
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Saglara S. Mandzhieva | + 1962 word(s) | 1962 | 2021-07-06 06:22:31 | | | |
| 2 | Dean Liu | + 67 word(s) | 2029 | 2021-08-06 09:42:11 | | |
Video Upload Options
Organoclays are effective adsorbents, prepared by intercalation or surface grafting of clays and clay minerals with various organic compounds. Organoclays have important practical applications as adsorbents of a wide range of organic pollutants and some inorganic contaminants.
1. Introduction
The properties of phyllosilicate (layered) clay minerals, such as great specific surface area, chemical stability, variable expansibility, reactive functional groups, and surface charge make them perfect adsorbents for a wide range of environmental pollutants [1][2][3]. The advantages of clays as adsorbents are also related to the following: they are naturally widespread, commonly nontoxic, easily mined, and relatively cheap materials. For example, more than 16 Mt of bentonite clays and 44 Mt of kaolin clays are mined annually at present [4]. The price of clay is 0.05–0.46 USD/kg, and the cost of montmorillonite is about 0.04–0.12
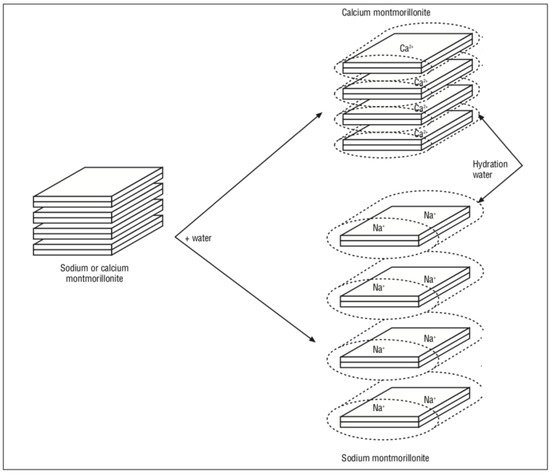
Most clay minerals’ outer and inner surface is hydrophilic and polar, resulting in high affinities towards low and high-molecular, mainly cationic, substances. However, their anionic substance-adsorbing capacity is low: less than 5 cmol/kg for smectites [5] and not more than 2 cmol/kg for kaolinites [6]. Although clay minerals have a hydrophile surface, their significant area and volume of small pores allow the clays to adsorb some amount of the nonionogenic substances [7]. The modification of natural clays by organic surface-active substances (surfactants) makes it possible to refine their pore structure and impart the hydrophobic property to their surface if necessary.
The synthesis of special organo-mineral materials (organoclays) by intercalation or by grafting organic surfactants into expanding clay minerals has attracted a great deal of attention over the past two decades [8]. Organoclays have important practical applications as adsorbents of organic pollutants [9][10] and as components in the formation of clay polymer nanocomposites [11]. The theoretical basics for intercalating surfactants into the interlayer space of clay minerals are well understood ([8][10][11] etc.). Recently, there has been growing interest in the synthesis of organoclays using zwitterionic substances that form complex adsorption positions and nonionic surfactants with low toxicity and biodegradability [8][12].
2. Organoclays and Their Properties
| Surfactants | Chemical Formula | Surfactants | Chemical Formula |
|---|---|---|---|
| Methyl tallow bis-2-hydroxyethyl quaternary ammonium | 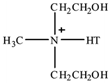 |
Polyoxy propylene methyl diethyl ammonium | 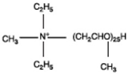 |
| Dimethyl dehydrogenated tallow quaternary ammonium | 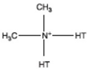 |
Octadecyl amine | 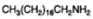 |
| Dimethyl dehydrogenated tallow 2–ethylhexyl quaternary ammonium | 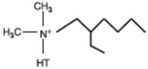 |
Dimethyl octadecyl amine | 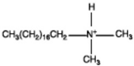 |
| Dimethyl benzyl hydrogenated tallow quaternary ammonium | 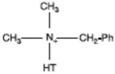 |
Hexadecyl trimetyl ammonium | 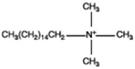 |
| Dimethyl dialkyl (tallow, presented by T) ammonium | 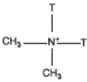 |
Dodecyl triphnyl phosphonium | 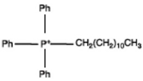 |
| Trioctyl methyl ammonium | 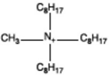 |
Hexadecyl tributyl phosphonium | 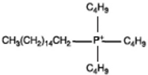 |
| Dipilyoxy ethylene alkyl (COCO) methyl ammonium | 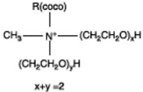 |
Dodecyl trimethyl phosphonium | 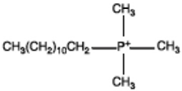 |
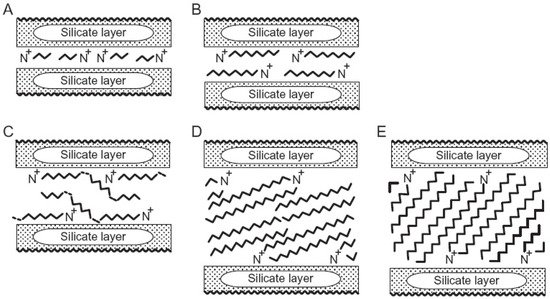
References
- Churchman, G.J.; Gates, W.P.; Theng, B.K.G.; Yuan, G. Clays and clay minerals for pollution control. In Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 625–675.
- Srinivasan, R. Advances in Application of Natural Clay and Its Composites in Removal of Biological, Organic, and Inorganic Contaminants from Drinking Water. Adv. Mater. Sci. Eng. 2011, 2011, 1–17.
- Sarkar, B.; Rusmin, R.; Ugochukwu, U.C.; Mukhopadhyay, R.; Manjaiah, K.M. Modified clay minerals for environmental applications. In Modified Clay and Zeolite Nanocomposite Materials: Environmental and Pharmaceutical Applications; Mercurio, M., Sarkar, B., Langella, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–127.
- United States Geological Survey. National Minerals Information Center. Clays Statistics and Information. Annual Publications. Mineral Commodity Summaries. 2021. Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-clays.pdf (accessed on 1 January 2021).
- Borchardt, G. Smectites. In Minerals in Soil Environments, 2nd ed.; Dixon, J.B., Weed, S.B., Eds.; SSSA Book Series; Soil Science Society of America: Madison, WI, USA, 1989; pp. 675–727.
- Dixon, J.B. Kaolin and Serpentine Group Minerals. Methods Soil Anal. Part Mineral. Methods 2018, 467–525.
- Dehmani, Y.; Ed-Dra, A.; Zennouhi, O.; Bouymajane, A.; Rhazi Filali, F.; Nassiri, L.; Abouarnadasse, S. Chemical character-ization and adsorption of oil mill wastewater on Moroccan clay in order to be used in the agricultural field. Heliyon 2020, 6, e03164.
- He, H.; Ma, L.; Zhu, J.; Frost, R.L.; Theng, B.K.; Bergaya, F. Synthesis of organoclays: A critical review and some unresolved issues. Appl. Clay Sci. 2014, 100, 22–28.
- Bergaya, F.; Lagaly, G. Chapter 1. General introduction: Clays, clay minerals and clay science. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5A, pp. 1–19.
- Theng, B.K.G.; Churchman, G.J.; Gates, W.P.; Yuan, G. Organically modified clays for pollutant uptake and environmental protection. In Soil Mineral-Microbe-Organic Interactions: Theories and Applications; Huang, Q., Huang, P.M., Violante, A., Eds.; Springer: Berlin, Germany, 2008; pp. 145–174.
- Theng, B.K.G. Formation and Properties of Clay-Polymer Complexes, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; 526p.
- Guégan, R.; Veron, E.; Le Forestier, L.; Ogawa, M.; Cadars, S. Structure and Dynamics of Nonionic Surfactant Aggregates in Layered Materials. Langmuir 2017, 33, 9759–9771.
- Barrer, R.M.; MacLeod, D.M. Intercalation and sorption by montmorillonite. Trans. Faraday Soc. 1954, 50, 980–989.
- Barrer, R.M.; MacLeod, D.M. Activation of montmorillonite by ion exchange and sorption complexes of tetra-alkyl ammonium montmorillonites. Trans. Faraday Soc. 1955, 51, 1290–1300.
- Guégan, R. Organoclay applications and limits in the environment. Comptes Rendus Chim. 2019, 22, 132–141.
- Lagaly, G.; Ogawa, M.; Dékány, I. Clay mineral–organic interactions. In Handbook of Clay Science. Developments in Clay Science; Bergaya, F., Lagaly., G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 435–505.
- Brixie, J.M.; Boyd, S.A. Treatment of Contaminated Soils with Organoclays to Reduce Leachable Pentachlorophenol. J. Environ. Qual. 1994, 23, 1283–1290.
- Boeva, N.M.; Bocharnikova, Y.I.; Nasedkin, V.V.; Belousov, P.E.; Demidenok, K.V. Thermal analysis as an express method for assessing the quality and quantity of natural and synthesized organoclays. Nanotechnologies Russ. 2013, 8, 205–208.
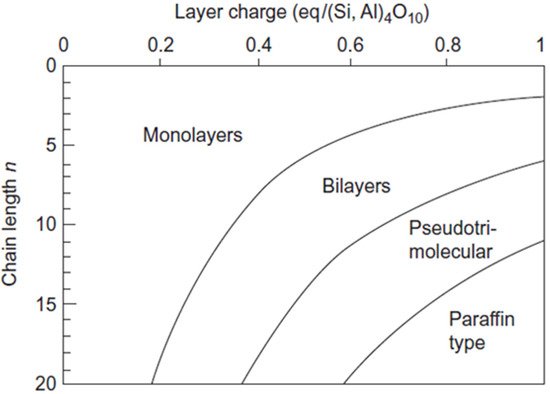
- Lagaly, G. Layer charge determination by alkylammonium ions. In Clay Minerals Society Workshop Lectures: Layer Charge Characteristics of 2:1 Silicate Clay Minerals; Mermut, A.R., Ed.; The Clay Minerals Society: Boulder, CO, USA, 1994; Volume 6, pp. 1–46.
- Lee, S.Y.; Kim, S.J. Expansion characteristics of organoclay as a precursor to nanocomposites. Colloids Surfaces A Physicochem. Eng. Asp. 2002, 211, 19–26.
- Churakov, S.V. Ab initio study of edge sites reactivity on pyrophyllite. In Proceedings of the International Meeting “Clays in Natural & Engineered Barriers for Radioactive Waste Confinement”, Tours, France, 14–18 March 2005; pp. 219–220.
- Popov, V.G.; Abdrakhmanov, R.F. Ion Exchange Concept in Genetic Hydrogeochemistry; Gilem, Bashkir Encyclopedia: Ufa, Russia, 2013; 356p. (In Russian)
- Civan, F. Reservoir Formation Damage: Fundamentals, Modeling, Assessment, and Mitigation, 3rd ed.; Gulf Professional Publishing: Houston, TX, USA, 2015; 1042p.
- Sparks, D.L. Environmental Soil Chemistry; Elsevier: Amsterdam, The Netherlands, 2003; 352p.
- Ray, S.S. Introduction to environmentally friendly polymer nanocomposites. In Environmentally Friendly Polymer Nanocomposites, Types, Processing and Properties, 1st ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 3–24.
- Brindley, G.W. Ethylene glycol and glycerol complexes of smectites and vermiculites. Clay Miner. 1966, 6, 237–259.
- Cloos, P.; Laura, R.D.; Badot, C. Adsorption of ethylene diamine on montmorillonite saturated with different cations-V: Ammonium- and triethylammonium-montmorillonite: Ion exchange, protonation and hydrogen-bonding. Clays Clay Miner. 1975, 23, 417–423.
- Farmer, V.C.; Mortland, M.M. An infrared study of the co-ordination of pyridine and water to exchangeable cations in montmorillonite and saponite. J. Chem. Soc. A 1966, 344–351.
- Guégan, R.; De Oliveira, T.; Le Gleuher, J.; Sugahara, Y. Tuning down the environmental interests of organoclays for emerging pollutants: Pharmaceuticals in presence of electrolytes. Chemosphere 2020, 239, 124730.
- Kowalska, M.; Güler, H.; Cocke, D.L. Interactions of clay minerals with organic pollutants. Sci. Total. Environ. 1994, 141, 223–240.
- Gerasin, V.A.; Antipov, E.M.; Karbushev, V.V.; Kulichikhin, V.; Karpacheva, G.P.; Talroze, R.V.; Kudryavtsev, Y.V. New approaches to the development of hybrid nanocomposites: From structural materials to high-tech applications. Russ. Chem. Rev. 2013, 82, 303–332.
- Brindley, G.W.; Moll, W.F. Complexes of natural and synthetic Ca-montmorillonites with fatty acids. Am. Mineral. 1965, 50, 1355–1370.
- Theng, B.K.G. Polymer–clay nanocomposites. In Developments in Clay Science; Theng, B.K.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 4, pp. 201–241.
- He, H.; Ma, Y.; Zhu, J.; Yuan, P.; Qing, Y. Organoclays prepared from montmorillonites with different cation exchange capacity and surfactant configuration. Appl. Clay Sci. 2010, 48, 67–72.
- Gougeon, R.D.; Soulard, M.; Reinholdt, M.; Miehé-Brendlé, J.; Chézeau, J.-M.; Le Dred, R.; Marchal, R.; Jeandet, P. Polypeptide Adsorption on a Synthetic Montmorillonite: A Combined Solid-State NMR Spectroscopy, X-ray Diffraction, Thermal Analysis and N2 Adsorption Study. Eur. J. Inorg. Chem. 2003, 2003, 1366–1372.
- Lagaly, G. Interaction of alkylamines with different types of layered compounds. Solid State Ionics 1986, 22, 43–51.




