJU Int. 2020, 125, 920–924.
- Keller, E.X.; Doizi, S.; Villa, L.; Traxer, O. Which flexible ureteroscope is the best for upper tract urothelial carcinoma treatment? World J. Urol. 2019, 37, 2325–2333.
- Marchini, G.S.; Batagello, C.A.; Monga, M.; Torricelli, F.C.M.; Vicentini, F.C.; Danilovic, A.; Srougi, M.; Nahas, W.C.; Mazzucchi, E. In Vitro Evaluation of Single-Use Digital Flexible Ureteroscopes: A Practical Comparison for a Patient-Centered Approach. J. Endourol. 2018, 32, 184–191.
- Dragos, L.B.; Somani, B.; Keller, E.X.; De Coninck, V.M.J.; Herrero, M.R.-M.; Kamphuis, G.M.; Bres-Niewada, E.; Sener, E.T.; Doizi, S.; Wiseman, O.J.; et al. Characteristics of current digital single-use flexible ureteroscopes versus their reusable counterparts: An in-vitro comparative analysis. Transl. Androl. Urol. 2019, 8 (Suppl. 4), S359–S370.
- Patil, A.; Agrawal, S.; Singh, A.; Ganpule, A.; Sabnis, R.; Desai, M. A Single-Center Prospective Comparative Study of Two Single-Use Flexible Ureteroscopes: LithoVue (Boston Scientific, USA) and Uscope PU3022a (Zhuhai Pusen, China). J. Endourol. 2021, 35, 274–278.
More
Information
Subjects:
Medicine, General & Internal
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
757
Revisions:
2 times
(View History)
Update Date:
10 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




