
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giuseppinella Melita | + 5736 word(s) | 5736 | 2021-07-13 04:25:23 | | | |
| 2 | Bruce Ren | -21 word(s) | 5715 | 2021-08-05 03:14:03 | | | | |
| 3 | Bruce Ren | Meta information modification | 5715 | 2021-08-09 05:36:29 | | |
Video Upload Options
Pancreatic neuroendocrine neoplasms (PanNENs) are relatively rare, but their incidence has increased significantly in the last decades. Precise diagnosis and prognostic stratification are crucial for proper patient management. Endoscopic ultrasound (EUS) is the modality of choice for diagnosis of solid pancreatic tumors, showing a higher tumor detection rate than other imaging modalities, especially for small size lesions. EUS also serves as a guide for preoperative sampling and other interventions. EUS-tissue acquisition is a safe and highly accurate technique for cyto/histological diagnosis of PanNENs with a well-demonstrated correlation between Ki-67 proliferation index values and tumor grading on EUS and surgical specimens according to the WHO 2017 classification. Furthermore, the possibility of a preoperative EUS-guided fine needle tattooing or fiducial markers placement may help the surgeon to locate small and deep tumors, thus avoiding formal pancreatic resections in favor of parenchymal-sparing surgery. Finally, locoregional ablative treatments using either ethanol injection or radiofrequency ablation have been proposed in recent studies with promising results in order to control symptoms or reduce tumor burden in selected patients unfit for surgery with functioning or non-functioning PanNENs.
1. Introduction
| Terminology | Differentiation | Grade | Mitotic Rate | Ki-67 Index |
|---|---|---|---|---|
| NET G1 | Well differentiated | Low | >2/10 HPF | <3% |
| NET G2 | Well differentiated | Intermediate | 2–20/10 HPF | 3–20% |
| NET G3 | Well differentiated | High | >20/10 HPF | >20% |
| NEC G3 | Poorly differentiated | High | >20/10 HPF | >20% |
2. EUS Technique and B-Mode Evaluation
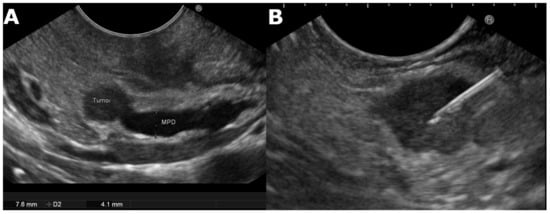
2.1. Endoscopic Ultrasound-Guided Tissue Acquisition
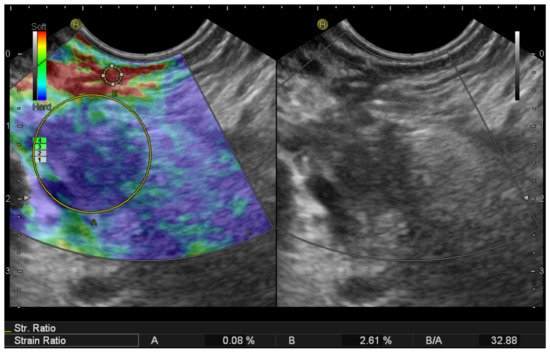
2.2. EUS Elastography
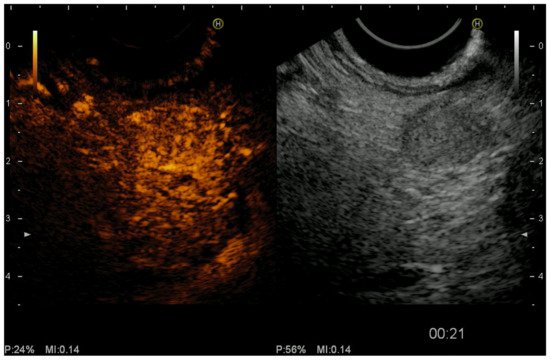
2.3. Color Doppler EUS and Contrast-Harmonic EUS (CH-EUS)
3. Interventional Endoscopic Ultrasound for Pancreatic Neuroendocrine Tumors
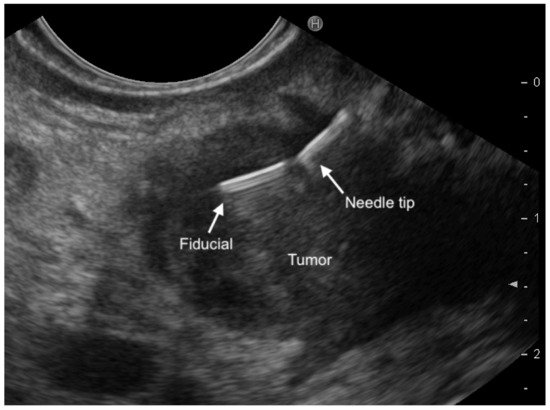
3.1. EUS-Fine-Needle-Tattooing (EUS-FNT) and EUS-Guided Fiducial Implantation (EUS-FI)
3.2. EUS-Guided Ethanol Ablation
3.3. EUS-Guided Radiofrequency Ablation (EUS-RFA)
References
- Ito, T.; Sasano, H.; Tanaka, M.; Osamura, R.Y.; Sasaki, I.; Kimura, W.; Takano, K.; Obara, T.; Ishibashi, M.; Nakao, K.; et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J. Gastroenterol. 2010, 45, 234–243.
- Yao, J.C.; Hassan, M.M.; Phan, A.T.; Dagohoy, C.G.; Leary, C.C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One Hundred Years After “Carcinoid”: Epidemiology of and Prognostic Factors for Neuroendocrine Tumors in 35,825 Cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072.
- Metz, D.C.; Jensen, R.T. Gastrointestinal Neuroendocrine Tumors: Pancreatic Endocrine Tumors. Gastroenterology 2008, 135, 1469–1492.
- Öberg, K. Pancreatic Endocrine Tumors. Semin. Oncol. 2010, 37, 594–618.
- De Wilde, R.F.; Edil, B.H.; Hruban, R.H.; Maitra, A. Well-differentiated pancreatic neuroendocrine tumors: From genetics to therapy. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 199–208.
- Jensen, R.T.; Cadiot, G.; Brandi, M.L.; De Herder, W.W.; Kaltsas, G.; Komminoth, P.; Scoazec, J.-Y.; Salazar, R.; Sauvanet, A.; Kianmanesh, R. ENETS Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Neoplasms: Functional Pancreatic Endocrine Tumor Syndromes. Neuroendocrinology 2012, 95, 98–119.
- Falconi, M.; Bartsch, D.K.; Eriksson, B.; Klöppel, G.; Lopes, J.M.; O’Connor, J.M.; Salazar, R.; Taal, B.G.; Vullierme, M.P.; O’Toole, D.; et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: Well-differentiated pancreatic non-functioning tumors. Neuroendocrinology 2012, 95, 120–134.
- Ehehalt, F.; Saeger, H.D.; Schmidt, C.M.; Grützmann, R. Neuroendocrine Tumors of the Pancreas. Oncologist 2009, 14, 456–467.
- Kulke, M.H.; Anthony, L.B.; Bushnell, D.L.; de Herder, W.W.; Goldsmith, S.J.; Klimstra, D.S.; Marx, S.J.; Pasieka, J.L.; Pommier, R.F.; Yao, J.C.; et al. NANETS Treatment Guidelines. Pancreas 2010, 39, 735–752.
- Vanderveen, K.; Grant, C. Insulinoma. Cancer Treat. Res. 2009, 153, 235–252.
- De Herder, W.W.; Niederle, B.; Scoazec, J.-Y.; Pauwels, S.; Klöppel, G.; Falconi, M.; Kwekkeboom, D.J.; Öberg, K.; Eriksson, B.; Wiedenmann, B.; et al. Well-Differentiated Pancreatic Tumor/Carcinoma: Insulinoma. Neuroendocrinology 2006, 84, 183–188.
- Attili, F.; Capurso, G.; Vanella, G.; Fuccio, L.; Fave, G.D.; Costamagna, G.; Larghi, A. Diagnostic and therapeutic role of endoscopy in gastroenteropancreatic neuroendocrine neoplasms. Dig. Liver Dis. 2014, 46, 9–17.
- Zhao, Y.-P.; Zhan, H.-X.; Zhang, T.-P.; Cong, L.; Dai, M.-H.; Liao, Q.; Cai, L.-X. Surgical management of patients with insulinomas: Result of 292 cases in a single institution. J. Surg. Oncol. 2010, 103, 169–174.
- Niederle, M.B.; Hackl, M.; Kaserer, K.; Niederle, B. Gastroenteropancreatic neuroendocrine tumours: The current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: An analysis based on prospectively collected parameters. Endocr. Relat. Cancer 2010, 17, 909–918.
- Cheema, A.; Weber, J.; Strosberg, J.R. Incidental Detection of Pancreatic Neuroendocrine Tumors: An Analysis of Incidence and Outcomes. Ann. Surg. Oncol. 2012, 19, 2932–2936.
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, M.K.; Carneiro, F.; Cree, I.A.; The WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188.
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumors of the Digestive System. In WHO Classification of Tumors; Bosman, F.T., Hruban, R.H., Theise, N.D., Eds.; WHO IARC: Geneva, Switzerland, 2010; Volume 3, ISBN 9789283224327.
- Casadei, R.; Ricci, C.; Rega, D.; D’Ambra, M.; Pezzilli, R.; Tomassetti, P.; Campana, D.; Nori, F.; Minni, F. Pancreatic endocrine tumors less than 4 cm in diameter: Resect or enucleate? A single-center experience. Pancreas 2010, 39, 825–828.
- Zerbi, A.; Falconi, M.; Rindi, G.; Fave, G.D.; Tomassetti, P.; Pasquali, C.; Capitanio, V.; Boninsegna, L.; Di Carlo, V. Clinicopathological Features of Pancreatic Endocrine Tumors: A Prospective Multicenter Study in Italy of 297 Sporadic Cases. Am. J. Gastroenterol. 2010, 105, 1421–1429.
- Jonkers, Y.M.H.; Claessen, S.M.H.; Perren, A.; Schmitt, A.M.; Hofland, L.J.; De Herder, W.; De Krijger, R.R.; Verhofstad, A.A.J.; Hermus, A.R.; Kummer, J.A.; et al. DNA copy number status is a powerful predictor of poor survival in endocrine pancreatic tumor patients. Endocr. Relat. Cancer 2007, 14, 769–779.
- Panzuto, F.; Boninsegna, L.; Fazio, N.; Campana, D.; Brizzi, M.P.; Capurso, G.; Scarpa, A.; de Braud, F.G.M.; Dogliotti, L.; Tomassetti, P.; et al. Metastatic and Locally Advanced Pancreatic Endocrine Carcinomas: Analysis of Factors Associated with Disease Progression. J. Clin. Oncol. 2011, 29, 2372–2377.
- Rindi, G.; Falconi, M.; Klersy, C.; Albarello, L.; Boninsegna, L.; Buchler, M.W.; Capella, C.; Caplin, M.; Couvelard, A.; Doglioni, C.; et al. TNM staging of neoplasms of the endocrine pancreas: Results from a large international cohort study. J. Nat. Cancer Inst. 2012, 104, 764–777.
- Boninsegna, L.; Panzuto, F.; Partelli, S.; Capelli, P.; Fave, G.D.; Bettini, R.; Pederzoli, P.; Scarpa, A.; Falconi, M. Malignant pancreatic neuroendocrine tumour: Lymph node ratio and Ki67 are predictors of recurrence after curative resections. Eur. J. Cancer 2012, 48, 1608–1615.
- Marthur, A.; Gorden, P.; Libuti, S.K. Insulinoma. Surg. Clin. N. Am. 2009, 89, 1105–1121.
- Sundin, A.; Vulleierme, M.P.; Kaltsas, G.; Plockinger, U. Mallorca Consensus Conference Partecipants; European Neuroendocrine Tumor Society: ENETS Consensus Guidelines for the standards of care in neuroendocrine tumors: Radiological examinations. Neuroendocrinology 2009, 90, 167–183.
- Ito, T.; Igarashi, H.; Jensen, R.T. Pancreatic neuroendocrine tumors Consensus Guidelines clinical features, diagnosis and medical treatment advances. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 737–753.
- Iglesias-Garcia, J.; Larino-Noia, J.; Abdulkader, I.; Forteza, J.; Dominguez-Munoz, J.E. Quantitative Endoscopic Ultrasound Elastography: An Accurate Method for the Differentiation of Solid Pancreatic Masses. Gastroenterology 2010, 139, 1172–1180.
- Itoi, T.; Sofuni, A.; Itokawa, F.; Irisawa, A.; Khor, C.J.; Rerknimitr, R. Current status of diagnostic endoscopic ultrasonography in the evaluation of pancreatic mass lesions. Dig. Endosc. 2011, 23, 17–21.
- Wang, W.; Shpaner, A.; Krishna, S.G.; Ross, W.A.; Bhutani, M.S.; Tamm, E.P.; Raju, G.S.; Xiao, L.; Wolff, R.A.; Fleming, J.B.; et al. Use of EUS-FNA in diagnosing pancreatic neoplasm without a definitive mass on CT. Gastrointest. Endosc. 2013, 78, 73–80.
- Aso, A.; Ihara, E.; Osoegawa, T.; Nakamura, K.; Itaba, S.; Igarashi, H.; Ito, T.; Aishima, S.; Oda, Y.; Tanaka, M.; et al. Key endoscopic ultrasound features of pancreatic ductal adenocarcinoma smaller than 20 mm. Scand. J. Gastroenterol. 2014, 49, 332–338.
- Zilli, A.; Arcidiacono, P.G.; Conte, D.; Massironi, S. Clinical impact of endoscopic ultrasonography on the management of neuroendocrine tumors: Lights and shadows. Dig. Liver Dis. 2018, 50, 6–14.
- Fujimori, N.; Osoegawa, T.; Lee, L.; Tachibana, Y.; Aso, A.; Kubo, H.; Kawabe, K.; Igarashi, H.; Nakamura, K.; Oda, Y.; et al. Efficacy of endoscopic ultrasonography and endoscopic ultrasonography-guided fine-needle aspiration for the diagnosis and grading of pancreatic neuroendocrine tumors. Scand. J. Gastroenterol. 2015, 51, 245–252.
- Manta, R.; Nardi, E.; Pagano, N.; Ricci, C.; Sica, M.; Castellani, D.; Bertani, H.; Piccoli, M.; Mullineris, B.; Tringali, A.; et al. Pre-operative Diagnosis of Pancreatic Neuroendocrine Tumors with Endoscopic Ultrasonography and Computed Tomography in a Large Series. J. Gastrointest. Liver Dis. 2016, 25, 317–321.
- Giuliani, T.; Marchegiani, G.; Girgis, M.D.; Crinò, S.F.; Muthusamy, V.R.; Bernardoni, L.; Pea, A.; Ramera, M.; Paiella, S.; Landoni, L.; et al. Endoscopic placement of pancreatic stent for “Deep” pancreatic enucleations operative technique and preliminary experience at two high-volume centers. Surg. Endosc. 2020, 34, 2796–2802.
- Figueredo, F.A.; Giovannini, M.; Monges, G.; Charfi, S.; Bories, E.; Pesenti, C.; Caillol, F.; Delpero, J.R. Pancreatic endocrine tumors: A large single-center experience. Pancreas 2009, 38, 936–940.
- Figueiredo, F.A.; Giovannini, M.; Monges, G.; Bories, E.; Pesenti, C.; Caillol, F.; Delpero, J.R. EUS-FNA predicts 5-year survival in pancreatic endocrine tumors. Gastrointest. Endosc. 2009, 70, 907–914.
- Piani, C.; Franchi, G.M.; Cappelletti, C.; Scavini, M.; Albarello, L.; Zerbi, A.; Arcidacono, P.G.; Bosi, E.; Manzoni, M.F. Cytological Ki-67 in pancreatic endocrine tumors: An opportunity for pre-operative grading. Endocr. Relat. Cancer 2008, 15, 175–181.
- Alexiev, B.A.; Darwin, P.E.; Goloubeva, O.; Ioffe, O.B. Proliferative rate in endoscopic ultrasound fine-needle aspiration of pancreatic endocrine tumors. Cancer Cytopathol. 2009, 117, 40–45.
- Hamburg, M.A.; Collins, F.S. The Path to Personalized Medicine. N. Engl. J. Med. 2010, 363, 301–304.
- Vilmann, P.; Jacobsen, G.K.; Henriksen, F.W.; Hancke, S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest. Endosc. 1992, 38, 172–173.
- Varadarajulu, S.; Eloubeidi, M.A. The Role of Endoscopic Ultrasonography in the Evaluation of Pancreatico-Biliary Cancer. Gastrointest. Endosc. Clin. N. Am. 2005, 15, 497–511.
- Kim, M.K. Endoscopic Ultrasound in Gastroenteropancreatic Neuroendocrine Tumors. Gut Liver 2012, 6, 405–410.
- Crinó, S.F.; Brandolese, A.; Vieceli, F.; Paiella, S.; Bellocchi, M.C.C.; Manfrin, E.; Bernardoni, L.; Sina, S.; D’Onofrio, M.; Marchegiani, G.; et al. Endoscopic Ultrasound Features Associated with Malignancy and Aggressiveness of Nonhypovascular Solid Pancreatic Lesions: Results from a Prospective Observational Study. Ultraschall Med. Eur. J. Ultrasound 2021, 42, 167–177.
- Cosgrove, D.; Piscaglia, F.; Bamber, J.; Bojunga, J.; Correas, J.-M.; Gilja, O.H.; Klauser, A.; Sporea, I.; Calliada, F.; Cantisani, V.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography. Part 2: Clinical Applications. Ultraschall der Med. Eur. J. Ultrasound 2013, 34, 238–253.
- Di Leo, M.; Poliani, L.; Rahal, D.; Auriemma, F.; Anderloni, A.; Ridolfi, C.; Spaggiari, P.; Capretti, G.; Di Tommaso, L.; Preatoni, P.; et al. Pancreatic Neuroendocrine Tumours: The Role of Endoscopic Ultrasound Biopsy in Diagnosis and Grading Based on the WHO 2017 Classification. Dig. Dis. 2019, 37, 325–333.
- Artale, S.; Giannetta, L.; Cerea, G.; Maggioni, D.; Pedrazzoli, P.; Schiavetto, I.; Napolitano, M.; Veronese, S.; Bramerio, E.; Gambacorta, M.; et al. Treatment of metastatic neuroendocrine carcinoma based on WHO classification. Anticancer Res. 2005, 25, 4463–4469.
- Chaudhry, A.; Öberg, K.; Wilander, E. A Study of Biological Behavior Based on the Expression of a Proliferating Antigen in Neuroendocrine Tumors of the Digestive System. Tumor Biol. 1992, 13, 27–35.
- Couvelard, A.; Deschamps, L.; Ravaud, P.; Baron, G.; Sauvanet, A.; Hentic, O.; Colnot, N.; Paradis, V.; Belghiti, J.; Bedossa, P.; et al. Heterogeneity of tumor prognostic markers: A reproducibility study applied to liver metastases of pancreatic endocrine tumors. Mod. Pathol. 2008, 22, 273–281.
- Yang, Z.; Tang, L.H.; Klimstra, D.S. Effect of tumor heterogeneity on the assessment of Ki-67 labeling index in well differentiated neuroendocrine tumors metastatic to the liver: Implications for prognostic stratification. Am. J. Surg. Pathol. 2011, 35, 853–860.
- Benini, L.; Gabbrielli, A.; Cristofori, C.; Amodio, A.; Butturini, G.; Cardobi, N.; Sozzi, C.; Frulloni, L.; Mucelli, R.P.; Crinò, S.; et al. Residual pancreatic function after pancreaticoduodenectomy is better preserved with pancreaticojejunostomy than pancreaticogastrostomy: A long-term analysis. Pancreatology 2019, 19, 595–601.
- Adsay, V. Ki67 Labeling Index in Neuroendocrine Tumors of the Gastrointestinal and Pancreatobiliary Tract. Am. J. Surg. Pathol. 2012, 36, 1743–1746.
- Ekeblad, S.; Skogseid, B.; Dunder, K.; Öberg, K.; Eriksson, B. Prognostic Factors and Survival in 324 Patients with Pancreatic Endocrine Tumor Treated at a Single Institution. Clin. Cancer Res. 2008, 14, 7798–7803.
- La Rosa, S.; Klersy, C.; Uccella, S.; Dainese, L.; Albarello, L.; Sonzogni, A.M.; Doglioni, C.; Capella, C.; Solcia, E. Improved histologic and clinicopathologic criteria for prognostic evaluation of pancreatic endocrine tumors. Hum. Pathol. 2009, 40, 30–40.
- Pape, U.-F.; Berndt, U.; Müller-Nordhorn, J.; Böhmig, M.; Roll, S.; Koch, M.; Willich, S.N.; Wiedenmann, B. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr. Relat. Cancer 2008, 15, 1083–1097.
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Klöppel, G.; et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171.
- Larghi, A.; Manfrin, E.; Fabbri, C.; Crinò, S.F.; Correale, L.; Chiarello, G.; Barresi, L.; Van Velthuysen, M.-L.; Poley, J.W.; Rahal, D.; et al. Interobserver agreement among expert pathologists on through-the-needle microforceps biopsy samples for evaluation of pancreatic cystic lesions. Gastrointest. Endosc. 2019, 90, 784–792.e4.
- Capurso, G.; Archibugi, L.; Petrone, M.C.; Arcidiacono, P.G. Slow-pull compared to suction technique for EUS-guided sampling of pancreatic solid lesions: A meta-analysis of randomized controlled trials. Endosc. Int. Open 2020, 8, E636–E643.
- Polkowski, M.; Jenssen, C.C.; Kaye, P.V.; Carrara, S.; Deprez, P.; Ginès, A.; Fernández-Esparrach, G.G.; Eisendrath, P.; Aithal, G.P.; Arcidiacono, P.P.; et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline–March 2017. Endoscopy 2017, 49, 989–1006.
- Paiella, S.; Landoni, L.; Rota, R.; Valenti, M.; Elio, G.; Crinò, S.F.; Manfrin, E.; Parisi, A.; Cingarlini, S.; D’Onofrio, M.; et al. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis and grading of pancreatic neuroendocrine tumors: A retrospective analysis of 110 cases. Endoscopy 2020, 52, 988–994.
- Pezzilli, R.; Partelli, S.; Cannizzaro, R.; Pagano, N.; Crippa, S.; Pagnanelli, M.; Falconi, M. Ki-67 prognostic and therapeutic decision driven marker for pancreatic neuroendocrine neoplasms (PNENs): A systematic review. Adv. Med. Sci. 2016, 61, 147–153.
- Larghi, A.; Rimbaş, M.; Crino, S.F.; Gasbarrini, A.; Costamagna, G.; Scarpa, A. EUS-guided fine-needle tissue acquisition for solid pancreatic lesions: Finally moving from fine-needle aspiration to fine-needle biopsy? Endosc. Ultrasound 2018, 7, 137–140.
- Crinò, S.F.; Le Grazie, M.; Manfrin, E.; Bellocchi, M.C.C.; Bernardoni, L.; Granato, A.; Locatelli, F.; Parisi, A.; Di Stefano, S.; Frulloni, L.; et al. Randomized trial comparing fork-tip and side-fenestrated needles for EUS-guided fine-needle biopsy of solid pancreatic lesions. Gastrointest. Endosc. 2020, 92, 648–658.e2.
- Eusebi, L.H.; Thorburn, D.; Toumpanakis, C.; Frazzoni, L.; Johnson, G.; Vessal, S.; Luong, T.V.; Caplin, M.; Pereira, S.P. Endoscopic ultrasound-guided fine-needle aspiration vs fine-needle biopsy for the diagnosis of pancreatic neuroendocrine tumors. Endosc. Int. Open 2019, 7, E1393–E1399.
- Crinò, S.F.; Ammendola, S.; Meneghetti, A.; Bernardoni, L.; Bellocchi, M.C.C.; Gabbrielli, A.; Landoni, L.; Paiella, S.; Pin, F.; Parisi, A.; et al. Comparison between EUS-guided fine-needle aspiration cytology and EUS-guided fine-needle biopsy histology for the evaluation of pancreatic neuroendocrine tumors. Pancreatology 2021, 21, 443–450.
- Leeds, J.S.; Nayar, M.K.; Bekkali, N.L.; Wilson, C.H.; Johnson, S.J.; Haugk, B.; Darne, A.; Oppong, K.W. Endoscopic ultrasound-guided fine-needle biopsy is superior to fine-needle aspiration in assessing pancreatic neuroendocrine tumors. Endosc. Int. Open 2019, 7, E1281–E1287.
- Crinò, S.F.; Larghi, A.; Bernardoni, L.; Parisi, A.; Frulloni, L.; Gabbrielli, A.; Parcesepe, P.; Scarpa, A.; Manfrin, E. Touch imprint cytology on endoscopic ultrasound fine-needle biopsy provides comparable sample quality and diagnostic yield to standard endoscopic ultrasound fine-needle aspiration specimens in the evaluation of solid pancreatic lesions. Cytopathology 2019, 30, 179–186.
- Crinò, S.F.; Di Mitri, R.; Nguyen, N.Q.; Tarantino, I.; de Nucci, G.; Deprez, P.H.; Carrara, S.; Kitano, M.; Shami, V.M.; Fernández-Esparrach, G.; et al. EUS-guided fine-needle biopsy with or without rapid on-site evaluation for diagnosis of solid pancreatic lesions: A randomized controlled non-inferiority trial. Gastroenterology 2021.
- Iglesias-Garcia, J.; Lindkvist, B.; Lariño-Noia, J.; Domínguez-Muñoz, J. Endoscopic ultrasound elastography. Endosc. Ultrasound 2012, 1, 8.
- Hirooka, Y.; Kuwahara, T.; Irisawa, A.; Itokawa, F.; Uchida, H.; Sasahira, N.; Kawada, N.; Itoh, Y.; Shiina, T. JSUM ultrasound elastography practice guidelines: Pancreas. J. Med. Ultrason. 2015, 42, 151–174.
- Carrara, S.; Di Leo, M.; Grizzi, F.; Correale, L.; Rahal, D.; Anderloni, A.; Auriemma, F.; Fugazza, A.; Preatoni, P.; Maselli, R.; et al. EUS elastography (strain ratio) and fractal-based quantitative analysis for the diagnosis of solid pancreatic lesions. Gastrointest. Endosc. 2018, 87, 1464–1473.
- Ignee, A.; Jenssen, C.; Arcidiacono, P.G.; Hocke, M.; Möller, K.; Saftoiu, A.; Will, U.; Fusaroli, P.; Iglesias-Garcia, J.; Ponnudurai, R.; et al. Endoscopic ultrasound elastography of small solid pancreatic lesions: A multicenter study. Endoscopy 2018, 50, 1071–1079.
- Rana, S.S.; Vilmann, P. Endoscopic ultrasound features of chronic pancreatitis: A pictorial review. Endosc. Ultrasound 2015, 4, 10–14.
- Giovannini, M.; Hookey, L.C.; Bories, E.; Pesenti, C.; Monges, G.; Delpero, J.R. Endoscopic ultrasound elastography: The first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy 2006, 38, 344–348.
- Ohno, E.; Kawashima, H.; Ishikawa, T.; Iida, T.; Suzuki, H.; Uetsuki, K.; Yashika, J.; Yamada, K.; Yoshikawa, M.; Gibo, N.; et al. Diagnostic performance of endoscopic ultrasonography-guided elastography for solid pancreatic lesions: Shear-wave measurements versus strain elastography with histogram analysis. Dig. Endosc. 2021, 33, 629–638.
- Agarwala, R.; Rana, S.S. Endoscopic Ultrasound Elastography for Solid Pancreatic Lesions: Ready to Replace Fineneedle Biopsy? J. Dig. Endosc. 2018, 9, 141–143.
- Saftoiu, A.; Napoleon, B.; Arcidiacono, P.G.; Braden, B.; Burmeister, S.; Carrara, S.; Cui, X.W.; Fusaroli, P.; Gottschalk, U.; Hocke, M.; et al. Do we need contrast for EUS? Endosc. Ultrasound 2020, 9, 361–368.
- Palazzo, M.; Napoléon, B.; Gincul, R.; Pioche, M.; Pujol, B.; Lefort, C.; Fumex, F.; Hautefeuille, V.; Fabre, M.; Cros, J.; et al. Contrast harmonic EUS for the prediction of pancreatic neuroendocrine tumor aggressiveness (with videos). Gastrointest. Endosc. 2018, 87, 1481–1488.
- Crinò, S.F.; Bernardoni, L.; Manfrin, E.; Parisi, A.; Gabbrielli, A. Endoscopic ultrasound features of pancreatic schwannoma. Endosc. Ultrasound 2016, 5, 396–398.
- Yamashita, Y.; Shimokawa, T.; Napoléon, B.; Fusaroli, P.; Gincul, R.; Kudo, M.; Kitano, M. Value of contrast-enhanced harmonic endoscopic ultrasonography with enhancement pattern for diagnosis of pancreatic cancer: A meta-analysis. Dig. Endosc. 2019, 31, 125–133.
- Fusaroli, P.; Napoleon, B.; Gincul, R.; Lefort, C.; Palazzo, L.; Palazzo, M.; Kitano, M.; Minaga, K.; Caletti, G.; Lisotti, A. The clinical impact of ultrasound contrast agents in EUS: A systematic review according to the levels of evidence. Gastrointest. Endosc. 2016, 84, 587–596.e10.
- Sakamoto, H.; Kitano, M.; Suetomi, Y.; Maekawa, K.; Takeyama, Y.; Kudo, M. Utility of Contrast-Enhanced Endoscopic Ultrasonography for Diagnosis of Small Pancreatic Carcinomas. Ultrasound Med. Biol. 2008, 34, 525–532.
- Kitano, M.; Kudo, M.; Yamao, K.; Takagi, T.; Sakamoto, H.; Komaki, T.; Kamata, K.; Imai, H.; Chiba, Y.; Okada, M.; et al. Characterization of Small Solid Tumors in the Pancreas: The Value of Contrast-Enhanced Harmonic Endoscopic Ultrasonography. Am. J. Gastroenterol. 2012, 107, 303–310.
- Ishikawa, T.; Itoh, A.; Kawashima, H.; Ohno, E.; Matsubara, H.; Itoh, Y.; Nakamura, Y.; Nakamura, M.; Miyahara, R.; Hayashi, K.; et al. Usefulness of EUS combined with contrast-enhancement in the differential diagnosis of malignant versus benign and preoperative localization of pancreatic endocrine tumors. Gastrointest. Endosc. 2010, 71, 951–959.
- Palazzo, M.; Palazzo, L.; Aubert, A.; Maire, F.; Fabre, M.; Felce, M.; Cros, J.; Couvelard, A.; Hentic-Dhomé, O.; Hammel, P.; et al. 665 Contrast Harmonic Endoscopic Ultrasonography Is Able to Predict Aggressivity in Pancreatic Neuroendocrine Tumors. Gastroenterology 2015, 148.
- Takada, S.; Kato, H.; Saragai, Y.; Muro, S.; Uchida, D.; Tomoda, T.; Matsumoto, K.; Horiguchi, S.; Tanaka, N.; Okada, H. Contrast-enhanced harmonic endoscopic ultrasound using time–intensity curve analysis predicts pathological grade of pancreatic neuroendocrine neoplasm. J. Med. Ultrason. 2019, 46, 449–458.
- Matsubara, H.; Itoh, A.; Kawashima, H.; Kasugai, T.; Ohno, E.; Ishikawa, T.; Itoh, Y.; Nakamura, Y.; Hiramatsu, T.; Nakamura, M.; et al. Dynamic Quantitative Evaluation of Contrast-Enhanced Endoscopic Ultrasonography in the Diagnosis of Pancreatic Diseases. Pancreas 2011, 40, 1073–1079.
- Hill, J.S.; Mc Phee, J.T.; Mc Dade, T.P.; Zhou, Z.; Sullivan, M.E.; Whalen, G.F.; Tseng, J.F. Pancreatic neuroendocrine tumors: The impact of surgical resection on survival. Cancer 2009, 115, 741–745.
- Jilesen, A.P.; van Eijck, C.H.; Bush, O.R.; van Gulik, T.M.; Gouma, D.J.; van Dijkum, E.J. Postoperative outcomes of enucleation and standard resections in patients with a pancreatic neuroendocrine tumor. World J. Surg. 2016, 40, 715–728.
- Jilesen, A.P.; van Eijck, C.H.; In’t Hof, K.H.; van Dieren, S.; Gouma, D.J.; van Dijkum, E.J.M. Postoperative complications, in hospital mortality and 5 years survival after surgical resection for patients with pancreatic neuroendocrine tumor. A systematic review. World J. Surg. 2016, 40, 729–748.
- Law, J.K.; Singh, V.K.; Khashab, M.A.; Hruban, R.H.; Canto, M.I.; Shin, E.J.; Saxena, P.; Weiss, M.J.; Pawlik, T.M.; Wolfgang, C.L.; et al. Endoscopic ultrasound (EUS)-guided fiducial placement allows localization of small neuroendocrine tumors during parenchymal-sparing pancreatic surgery. Surg. Endosc. 2013, 27, 3921–3926.
- Leelasinjaroen, P.; Manatsathit, W.; Berri, R.; Barawi, M.; Gress, F.G. Role of preoperative endoscopic ultrasound-guided fine-needle tattoing of pancreatic head insulinoma. World J. Gastrointest Endosc. 2014, 6, 506–509.
- Nikfarjam, M.; Warshaw, A.L.; Axelrod, L.; Deshpande, V.; Thayer, S.; Ferrone, C.R.; Fernandez-del, C.C. Improved contemporary surgical management of insulinomas: A 25-year experience at the Massachussetts General Hospital. Ann. Surg. 2008, 247, 165–172.
- Falconi, M.; Mantovani, W.; Crippa, S.; Mascetta, G.; Salvia, R.; Pederzoli, P. Pancreatic insufficiency after different resections for benign tumors. Br. J. Surg. 2008, 95, 85–91.
- Rimbaş, M.; Horumbă, M.; Rizzatti, G.; Crinò, S.F.; Gasbarrini, A.; Costamagna, G.; Larghis, A. Interventional endoscopic ultrasound for pancreatic neuroendocrine neoplasms. Dig. Endosc. 2020, 32, 1031–1041.
- Gress, F.G.; Barawi, M.; Kim, D.; Grendell, J.H. Preoperative localization of neuroendocrine tumor of the pancreas with EUS-guided fine needle Tattoing. Gastrointest. Endosc. 2002, 55, 594–597.
- Lennon, A.M.; Newman, N.; Makary, M.A.; Edil, B.H.; Shin, E.J.; Khashab, M.A.; Hruban, R.H.; Wolfgang, C.L.; Schulick, R.D.; Cando, S.G.M.I. EUS-guided tattoing before laparoscopic distal pancreatic resection. Gastrointest. Endosc. 2010, 72, 1089–1094.
- Rimbaş, M.; Larghi, A.; Fusaroli, P.; Dong, Y.; Hollerbach, S.; Jenssen, C.; Săftoiu, A.; Sahai, A.V.; Napoleon, B. How to perform EUS guided tattooing? Endosc. Ultrasound 2020, 9, 291–297.
- Han, J.; Chang, K.J. Endoscopic Ultrasound-Guided Direct Intervention for Solid Pancreatic Tumors. Clin. Endosc. 2017, 50, 126–137.
- Zhang, W.-Y.; Li, Z.-S.; Jin, Z.-D. Endoscopic ultrasound-guided ethanol ablation therapy for tumors. World J. Gastroenterol. 2013, 19, 3397.
- Gelczer, R.K.; Charboneau, J.W.; Hussain, S.; Brown, D.L. Complication of percutaneus ethanol ablation. J. Ultrasound Med. 1998, 17, 531–533.
- Jürgensen, C.; Schuppan, D.; Neser, F.; Ernstberger, J.; Junghans, U.; Stölzel, U. EUS-guided alcohol ablation of an insulinoma. Gastrointest. Endosc. 2006, 63, 1059–1062.
- Muscatiello, N.; Salcuni, A.; Macarini, L.; Cignarelli, M.; Prencipe, S.; Di Maso, M.; Castriota, M.; D’Agnessa, V.; Ierardi, E. Treatment of a pancreatic endocrine tumor by ethanol injection guided by endoscopic ultrasound. Endoscopy 2008, 40 (Suppl. 2), E258–E259.
- Deprez, P.H.; Claessens, A.; Borbath, I.; Gigot, J.F.; Maiter, D. Successful endoscopic ultrasound-guided ethanol ablation of a sporadic insulinoma. Acta Gastro Enterol. Belg. 2009, 71, 333–337.
- Vleggaar, F.P.; De Vaate, E.A.B.; Valk, G.D.; Leguit, R.J.; Siersema, P.D. Endoscopic ultrasound-guided ethanol ablation of a symptomatic sporadic insulinoma. Endoscopy 2011, 43 (Suppl. 2), E328–E329.
- Levy, M.J.; Thompson, G.B.; Topazian, M.D.; Callstrom, M.R.; Grant, C.S.; Vella, A. US-guided ethanol ablation of insulinomas: A new treatment option. Gastrointest. Endosc. 2012, 75, 200–206.
- Park, D.H.; Choi, J.-H.; Oh, D.; Lee, S.S.; Seo, D.-W.; Lee, S.K.; Kim, M.-H. Endoscopic Ultrasonography-Guided Ethanol Ablation for Small Pancreatic Neuroendocrine Tumors: Results of a Pilot Study. Clin. Endosc. 2015, 48, 158–164.
- Choi, J.; Park, D.H.; Kim, M.; Hwang, H.S.; Hong, S.; Song, T.J.; Lee, S.S.; Seo, D.; Lee, S.K. Outcomes after endoscopic ultrasound-guided ethanol-lipiodol ablation of small pancreatic neuroendocrine tumors. Dig. Endosc. 2018, 30, 652–658.
- Larghi, A.; Rizzatti, G.; Rimbaş, M.; Crino, S.F.; Gasbarrini, A.; Costamagna, G. EUS_guided radiofrequency ablation as ana alternative to surgery for pancreatic neuroendocrine neoplasms: Who should we treat? Endosc. Ultrasound 2019, 8, 220–226.
- Lakhtakia, S. Therapy of Pancreatic Neuroendocrine Tumors: Fine Needle Intervention including Ethanol and Radiofrequency Ablation. Clin. Endosc. 2017, 50, 546–551.
- Armellini, E.S.F.C.E.; Crinò, S.F.; Ballarè, M.; Pallio, S.; Occhipinti, P. Endoscopic ultrasound-guided ethanol ablation of pancreatic neuroendocrine tumours: A case study and literature review. World J. Gastrointest. Endosc. 2016, 8, 192–197.
- Simon, C.J.; Dupuy, D.E.; Mayo-Smith, W.W. Microwave Ablation: Principles and Applications. Radiographics 2005, 25 (Suppl. 1), S69–S83.
- Gazelle, G.S.; Goldberg, S.N.; Solbiati, L.; Livraghi, T. Tumor Ablation with Radio-frequency Energy. Radiology 2000, 217, 633–646.
- Lakhtakia, S.; Seo, D. Endoscopic ultrasonography-guided tumor ablation. Dig. Endosc. 2017, 29, 486–494.
- Lakhtakia, S.; Ramchandani, M.; Galasso, D.; Gupta, R.; Venugopal, S.; Kalpala, R.; Reddy, D.N. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos). Gastrointest. Endosc. 2016, 83, 234–239.
- Paiella, S.; De Pastena, M.; D’Onofrio, M.; Crinò, S.F.; Pan, T.L.; De Robertis, R.; Elio, G.; Martone, E.; Bassi, C.; Salvia, R. Palliative therapy in pancreatic cancer—Interventional treatment with radiofrequency ablation/irreversible electroporation. Transl. Gastroenterol. Hepatol. 2018, 3, 80.
- Armellini, E.; Crinò, S.F.; Ballarè, M.; Occhipinti, P. Endoscopic ultrasound-guided radiofrequency ablation of a pancreatic neuroendocrine tumor. Endoscopy 2015, 47 (Suppl. 1), E600–E601.
- Limmer, S.; Huppert, P.E.; Juette, V.; Lenhart, A.; Welte, M.; Wietholtz, H. Radiofrequency ablation of solitary pancreatic insulinoma in patient with episodes of severe hypoglicemia. Eur. J. Gastroenterol. Hepatol 2009, 21, 1097–1101.
- Rossi, S.; Viera, F.T.; Ghittoni, G.; Cobianchi, L.; Rosa, L.L.; Siciliani, L.; Bortolotto, C.; Veronese, L.; Vercelli, A.; Gallotti, A.; et al. Radiofrequency ablation of pancreatic neuroendocrine tumors: A pilot study of feasibility, efficacy and safety. Pancreas 2014, 43, 938–945.
- Pai, M.; Habib, N.; Senturk, H.; Lakhtakia, S.; Reddy, N.; Cicinnati, V.R.; Kaba, I.; Backebaum, S.; Drymousis, P.; Kaheleh, M.; et al. Endoscopic ultrasound-guided radiofrequency ablation, pancreatic cystic neoplasms and neuroendocrine tumors. World J. Gastrointest. Surg. 2015, 7, 52.
- Waung, J.A.; Todd, J.F.; Keane, M.G.; Pereira, S.P. Successful management of a sporadic pancreatic insulinoma by endoscopic ultrasound-guided radiofrequency ablation. Endoscopy 2016, 48 (Suppl. 1), E144–E145.
- Bas-Cutrina, F.; Bargalló, D.; Gornals, J.B. Small pancreatic insulinoma: Successful endoscopic ultrasound-guided radiofrequency ablation in a single session using a 22-G fine needle. Dig. Endosc. 2017, 29, 636–638.
- Choi, J.-H.; Seo, D.-W.; Song, T.J.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.-H. Endoscopic ultrasound-guided radiofrequency ablation for management of benign solid pancreatic tumors. Endoscopy 2018, 50, 1099–1104.
- Barthet, M.; Giovannini, M.; Lesavre, N.; Boustiere, C.; Napoleon, B.; Koch, S.; Gasmi, M.; Vanbiervliet, G.; Gonzalez, J.-M. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: A prospective multicenter study. Endoscopy 2019, 51, 836–842.
- Oleinikov, K.; Dancour, A.; Epshtein, J.; Benson, A.; Mazeh, H.; Tal, I.; Matalon, S.; Benbassat, C.A.; Livovsky, D.M.; Goldin, E.; et al. Endoscopic Ultrasound-Guided Radiofrequency Ablation: A New Therapeutic Approach for Pancreatic Neuroendocrine Tumors. J. Clin. Endocrinol. Metab. 2019, 104, 2637–2647.




