Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Siddabasave Gowda B. Gowda | + 1961 word(s) | 1961 | 2021-07-23 10:33:42 | | | |
| 2 | Rita Xu | -31 word(s) | 1930 | 2021-08-06 08:29:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
B. Gowda, S.G. Hydroxy Fatty Acid. Encyclopedia. Available online: https://encyclopedia.pub/entry/12858 (accessed on 08 February 2026).
B. Gowda SG. Hydroxy Fatty Acid. Encyclopedia. Available at: https://encyclopedia.pub/entry/12858. Accessed February 08, 2026.
B. Gowda, Siddabasave Gowda. "Hydroxy Fatty Acid" Encyclopedia, https://encyclopedia.pub/entry/12858 (accessed February 08, 2026).
B. Gowda, S.G. (2021, August 06). Hydroxy Fatty Acid. In Encyclopedia. https://encyclopedia.pub/entry/12858
B. Gowda, Siddabasave Gowda. "Hydroxy Fatty Acid." Encyclopedia. Web. 06 August, 2021.
Copy Citation
Fatty acid esters of hydroxy fatty acids (FAHFAs) are a new class of endogenous lipids with interesting physiological functions in mammals. Despite their structural diversity and links with nuclear factor erythroid 2-related factor 2 (NRF2) biosynthesis, FAHFAs are less explored as NRF2 activators.
FAHFAs
DHA
NRF2
lipid droplet
HRMS
LC/MS
1. Introduction
Fatty acid esters of hydroxy fatty acids (FAHFAs) are a family of endogenous lipids composed of a fatty acid esterified with a hydroxy fatty acid at various positions. They are structurally diverse, with more than 51 families and 300 regioisomers identified [1]. FAHFAs are reported to exhibit important biological properties such as antidiabetic, anti-inflammatory, anti-apoptotic, and antioxidant activities [2][3][4]. Several in vitro and in vivo studies demonstrated the FAHFAs de novo synthesis in mammals by the transferring a fatty acid from a fatty acid-CoA to a hydroxy fatty acid via lipid acyltransferases [5]. They are also present in dietary plant sources as well as animal meats. In addition, FAHFAs are known to be the endogenous substrates for hydrolases such as ADTRP (androgen-dependent TFPI-regulating protein), AIG1 (androgen-induced gene 1 protein), and pancreatic lipase CEL (carboxy-ester lipase) [6][7]. The most studied FAHFAs are the palmitic-hydroxy stearic acid (PAHSA) with ester linkage at C5 to C13 [1][5][8]. PAHSAs augmented intestinal crypt Paneth cell bactericidal potency via GPR 120 and reduced the colonic T-cell activation and pro-inflammatory cytokine or chemokine expression [9]. Oral administration of 5/9-PAHSAs improved glucose tolerance and enhanced insulin sensitivity in insulin resistant mice on a high-fat diet [10].
Polyunsaturated fatty acid (PUFA) esterified FAHFA such as 13-docosahexaenoic acid hydroxy linoleic acid (13-DHAHLA) is also inhibited lipopolysaccharide-induced secretion of pro-inflammatory cytokines and stimulated the immune cells to limit macrophage activation during the inflammatory responses [11]. Importantly, dietary supplementation of docosahexaenoic acid (DHA) in diabetic patients increased the blood levels of DHAHLA and played a protective role as antidiabetic agents [11]. Hence, PUFAs derived FAHFAs are demonstrated to be more potent anti-inflammatory properties than saturated ones. To defend against oxidative stress induced cellular damages in various pathological conditions such as chronic kidney diseases, inflammatory bowel disease, pulmonary hypertension, and cardiovascular and neurodegenerative disorders, NRF2 activation is needed [12]. Hence, there is a growing need for the most promising NRF2 activators. The activated NRF2 is known to be involved in upregulation of antioxidant enzymes and plays a protective cellular function. Biosynthesis of FAHFAs can be augment oxidative stress via the NRF2- mediated antioxidant defense system [13]. Although the studies on antioxidant properties of FAHFAs are limited, our recent report demonstrated that eicosapentaenoic acid esterified 12-hydroxy stearic acid (12-EPAHSA) or oleic acid (12-EPAHOA) activated NRF2 signaling and also suppressed the oxidation of small lipid droplets (LDs) and oxidative stress induced by H2O2 [4]. Despite the numerous biological activities of PUFA-derived FAHFAs, a limited number of molecular species were explored.
2. Docosahexaenoic Acid
The synthesis of docosahexaenoic acid (DHA) esters of 12-hydroxy stearic acid (12-HSA) and 12-hydroxy oleic acid (12-HOA) was performed by the method developed earlier in our laboratory for EPA-derived FAHFAs [4]. The column chromatographic purified compounds 12-DHAHOA and 12-DHAHSA were subjected to LC/MS analysis to confirm their structure. The extracted ion chromatograms, high-resolution masses, and MS/MS fragmentation pattern of 12-DHAHOA and 12-DHAHSA were provided in Figure 1. The compound 12-DHAHOA elutes at 18.3 min and is ionized in negative mode to produce [M-H]− ion having the acquired mass 607.4732 (theoretical m/z: 607.4732, mass error: 0 ppm). The MS/MS spectra of 12-DHAHOA showed that the two major fragment ions at m/z 297 and m/z 327 correspond to their free fatty acids 12-HOA and DHA, respectively.
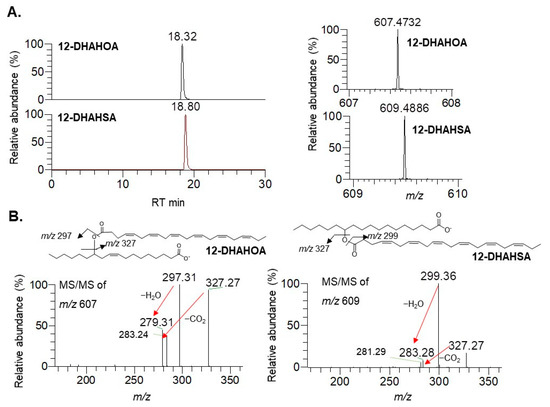
Figure 1. Structural characterization of DHA-derived FAHFAs. (A) Extracted ion chromatograms of 12-DHAHOA, 12-DHAHSA (left panel), and their respective high-resolution mass spectra (right panel). (B) MS/MS spectra of 12-DHAHOA and 12-DHAHSA.
The 12-HOA (m/z 297) loses a neutral molecule of water (18 Da) and produces a peak at m/z 279, whereas DHA (m/z 327) loses a neutral molecule of carbon dioxide (44 Da) to produce a peak at m/z 283, respectively. Hence, the observed MS and MS/MS patterns confirm the structure as 12-DHAHOA. Similarly, the compound 12-DHAHSA elutes at 18.8 min and is ionized in negative mode to produce [M-H]− ion having acquired m/z 609.4886 (theoretical m/z: 609.4888, mass error: −0.32 ppm). The MS/MS spectra of 12-DHAHSA showed that the two product ions at m/z 299 and m/z 327 corresponds to 12-HSA and DHA, respectively. These fragment ions are further ionized and lose their neutral molecules of H2O and CO2 to produce m/z 281 and m/z 283 fragment ions, respectively. This type of saturated/polyunsaturated fatty acid-specific fragmentation is quite commonly observed in previous reports [14]. Overall, both the acquired MS and MS/MS fragmentation patterns confirm the structure as 12-DHAHSA.
The cytotoxicity of DHA, 12-HSA, 12-DHAHSA, and 12-DHAHOA was evaluated against cultured C3A cells by CCK-8 assay and the values are found to be 124.5 µM, 161 µM, 252 µM, and 225 µM, respectively. The percentages of cell viability logarithmic plots for the compounds are shown in Figure 2. However, cytotoxicity of 12-HOA is not evaluated in the current study but it should be expected to be similar to 12-HSA. The results show that DHA-derived FAHFAs are almost two-folds less toxic when compared to their respective free fatty acids. The ability of each DHA-derived FAHFAs towards the activation of NRF2 was examined with a reporter gene assay using the Dual-Glo Luciferase Reporter Assay System (Promega) by the protocols established in our lab [4][15]. In this assay, the antioxidant response element (ARE) was applied and drove the transcription of the luciferase reporter gene. If the NRF2 is activated, then it translocated to the nucleus where it binds to ARE protein and activates antioxidant signaling. Hence, higher luciferase activity refers to the strong activation of NRF2. The results of the reporter gene assay are shown in Figure 3A,B. These data suggest the dose-dependent activation of NRF2 by both 12-DHAHOA and 12-DHAHSA. However, the 12-DHAHOA seems to have a more potent activator of NRF2 compared to 12-DHAHSA. Furthermore, cellular reactive oxygen species (ROS) levels were determined by the protocol established earlier in our lab with minor modifications [15]. The oxidative stress in C3A cells was induced by treatment with the known concentration of hydrogen peroxide (0.25 mM) and the effect of 12-DHAHOA on ROS levels was measured. These data are shown in Figure 3C The results show a significant decrease in ROS levels at 125 µM treatment of 12-DHAHOA compared to the hydrogen peroxide treated group.
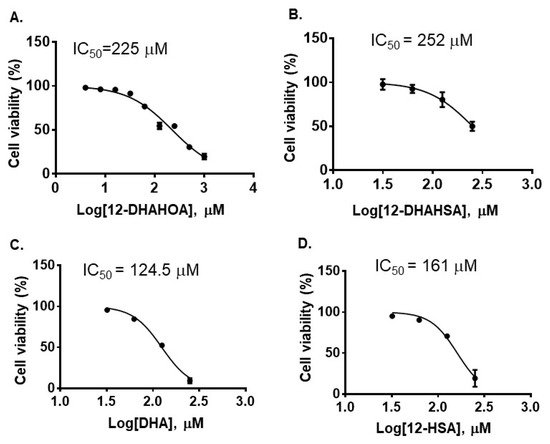
Figure 2. Cytotoxicity of DHA-derived FAHFAs and their free fatty acids. (A) 12-DHAHOA, (B) 12-DHAHSA, (C) DHA and (D) 12-HSA (mean ± SD (n = 6)).
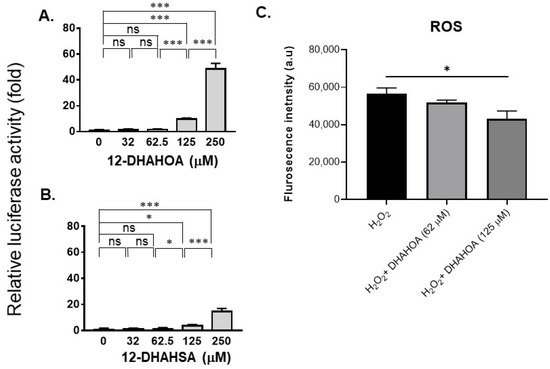
Figure 3. Evaluation of the antioxidant potential of DHA-derived FAHFAs in C3A cells. Reporter gene assay results of 12-DHAHOA (A) and 12-DHAHSA (B) treatment. (C) The fluorescence intensity responses to oxidative stress induced by H2O2 and the effect of 12-DHAHOA treatment. * p < 0.05, *** p < 0.001, ns: not significant (one-way ANOVA) (n = 6, mean ± SEM).
Next, in order to investigate the effect of DHA-derived FAHFAs on cellular lipid pathways, the cells were treated with the known concentration of 12-DHAHOA (77 µM) and incubated at 37 °C for 24 h. After that, cells were extracted for total lipids and untargeted LC/MS analysis was performed to know the relative levels of multiple lipid classes. In total, about 106 lipids were identified and annotated based on MS/MS fragmentation behavior. The volcanic plot (Figure 4A) shows the significantly altered lipids between control and 12-DHAHOA treated groups. Out of 106, about 44 lipids are significantly increased by 5-fold from the control group. In contrast, only two phosphoglycerols (PG) (PG (18:1/18:1) and PG (18:1/22:6)) were shown to be decreased in the 12-DHAHOA treated group compared to the control. Among significantly increased lipids, triacylglycerols (TAGs) and FA 22:6 derived lipids are the major species. Furthermore, multivariate analysis such as OPLS-DA was performed to find the separation between the groups based on the most significant variables. The OPLS-DA score plot (Figure 4B) shows the clear separation between the control and 12-DHAHOA treated groups. The feature importance box plot (also known as S-Plot) (Figure 4B) visualizes the variable influence in an Orthogonal PLS-DA model. It combines covariance and correlation loading profiles. This corresponds to combining the contribution with the effect and reliability for the model variables with respect to the model component scores. The larger positive or negative loading scores indicate that a variable has a strong effect on the orthogonal components. In our results, TAGs showed a large negative loading, whereas phospholipids such as PG, Phosphoethanolamine (PE), Phosphatidylinositol (PI), and FA 16:0 showed large positive loadings.
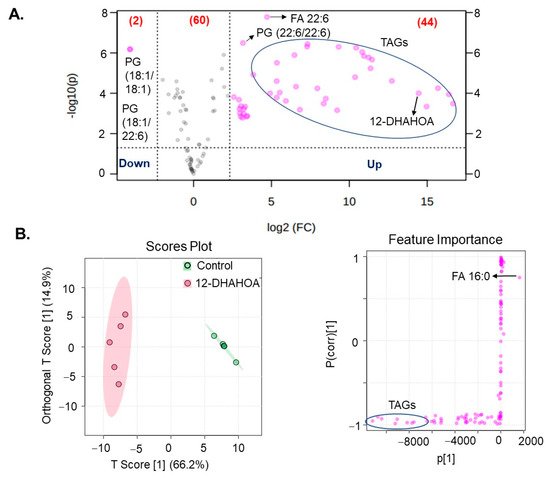
Figure 4. (A) Volcanic plot of all the annotated lipids (only species changed by 5-fold are highlighted with applied t-test of p < 0.05). (B) Orthogonal partial least squares discriminant analysis (OPLS-DA). Score plot and feature importance; colored circles display 95% confidence regions; Control (n = 5) and 12-DHAHOA (n = 5) (Predictive component 1: R2X = 0.662, R2Y = 0.965, Q2 = 0.954; Orthogonal component 1: R2X = 0.149 R2Y = 0.00218, Q2 = 0.0162; All components: R2X(cum): 0.811). Q2 and R2 values represent the predictability and goodness of fit, respectively.
The multivariate hierarchical cluster correlation analysis was performed to identify the species that increased or decreased and the results of the top 20 significantly altered lipids were shown in Figure 5A. As the results demonstrate, the mainly TAGs with polyunsaturated fatty acid (especially FA 22:6) were increased significantly in 12-DHAHOA treated group compared to the control, whereas PG (18:1/18:1) and PG (18:1/22:6) showed the decreasing trend in 12-DHAHOA treated cells. Moreover, a tight clustering is observed between the control and 12-DHAHOA groups. Figure 5B shows the total relative level of each lipid class. This data also shows a significant increase in total TAGs by almost 17-fold in 12-DHAHOA treated samples compare to control. However, no significant changes were observed in other lipid classes except PIs, which shows a slight decrease in 12-DHAHOA treated group.
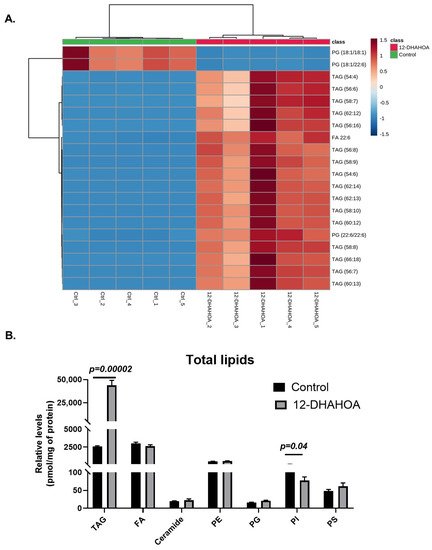
Figure 5. (A) Hierarchical cluster correlation analysis of top 20 significantly altered species (t-test, p < 0.05, distance measure: Euclidean; clustering algorithm: Ward. Each colored cell on the map corresponds to a concentration value of lipids, with samples in rows and compounds in columns. Red: increased, Blue: decreased). (B) Relative levels of total lipids in control (C3A) and 12-DHAHSA treated samples (t-test, p < 0.05) (mean ± SEM (n = 5). (TAG: Triacylglycerol, FA: Fatty acid, PE: Phosphatidylethanolamine, PG: Phosphatidylglycerol, PI: Phosphatidylinositol, and PS: Phosphatidylserine).
Furthermore, the antioxidant potential of 12-DHAHSA was evaluated by the fluorescent imaging technique. The 12-DHAHSA was selected because of its lower cytotoxicity compared to 12-DHAHOA. The lipid droplets (LDs) were induced by treatment of C3A cells with linoleic acid (LA) for 8 h and the effects of 12-DHAHSA on total and oxidized lipid droplets (oxLDs) were determined by the method established earlier in our laboratory [16]. There is a significant increase in the total number of LDs observed between the control and LA or 12-DHAHSA treated group (Figure 6A). However, a slightly decreasing trend is observed in total LDs with LA and the 12-DHAHSA treated group. Furthermore, the number of small oxLDs decreased with 12-DHAHSA treatment compared to LA treated group (Figure 6B). However, no significant changes were observed for large oxLDs. The degree of oxidation in small LDs was also decreased with 12-DHAHSA treatment. The fluorescent images showed the effect of 12-DHAHSA on LDs (SRfluor + Hoechst) and oxLDs (Liperfluo + Hoechst) are shown in Figure 6C.
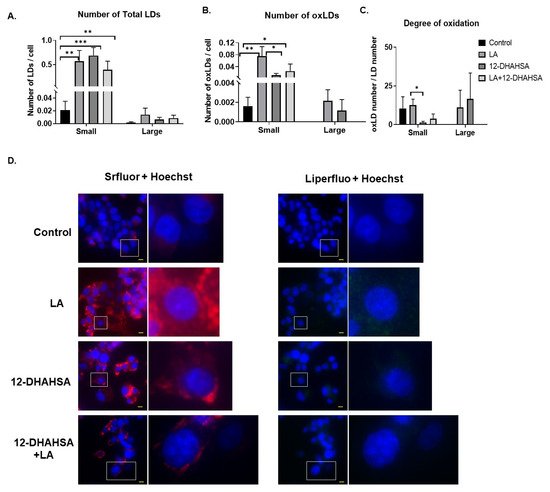
Figure 6. Effect of 12-DHAHSA (125 µM) on oxidation of LA-induced LDs in C3A cells. (A) Number of total LDs. (B) Number of oxLDs. (C) Degree of oxidation. Small LDs are < 3 µm2 and large LDs are ≥ 3 µm2 and are quantified by analyzing fluorescent images with ImageJ software. (D) Fluorescent images showed neutral lipid (red), lipid peroxide (green), and nuclei (blue). The scale bar shown in each image is 10 µm. Columns and bars represent the mean ± SEM (n = 3). Student’s t-test with * p < 0.1, ** p < 0.05, *** p < 0.01.
References
- Brejchova, K.; Balas, L.; Paluchova, V.; Brezinova, M.; Durand, T.; Kuda, O. Understanding FAHFAs: From structure to metabolic regulation. Prog. Lipid Res. 2020, 79, 1010153.
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 2014, 159, 318–332.
- Rodríguez, J.P.; Guijas, C.; Astudillo, A.M.; Rubio, J.M.; Balboa, M.A.; Balsinde, J. Sequestration of 9-hydroxystearic acid in fahfa (Fatty acid esters of hydroxy fatty acids) as a protective mechanism for colon carcinoma cells to avoid apoptotic cell death. Cancers 2019, 11, 524.
- Gowda, S.G.B.; Fuda, H.; Tsukui, T.; Chiba, H.; Hui, S.P. Discovery of eicosapentaenoic acid esters of hydroxy fatty acids as potent NRF2 activators. Antioxidants 2020, 9, 397.
- Benlebna, M.; Balas, L.; Gaillet, S.; Durand, T.; Coudray, C.; Casas, F.; Feillet-Coudray, C. Potential physio-pathological effects of branched fatty acid esters of hydroxy fatty acids. Biochimie 2021, 182, 13–22.
- Ertunc, M.E.; Kok, B.; Parsons, W.H.; Wang, J.G.; Tan, D.; Donaldson, C.J.; Pinto, A.F.M.; Vaughan, J.M.; Ngo, N.; Lum, K.M.; et al. AIg1 and adtrp are endogenous hydrolases of fatty acid esters of hydroxy fatty acids (fahfas) in mice. J. Biol. Chem. 2020, 295, 5891–5905.
- Kolar, M.; Kamat, S.; Parsons, W.; Homan, E.A.; Maher, T.; Peroni, O.D.; Syed, I.; Fjeld, K.; Molven, A.; Kahn, B.B.; et al. Branched Fatty Acid Esters of Hydroxy Fatty Acids Are Preferred Substrates of the MODY8 Protein Carboxyl Ester Lipase. Biochemistry 2016, 55, 4636–4641.
- Wood, P.L. Fatty acyl esters of hydroxy fatty acid (FAHFA) lipid families. Metabolites 2020, 10, 512.
- Lee, J.; Moraes-Vieira, P.M.; Castoldi, A.; Aryal, P.; Yee, E.U.; Vickers, C.; Parnas, O.; Donaldson, C.J.; Saghatelian, A.; Kahn, B.B. Branched fatty acid esters of hydroxy fatty acids (FAHFAs) protect against colitis by regulating gut innate and adaptive immune responses. J. Biol. Chem. 2016, 291, 22207–22217.
- Zhou, P.; Santoro, A.; Peroni, O.D.; Nelson, A.T.; Saghatelian, A.; Siegel, D.; Kahn, B.B. PAHSAs enhance hepatic and systemic insulin sensitivity through direct and indirect mechanisms. J. Clin. Investig. 2019, 129, 4138–4150.
- Kuda, O.; Brezinova, M.; Rombaldova, M.; Slavikova, B.; Posta, M.; Beier, P.; Janovska, P.; Veleba, J.; Kopecky, J., Jr.; Kudova, E.; et al. Docosahexaenoic acid-derived fatty acid esters of hydroxy fatty acids (FAHFAS) with anti-inflammatory properties. Diabetes 2016, 65, 2580–2590.
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxidative Med. Cell. Longev. 2019, 9372182.
- Kuda, O.; Brezinova, M.; Silhavy, J.; Landa, V.; Zidek, V.; Dodia, C.; Kreuchwig, F.; Vrbacky, M.; Balas, L.; Durand, T.; et al. NRF2-Mediated antioxidant defense and peroxiredoxin 6 are linked to biosynthesis of palmitic acid ester of 9-Hydroxystearic acid. Diabetes 2018, 67, 1190–1199.
- Gowda, S.G.B.; Liang, C.; Gowda, D.; Hou, F.; Kawakami, K.; Fukiya, S.; Yokota, A.; Chiba, H.; Hui, S.P. Identification of short chain fatty acid esters of hydroxy fatty acids (SFAHFAs) in murine model by nontargeted analysis using ultra-high-performance liquid chromatography/linear trap quadrupole-Orbitrap mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8831.
- Joko, S.; Watanabe, M.; Fuda, H.; Takeda, S.; Furukawa, T.; Hui, S.-P.; Shreshta, R.; Chiba, H. Comparison of chemical structures and cytoprotection abilities between direct and indirect antioxidants. J. Funct. Foods 2017, 35, 245–255.
- Tsukui, T.; Chen, Z.; Fuda, H.; Furukawa, T.; Oura, K.; Sakurai, T.; Hui, S.P.; Chiba, H. Novel Fluorescence-Based Method to Characterize the Antioxidative Effects of Food Metabolites on Lipid Droplets in Cultured Hepatocytes. J. Agric. Food Chem. 2019, 67, 9934–9941.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
06 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




