
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Takuma Shinozuka | + 2255 word(s) | 2255 | 2021-08-03 12:13:50 |
Video Upload Options
The roof plate is the most dorsal region of the neural tube. The Roof plate serves as an organizing center that controls proliferation and differentiation of dorsal interneurons. In addition, this region is involved in development of neural crest cells, which are the source of migratory neural crest cells. During early development of the spinal cord, roof plate cells secrete signaling molecules, such as Wnt and BMP family proteins, which regulate development of neural crest cells and dorsal spinal cord. After the dorso-ventral pattern is established, spinal cord dynamically changes its morphology. With this morphological transformation, the lumen of the spinal cord gradually shrinks to form the central canal. Accompanied by formation of the central canal, roof plate cells dramatically stretch along the dorso-ventral axis. During this stretching process, the tips of roof plate cells maintain contact with cells surrounding the shrinking lumen of the spinal cord, eventually exposed to the inner surface of the central canal. In this late stage of the spinal cord development, Wnt secreted by stretched roof plate cells regulates transformation of roof plate cells themselves and promotes proliferation of ependymal cells surrounding the central canal, including neural progenitor cells, in the spinal cord.
1. Introduction
In the beginning of neural development, the neural plate gradually invaginates and its lateral edges are transformed into the neural fold. The appearance of the neural fold is probably the first morphological indication of the dorsal region of neural tissues. Then, the tips of the neural fold fuse, resulting in formation of the neural tube, which develops into the brain in the head and the spinal cord in the trunk. In the mouse, anterior neural tube is generated by this process, called primary neurulation (Figure 1). On the other hand, the posterior neural tube is formed by a process called secondary neurogenesis, in which the neural tube is formed from precursors in the tail bud, followed by condensation of the mesenchyme and subsequent epithelialization [1]. After these processes, neuroepithelial cells adjacent to the lumen proliferate rapidly and differentiate into several distinct types of neuronal and glial cells. Roof plate cells are located in the most dorsal part of developing spinal cord and serve as the organizing center for surrounding neuroepithelial cells, promoting their proliferation and specification [2]. Prior to functioning as an organizing center, these cells give rise to neural crest cells, which migrate to many different tissues, where they give rise to neurons and glial cells of the sensory, sympathetic, and parasympathetic nervous systems, pigment-containing cells of the epidermis, and chromaffin cells of the adrenal gland [3][4][5][6]. Thus, cells in the roof plate, have markedly different roles and their behaviors are dynamic in early embryonic stages.
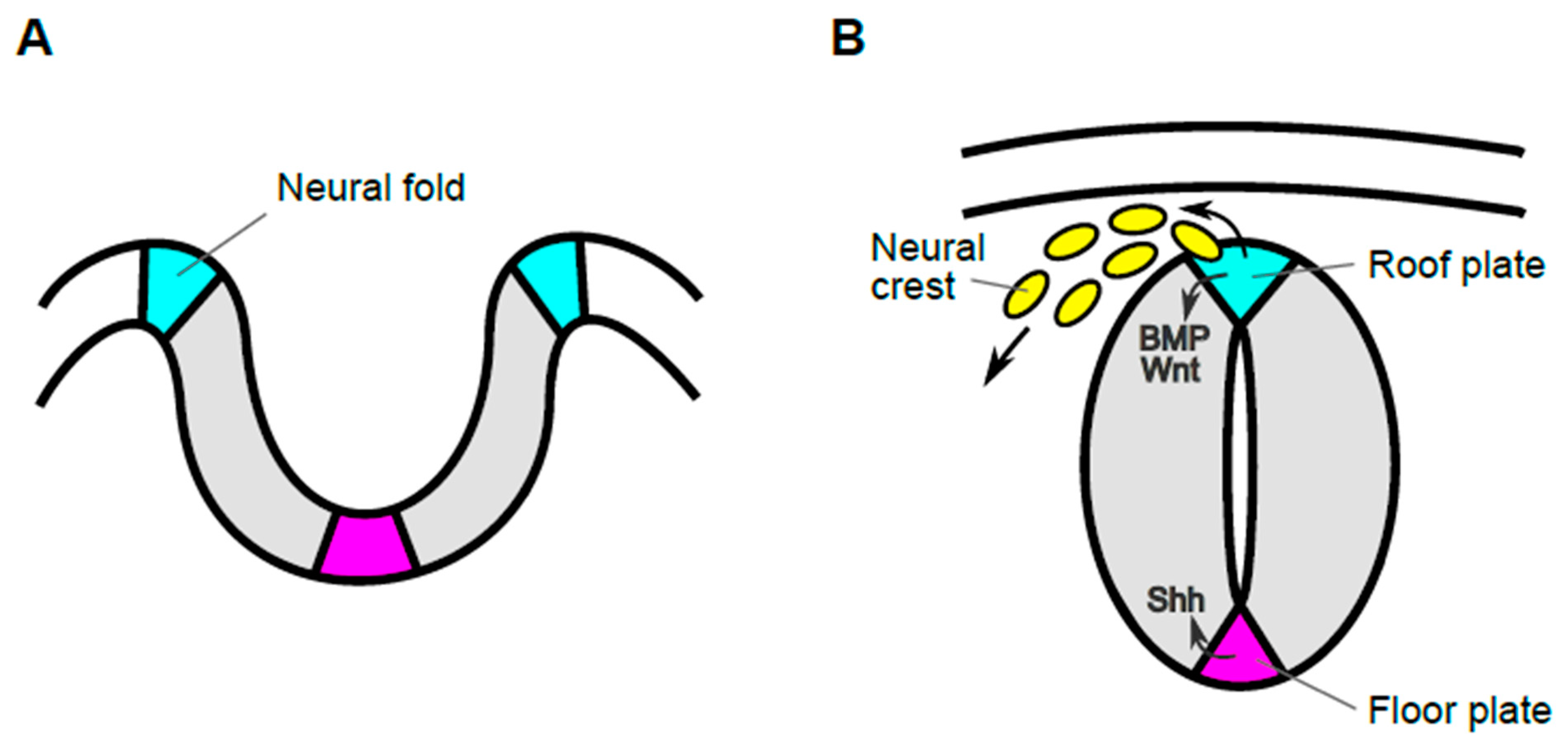
Figure.1 Transition in morphology of neural tissue and functions of cells in the most dorsal region during early developmental stages. Schematic images of transverse sections of developing spinal cord before (A) and after (B) closure of the neural tube during mouse development. The edge of the neural plate elevates and becomes the neural fold (cyan), from which neural crest cells are generated (A). Then, the lateral tips of the neural fold fuse, generating the neural tube (B). Cells in the most dorsal region of the neural tube, or roof plate (cyan), act as progenitors of migrating neural crest cells (yellow) and also as the organizing center for dorsal neuroepithelial cells. Roof plate cells secrete signaling molecules such as Wnt and BMP, which govern development of both neural crest and dorsal neuroepithelial cells. By contrast, cells in the most ventral region, the floor plate (magenta), act as the ventral organizer, by producing Shh.
After the dorso-ventral pattern is established, spinal cord dynamically changes its morphology. With this morphological transformation, the lumen of the spinal cord gradually shrinks to form the central canal, a cavity filled with cerebrospinal fluid that is connected to the ventricular system of the brain. The dorsal half of the spinal cord is separated by a glial structure called the dorsal (or posterior) median septum. Accompanied by reduction of the spinal cord lumen, roof plate cells dramatically stretch along the dorso-ventral axis. During this stretching process, the tips of roof plate cells maintain contact with cells surrounding the shrinking lumen, eventually exposed to the inner surface of the central canal.
2. Roof Plate Functions in the Early Developmental Stage of Spinal Cord
2.1. Generation of Trunk Neural Crest Cells
During development of the neural tube, premigratory neural crest cells first exist in the neural fold and undergo delamination and epithelial-mesenchymal transition to become migratory neural crest cells [7]. However, the timing and mechanism of fate determination in these neural crest cells remains controversial. Lineage tracing analysis using R26R-Confetti mice revealed that the vast majority of individual premigratory and even migratory neural crest cells are multipotent. Furthermore, in some clones with labeled progeny cells among neural crest derivatives, labeled progeny cells are retained in the dorsal neural tube, suggesting an asymmetric cell division of premigratory neural crest cells in the dorsal neural tube [6]. By contrast, lineage tracing analysis using avian embryos after electroporation with GFP reporter showed that pre-migratory neural crest cells generate progeny in single, rather than multiple derivatives, in most cases where delaminated neural crest cells are labeled. In these cases, no labeled cells remained in the neural tube [8]. This result with avian embryos suggests that premigratory neural crest cells are a distinct population from cells that remain in the neural tube, such as roof plate cells, which act as the organizing center. In addition, this transition from neural crest to roof plate accompanied by loss of responsiveness to BMP signaling in dorsal spinal cord [8][9][10]. These discrepancies may be due to differences in labeling techniques, in the stage and location of labeling, or in mechanisms of lineage segregation between mammalian and avian systems.
During development of neural crest cells, cells in the most dorsal region of the spinal cord produce secreted ligands such as BMP and Wnt [2][11][12] (Figure 1B). Several lines of evidence indicate that Wnt/β-catenin signaling is required for generation of neural crest cells [13][14][15][16][17][18] and also promotes segregation of sub-lineages of neural crest cells [19][20][21][22][23]. Furthermore, ligands of the BMP family are also involved in fate decision of neural crest cells [24][25].
2.2. Specification of Dorsal Interneurons
Direct evidence showing the requirement for roof plate cells in specification of dorsal neuroepithelial cells comes from genetic ablation of roof plate cells with Gdf7-DTA. Progenitors of dorsal interneurons are subclassified as dI1 to dI6, in dorsal-to-ventral order in developing spinal cord [26]. This ablation causes loss of progenitors of dorsal interneurons dI1-3 and compensatory occupation of a dorsal position by dI4-6 [27]. This specification, as well as proliferation of dorsal neuroepithelial cells, is regulated by roof plate-derived Wnt and BMP family proteins. For instance, Wnt1 and Wnt3a double-mutant embryos exhibit impaired proliferation and specification of cells in the dorsal spinal cord [28]. A similar phenotype is also observed in mutant embryos in which components of the Wnt/β-catenin pathway, including Wntless and β-catenin, are impaired [29][30]. In addition, activation of Wnt/β-catenin signaling in developing spinal cord indicates that this signaling can regulate specification and proliferation of neuroepithelial cells [30][31][32]. In terms of Wnt-mediated proliferation, it has been proposed that several Wnt ligands expressed in the dorsal spinal cord generate a proliferation gradient along the dorso-ventral axis [33].
BMP family proteins, including BMP4, BMP6, BMP7, and Gdf7, are also expressed in the surface ectoderm and the dorsal spinal cord and are involved in differentiation of dorsal interneurons [34][35][36][37][38]. Manipulation of BMP signaling can promote differentiation of dorsal interneurons in vitro [39][40]. Genetic analyses with mutant embryos showed that Gdf7 is required in formation of dI1 interneurons [36] and that Bmp7 is similarly essential for several subtypes of dorsal interneurons [41]. In contrast, inhibitory Smad6 and Smad7 are expressed in the neural tube, restricting the action of BMP signaling in the dorsal neural tube [42]. Bmp7 and Gdf7 are also required for dI1 axon growth [43]. Since activation of Wnt/β-catenin signaling in roof plate induces expansion of BMP signaling activity in dorsal spinal cord [44], combinatorial Wnt and BMP signaling appears to regulate dorsal interneuron specification and proliferation.3. Development of Roof Plate Cells in Formation of the Central Canal
3.1. Morphological Transformation of the Spinal Cord
During development of spinal cord, its size and morphology change dramatically. Neuroepithelial cells proliferate and give rise to migrating cells that accumulate around the original layer of neuroepithelial, or neuroprogenitor, cells. This accumulation results in formation of the mantle zone and the layer of neuroprogenitor cells remaining along the lumen, which is now called the ventricular layer The mantle zone, which will form the gray matter of the spinal cord, gradually becomes a butterfly-shaped structure, surrounded by the marginal zone, which will form the white matter (Figure 2C).
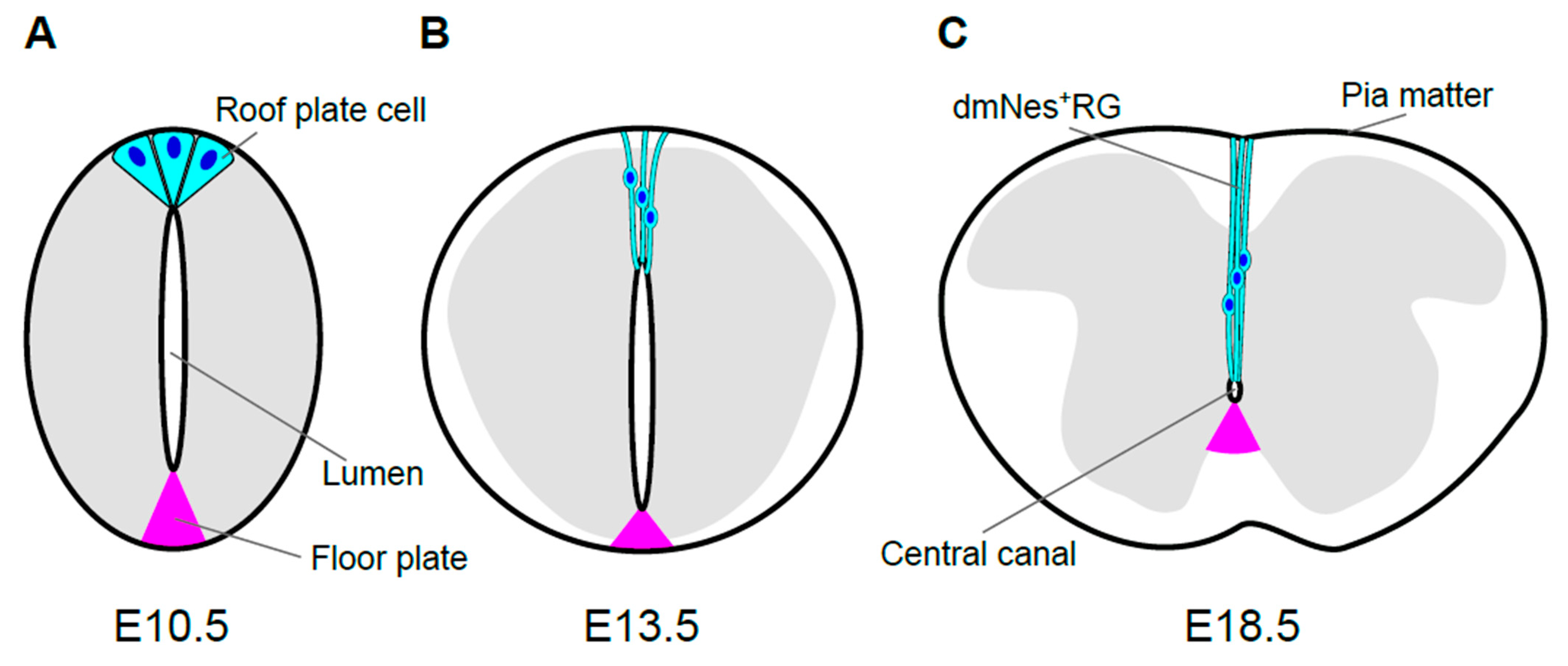
Figure 2. Morphological transformation of the mouse spinal cord. Schematic images of spinal cord morphology and roof plate shape are shown at E10.5 (A), E13.5 (B), and E18.5 (C) during mouse development. The gray matter of the spinal cord is shown in gray. Roof plate cells, or their descendants, and their nuclei are shown in cyan and blue, respectively. The size and morphology of spinal cord change dramatically during development. In accordance with these changes, morphology of roof plate cells also transforms. In mouse embryos, the apical sides of roof plate cells gradually constrict, such that roof plate cells assume a wedge-shaped form at E10.5 (A). Then, the lumen of the spinal cord shrinks gradually. Accompanying the reduction of the lumen, roof plate cells are stretched along the dorso-ventral axis and line up along the midline, resulting in morphological transformation. Compared to the dorsal side, reduction of the ventral ventricular zone and transformation of floor plate (magenta) are comparatively smaller (B). The stretched roof plate cells are also known as dorsomedial Nestin-positive radial glia (dmNes+RGs). At E18.5, the diameter of the lumen finally shrinks to roughly a few cell diameters, resulting in the central canal. At this stage, roof plate cells (dmNes+RGs) are stretched, maintaining contact with the inner surface of the central canal and also with the pia mater, which covers the outer surface of the spinal cord (C).
Contemporaneously with morphological transformation of the spinal cord, the lumen of the spinal cord, which is surrounded by the ventricular layer, gradually diminishes in size and finally becomes the central canal, a cavity filled with cerebrospinal fluid (CSF) that is connected to the ventricular system of the brain. In mouse embryos, this reduction starts on approximately embryonic day 13.5 (E13.5; Figure 2B). Prior to its reduction, the lumen extends over almost the entire dorsoventral axis of the spinal cord. The size of lumen, i.e., the dorso-ventral length of the ventricular layer, is dramatically reduced and the diameter of the lumen has shrunk to roughly a few cell diameters, resulting in the central canal [45][46] (Figure 2).
The central canal is lined with the ependymal layer, which is composed of several distinct cell types, including ciliated ependymal cells, tanycytes (a subpopulation of radial glia), and CSF-contacting, neuron-like cells [47][48][49][50]. Of note, at the dorsal pole of the central canal, neuron-like cells with extensive projections are observed. These cells express Nestin, and their projections extend from the apical side facing the central canal toward the superficial regions of the spinal cord, as far as the pia matter [51][52][53]. These cells are referred to as dorsal midline Nestin (+) radial glia (dmNes+RG) [53].
3.2. Development of Roof Plate Cells in Formation of the Central Canal
Accompanying the reduction of the lumen, roof plate cells are elongated along the dorso-ventral axis and transformed into dmNes+RGs [29][54]. dmNes+RGs maintain contact with the central canal and the pia mater (Figure 2C), eventually becoming part of the dorsal (or posterior) median septum, a thin, dense septum dividing the dorsal side of the spinal cord [29]. Along the stretching of dmNes+RGs, cytoskeletal structures are well developed. Electron microscopic analysis with mouse embryos revealed enrichment of intermediate filament structure in this process [45][55]. This is consistent with enrichment of Nestin, which is a component of intermediate filaments, and directional organization of actin filaments in this process [29][51]. In zebrafish, inhibition of Zic6 or Rock impairs the stretching morphogenesis of roof plate with disruption of the actin cytoskeleton [54][56]. Thus, these cytoskeletal structures apparently contribute to formation of the physically robust structure of the dorsal median septum.
3.3. Roof Plate Functions in the Late Developmental Stage of Spinal Cord
In the dorsal median septum, dmNes+RGs seem to act as a physical and molecular barrier, preventing decussation of developing long tracts of commissural axons [57][58]. In addition, dmNes+RGs apparently serve several different functions. For instance, dreher (Lmx1a-deficient) mice, which impair roof plate formation, show that dmNes+RGs regulate growth of long-range dorsal column axons [59][60].
In the late developmental stage of spinal cord development, expression of Wnt proteins remains in dmNes+RGs [29]. These Wnt proteins secreted by roof plate cells are required for the change in morphology of these cells along the midline [29]. In addition, in the ependymal layer surrounding the central canal, Wnt/β-catenin signaling is activated, resulting in proliferation of ependymal cells [29][61]. This activation depends partly on Wnt secretion from dmNes+RGs, apical ends of which face the dorsal surface of the central canal. On the other hand, since several Wnt ligands are also expressed in spinal cord, except dmNes+RGs in mouse embryos [62], it seems possible that these Wnt ligands promote Wnt/β-catenin signaling in ependymal cells. In addition, it is possible that a small number of ependymal cells, distinct from dmNes+RG, arise from the roof plate and maintain Wnt activity in the ependymal layer [61].
References
- Copp, A.J.; Greene, N.D.E.; Murdoch, J.N. The Genetic Basis of Mammalian Neurulation. Nat. Rev. Genet. 2003, 4, 784–793.
- Chizhikov, V.V.; Millen, K.J. Mechanisms of Roof Plate Formation in the Vertebrate CNS. Nat. Rev. Neurosci. 2004, 5, 808–812.
- Serbedzija, G.N.; Fraser, S.E.; Bronner-Fraser, M. Pathways of Trunk Neural Crest Cell Migration in the Mouse Embryo as Revealed by Vital Dye Labelling. Development 1990, 108, 605–612.
- Serbedzija, G.N.; Bronner-Fraser, M.; Fraser, S.E. Developmental Potential of Trunk Neural Crest Cells in the Mouse. Development 1994, 120, 1709–1718.
- Wilson, Y.M.; Richards, K.L.; Ford-Perriss, M.L.; Panthier, J.-J.; Murphy, M. Neural Crest Cell Lineage Segregation in the Mouse Neural Tube. Development 2004, 131, 6153–6162.
- Baggiolini, A.; Varum, S.; Mateos, J.M.; Bettosini, D.; John, N.; Bonalli, M.; Ziegler, U.; Dimou, L.; Clevers, H.; Furrer, R.; et al. Premigratory and Migratory Neural Crest Cells Are Multipotent In Vivo. Cell Stem Cell 2015, 16, 314–322.
- Martik, M.L.; Bronner, M.E. Regulatory Logic Underlying Diversification of the Neural Crest. Trends Genet. 2017, 33, 715–727.
- Krispin, S.; Nitzan, E.; Kassem, Y.; Kalcheim, C. Evidence for a Dynamic Spatiotemporal Fate Map and Early Fate Restrictions of Premigratory Avian Neural Crest. Development 2010, 137, 585–595.
- Nitzan, E.; Avraham, O.; Kahane, N.; Ofek, S.; Kumar, D.; Kalcheim, C. Dynamics of BMP and Hes1/Hairy1 Signaling in the Dorsal Neural Tube Underlies the Transition from Neural Crest to Definitive Roof Plate. BMC Biol. 2016, 14, 23.
- Rekler, D.; Kalcheim, C. From Neural Crest to Definitive Roof Plate: The Dynamic Behavior of the Dorsal Neural Tube. Int. J. Mol. Sci. 2021, 22, 3911.
- Caspary, T.; Anderson, K.V. Patterning Cell Types in the Dorsal Spinal Cord: What the Mouse Mutants Say. Nat. Rev. Neurosci. 2003, 4, 289–297.
- Chizhikov, V.V.; Millen, K.J. Roof Plate-Dependent Patterning of the Vertebrate Dorsal Central Nervous System. Dev. Biol. 2005, 277, 287–295.
- Ikeya, M.; Lee, S.M.K.; Johnson, J.E.; McMahon, A.P.; Takada, S. Wnt Signalling Required for Expansion of Neural Crest and CNS Progenitors. Nature 1997, 389, 966–970.
- LaBonne, C.; Bronner-Fraser, M. Neural Crest Induction in Xenopus: Evidence for a Two-Signal Model. Development 1998, 125, 2403–2414.
- Deardorff, M.A.; Tan, C.; Saint-Jeannet, J.P.; Klein, P.S. A Role for Frizzled 3 in Neural Crest Development. Development 2001, 128, 3655–3663.
- García-Castro, M.I.; Marcelle, C.; Bronner-Fraser, M. Ectodermal Wnt Function as a Neural Crest Inducer. Science 2002, 297, 848–851.
- Wu, J.; Yang, J.; Klein, P.S. Neural Crest Induction by the Canonical Wnt Pathway Can Be Dissociated from Anterior–Posterior Neural Patterning in Xenopus. Dev. Biol. 2005, 279, 220–232.
- Hassler, C.; Cruciat, C.-M.; Huang, Y.-L.; Kuriyama, S.; Mayor, R.; Niehrs, C. Kremen Is Required for Neural Crest Induction in Xenopus and Promotes LRP6-Mediated Wnt Signaling. Development 2007, 134, 4255–4263.
- Dorsky, R.I.; Moon, R.T.; Raible, D.W. Control of Neural Crest Cell Fate by the Wnt Signalling Pathway. Nature 1998, 396, 370–373.
- Hari, L.; Brault, V.; Kléber, M.; Lee, H.-Y.; Ille, F.; Leimeroth, R.; Paratore, C.; Suter, U.; Kemler, R.; Sommer, L. Lineage-Specific Requirements of Beta-Catenin in Neural Crest Development. J. Cell Biol. 2002, 159, 867–880.
- Lee, H.-Y.; Kléber, M.; Hari, L.; Brault, V.; Suter, U.; Taketo, M.M.; Kemler, R.; Sommer, L. Instructive Role of Wnt/Beta-Catenin in Sensory Fate Specification in Neural Crest Stem Cells. Science 2004, 303, 1020–1023.
- Sommer, L. Generation of Melanocytes from Neural Crest Cells: Generation of Melanocytes from Neural Crest Cells. Pigment Cell Melanoma Res. 2011, 24, 411–421.
- Hari, L.; Miescher, I.; Shakhova, O.; Suter, U.; Chin, L.; Taketo, M.; Richardson, W.D.; Kessaris, N.; Sommer, L. Temporal Control of Neural Crest Lineage Generation by Wnt/β-Catenin Signaling. Development 2012, 139, 2107–2117.
- Lo, L.; Dormand, E.L.; Anderson, D.J. Late-Emigrating Neural Crest Cells in the Roof Plate Are Restricted to a Sensory Fate by GDF7. Proc. Natl. Acad. Sci. USA 2005, 102, 7192–7197.
- Kléber, M.; Lee, H.-Y.; Wurdak, H.; Buchstaller, J.; Riccomagno, M.M.; Ittner, L.M.; Suter, U.; Epstein, D.J.; Sommer, L. Neural Crest Stem Cell Maintenance by Combinatorial Wnt and BMP Signaling. J. Cell Biol. 2005, 169, 309–320
- Helms, A.W.; Johnson, J.E. Specification of Dorsal Spinal Cord Interneurons. Curr. Opin. Neurobiol. 2003, 13, 42–49.
- Lee, K.J.; Dietrich, P.; Jessell, T.M. Genetic Ablation Reveals That the Roof Plate Is Essential for Dorsal Interneuron Specification. Nature 2000, 403, 734–740.
- Muroyama, Y.; Fujihara, M.; Ikeya, M.; Kondoh, H.; Takada, S. Wnt Signaling Plays an Essential Role in Neuronal Specification of the Dorsal Spinal Cord. Genes Dev. 2002, 16, 548–553.
- Shinozuka, T.; Takada, R.; Yoshida, S.; Yonemura, S.; Takada, S. Wnt Produced by Stretched Roof-Plate Cells Is Required for the Promotion of Cell Proliferation around the Central Canal of the Spinal Cord. Development 2019, 146, dev159343.
- Zechner, D.; Müller, T.; Wende, H.; Walther, I.; Taketo, M.M.; Crenshaw, E.B.; Treier, M.; Birchmeier, W.; Birchmeier, C. Bmp and Wnt/β-Catenin Signals Control Expression of the Transcription Factor Olig3 and the Specification of Spinal Cord Neurons. Dev. Biol. 2007, 303, 181–190.
- Dickinson, M.E.; Krumlauf, R.; McMahon, A.P. Evidence for a Mitogenic Effect of Wnt-1 in the Developing Mammalian Central Nervous System. Development 1994, 120, 1453–1471.
- Grigoryan, T.; Wend, P.; Klaus, A.; Birchmeier, W. Deciphering the Function of Canonical Wnt Signals in Development and Disease: Conditional Loss- and Gain-of-Function Mutations of Beta-Catenin in Mice. Genes Dev. 2008, 22, 2308–2341.
- Megason, S.G.; McMahon, A.P. A Mitogen Gradient of Dorsal Midline Wnts Organizes Growth in the CNS. Development 2002, 129, 2087–2098.
- Winnier, G.; Blessing, M.; Labosky, P.A.; Hogan, B.L. Bone Morphogenetic Protein-4 Is Required for Mesoderm Formation and Patterning in the Mouse. Genes Dev. 1995, 9, 2105–2116.
- Dudley, A.T.; Robertson, E.J. Overlapping Expression Domains of Bone Morphogenetic Protein Family Members Potentially Account for Limited Tissue Defects in BMP7 Deficient Embryos. Dev. Dyn. 1997, 208, 349–362.
- Lee, K.J.; Mendelsohn, M.; Jessell, T.M. Neuronal Patterning by BMPs: A Requirement for GDF7 in the Generation of a Discrete Class of Commissural Interneurons in the Mouse Spinal Cord. Genes Dev. 1998, 12, 3394–3407.
- Hu, Q.; Ueno, N.; Behringer, R.R. Restriction of BMP4 Activity Domains in the Developing Neural Tube of the Mouse Embryo. EMBO Rep. 2004, 5, 734–739.
- Le Dréau, G.; Martí, E. The Multiple Activities of BMPs during Spinal Cord Development. Cell. Mol. Life Sci. 2013, 70, 4293–4305.
- Liem, K.F.; Tremml, G.; Roelink, H.; Jessell, T.M. Dorsal Differentiation of Neural Plate Cells Induced by BMP-Mediated Signals from Epidermal Ectoderm. Cell 1995, 82, 969–979.
- Liem, K.F.; Tremml, G.; Jessell, T.M. A Role for the Roof Plate and Its Resident TGFbeta-Related Proteins in Neuronal Patterning in the Dorsal Spinal Cord. Cell 1997, 91, 127–138.
- Le Dréau, G.; Garcia-Campmany, L.; Rabadán, M.A.; Ferronha, T.; Tozer, S.; Briscoe, J.; Martí, E. Canonical BMP7 Activity Is Required for the Generation of Discrete Neuronal Populations in the Dorsal Spinal Cord. Development 2012, 139, 259–268.
- Hazen, V.M.; Phan, K.D.; Hudiburgh, S.; Butler, S.J. Inhibitory Smads Differentially Regulate Cell Fate Specification and Axon Dynamics in the Dorsal Spinal Cord. Dev. Biol. 2011, 356, 566–575.
- Butler, S.J.; Dodd, J. A Role for BMP Heterodimers in Roof Plate-Mediated Repulsion of Commissural Axons. Neuron 2003, 38, 389–401.
- Ille, F.; Atanasoski, S.; Falk, S.; Ittner, L.M.; Märki, D.; Büchmann-Møller, S.; Wurdak, H.; Suter, U.; Taketo, M.M.; Sommer, L. Wnt/BMP Signal Integration Regulates the Balance between Proliferation and Differentiation of Neuroepithelial Cells in the Dorsal Spinal Cord. Dev. Biol.
- Sturrock, R.R. An Electron Microscopic Study of the Development of the Ependyma of the Central Canal of the Mouse Spinal Cord. J. Anat. 1981, 132, 119–136.
- Cañizares, M.A.; Albors, A.R.; Singer, G.; Suttie, N.; Gorkic, M.; Felts, P.; Storey, K.G. Multiple Steps Characterise Ventricular Layer Attrition to Form the Ependymal Cell Lining of the Adult Mouse Spinal Cord Central Canal. J. Anat. 2020, 236, 334–350.
- Meletis, K.; Barnabé-Heider, F.; Carlén, M.; Evergren, E.; Tomilin, N.; Shupliakov, O.; Frisén, J. Spinal Cord Injury Reveals Multilineage Differentiation of Ependymal Cells. PLoS Biol. 2008, 6, e182.
- Hamilton, L.K.; Truong, M.K.V.; Bednarczyk, M.R.; Aumont, A.; Fernandes, K.J.L. Cellular Organization of the Central Canal Ependymal Zone, a Niche of Latent Neural Stem Cells in the Adult Mammalian Spinal Cord. Neuroscience 2009, 164, 1044–1056.
- Sabourin, J.-C.; Ackema, K.B.; Ohayon, D.; Guichet, P.-O.; Perrin, F.E.; Garces, A.; Ripoll, C.; Charité, J.; Simonneau, L.; Kettenmann, H.; et al. A Mesenchymal-like ZEB1(+) Niche Harbors Dorsal Radial Glial Fibrillary Acidic Protein-Positive Stem Cells in the Spinal Cord. Stem Cells 2009, 27, 2722–2733.
- Hugnot, J.P.; Franzen, R. The Spinal Cord Ependymal Region: A Stem Cell Niche in the Caudal Central Nervous System. Front. Biosci. 2011, 16, 1044–1059.
- Sevc, J.; Daxnerová, Z.; Miklosová, M. Role of Radial Glia in Transformation of the Primitive Lumen to the Central Canal in the Developing Rat Spinal Cord. Cell Mol. Neurobiol. 2009, 29, 927–936.
- Ghazale, H.; Ripoll, C.; Leventoux, N.; Jacob, L.; Azar, S.; Mamaeva, D.; Glasson, Y.; Calvo, C.-F.; Thomas, J.-L.; Meneceur, S.; et al. RNA Profiling of the Human and Mouse Spinal Cord Stem Cell Niches Reveals an Embryonic-like Regionalization with MSX1+ Roof-Plate-Derived Cells. Stem Cell Rep. 2019, 12, 1159–1177.
- Tait, C.M.; Chinnaiya, K.; Manning, E.; Murtaza, M.; Ashton, J.-P.; Furley, N.; Hill, C.J.; Alves, C.H.; Wijnholds, J.; Erdmann, K.S.; et al. Crumbs2 Mediates Ventricular Layer Remodelling to Form the Spinal Cord Central Canal. PLoS Biol. 2020, 18, e3000470.
- Kondrychyn, I.; Teh, C.; Sin, M.; Korzh, V. Stretching Morphogenesis of the Roof Plate and Formation of the Central Canal. PLoS ONE 2013, 8, e56219.
- Böhme, G. Formation of the Central Canal and Dorsal Glial Septum in the Spinal Cord of the Domestic Cat. J. Anat. 1988, 159, 37–47.
- Korzh, V. Stretching Cell Morphogenesis during Late Neurulation and Mild Neural Tube Defects. Dev. Growth Differ. 2014, 56, 425–433.
- Snow, D.M.; Steindler, D.A.; Silver, J. Molecular and Cellular Characterization of the Glial Roof Plate of the Spinal Cord and Optic Tectum: A Possible Role for a Proteoglycan in the Development of an Axon Barrier. Dev. Biol. 1990, 138, 359–376.
- Sarnat, H.B. Role of Human Fetal Ependyma. Pediatr. Neurol. 1992, 8, 163–178.
- Millonig, J.H.; Millen, K.J.; Hatten, M.E. The Mouse Dreher Gene Lmx1a Controls Formation of the Roof Plate in the Vertebrate CNS. Nature 2000, 403, 764–769.
- Kridsada, K.; Niu, J.; Haldipur, P.; Wang, Z.; Ding, L.; Li, J.J.; Lindgren, A.G.; Herrera, E.; Thomas, G.M.; Chizhikov, V.V.; et al. Roof Plate-Derived Radial Glial-like Cells Support Developmental Growth of Rapidly Adapting Mechanoreceptor Ascending Axons. Cell Rep. 2018, 23, 2928–2941.
- Xing, L.; Anbarchian, T.; Tsai, J.M.; Plant, G.W.; Nusse, R. Wnt/β-Catenin Signaling Regulates Ependymal Cell Development and Adult Homeostasis. Proc. Natl. Acad. Sci. USA 2018, 115, E5954–E5962.
- GENSAT Brain Atlas of Gene Expression in EGFP Transgenic Mice. Available online: http://www.gensat.org/index.html (accessed on 29 July 2021).




