
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marsha Pierce | + 1790 word(s) | 1790 | 2021-08-04 08:23:37 | | | |
| 2 | Dean Liu | Meta information modification | 1790 | 2021-08-05 04:48:45 | | | | |
| 3 | Dean Liu | Meta information modification | 1790 | 2021-08-05 04:49:22 | | |
Video Upload Options
Traumatic brain injury (TBI) is defined as an injury caused by an external force that results in the disruption of normal brain function. In the United States, between 2016–2017, there were approximately 451,000 cases of TBI that resulted in hospitalization. The most common mechanisms of injury contributing to TBI were unintentional falls and motor vehicle crashes.
1. Traumatic Brain Injury and Consciousness
Traumatic brain injury (TBI) is defined as an injury caused by an external force that results in the disruption of normal brain function. In the United States, between 2016–2017, there were approximately 451,000 cases of TBI that resulted in hospitalization. The most common mechanisms of injury contributing to TBI were unintentional falls and motor vehicle crashes [1]. Following a severe TBI, disorders of consciousness (DoC) are common sequela [2][3]. Clinical features correlated with prognosis include age and severity of the TBI [2][4][5][6][7]. In several studies, there is an inverse correlation between the probability of recovering from a DoC and the duration after the injury [5][6][8]; however, some recovery has been observed in patients years after the initial injury [3][9]. The integrity and function of various neural structures and their relationship to consciousness are crucial for predicting outcomes and treating patients [10][11].
2. Consciousness
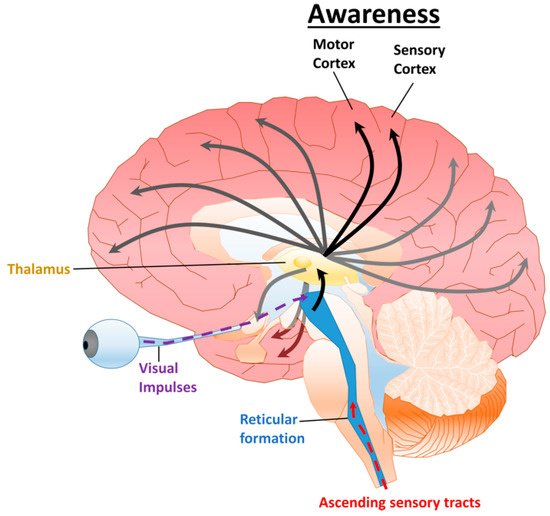
2.1. Disorders of Consciousness
| DoC | Arousal | Awareness | Apnea | Eye Opening | Communication |
|---|---|---|---|---|---|
| Brain Death | No | No | Artificial ventilation required | None | None |
| Coma | a No | b No | c Artificial ventilation required | None | None |
| VS/UWS | Yes | No | d Can breathe spontaneously without assistance | Spontaneous | Occasional moans and grunts |
| MCS− | Yes | Partial | d Can breathe spontaneously without assistance | Spontaneous | Occasional facial or vocal activity |
| MCS+ | Yes | Partial | d Can breathe spontaneously without assistance | Spontaneous | Some purposeful facial or vocal responses (inconsistent) |
2.2. Diagnosing Disorders of Consciousness
| Clinical Scoring System | Category | Score Range |
|---|---|---|
| Coma Recovery Scale-Revised (CRS-R) | Auditory Function Scale | 0–4 |
| Visual Function Scale | 0–5 | |
| Motor Function Scale | 0–6 | |
| Oromotor/Verbal Function Scale | 0–3 | |
| Communication Scale | 0–2 | |
| Arousal Scale | 0–3 | |
| Total Score | 0–23 | |
| Glasgow Coma Scale (GCS) | Eye Opening Response | 1–4 |
| Verbal Response | 1–5 | |
| Motor Response | 1–6 | |
| Total Score | 3–15 | |
| Simplified Evaluation of CONsciousness Disorders (SECONDs) | Observation | 0–1 |
| Command-Following | 0–1 | |
| Visual Pursuit | 0–1 | |
| Visual Fixation | 0–1 | |
| Oriented Behaviors | 0–1 | |
| Arousal | 0–1 | |
| * Communication | 0–1 | |
| * Localization of Pain | 0–1 | |
| Total Score | 0–8 | |
| Disability Rating Scale (DRS) | Eye Opening | 0–3 |
| Communication Ability | 0–4 | |
| Motor Response | 0–5 | |
| Feeding (Cognitive Ability Only) | 0–3 | |
| Toileting (Cognitive Ability Only) | 0–3 | |
| Grooming (Cognitive Ability Only) | 0–3 | |
| Level of Functioning (Physical, Mental, Emotional, Social) | 0–5 | |
| Employability | 0–3 | |
| Total Score | 0–29 |
| DoC | CRS-R | SECONDs | DRS |
| Coma | Not Applicable (N/A) | 0 | 29 |
| VS/UWS | N/A | 1 | 22–29 |
| MCS− | Eye Fixation | 2–5 | 17–21 |
| Attention | |||
| Automatic Motor Response | |||
| Localization of Noxious Stimulation | |||
| MCS+ | Consistent Movement to Command | 6–7 | 2–16 |
| Reproducible Movement to Command | |||
| Intelligible Verbalization | |||
| Non-Functional Intentional Communication | |||
| Emerging from MCS | Functional Object Use | 8 | <12 |
3. Pharmaceuticals
4. Electroceuticals
References
- Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Surveillance Report of Traumatic Brain Injury-Related Hospitalizations and Death by Agre Group, Sex, and Mechanism of Injury—United States, 2016 and 2017; Centers for Disease Control and Prevention, U.S. Department of Health and Human Services: Atlanta, GA, USA, 2021.
- Kowalski, R.G.; Hammond, F.M.; Weintraub, A.H.; Nakase-Richardson, R.; Zafonte, R.D.; Whyte, J.; Giacino, J.T. Recovery of Consciousness and Functional Outcome in Moderate and Severe Traumatic Brain Injury. JAMA Neurol. 2021, 78, 548–557.
- Hammond, F.M.; Giacino, J.T.; Nakase Richardson, R.; Sherer, M.; Zafonte, R.D.; Whyte, J.; Arciniegas, D.B.; Tang, X. Disorders of Consciousness due to Traumatic Brain Injury: Functional Status Ten Years Post-Injury. J. Neurotrauma 2019, 36, 1136–1146.
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK Coma Recovery Scale-Revised: Measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029.
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state (2). N. Engl. J. Med. 1994, 330, 1572–1579.
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state (1). N. Engl. J. Med. 1994, 330, 1499–1508.
- Ashwal, S.; Cranford, R. Medical aspects of the persistent vegetative state-a correction. The Multi-Society Task Force on PVS. New Engl. J. Med. 1995, 333, 130.
- Lucca, L.F.; Lofaro, D.; Pignolo, L.; Leto, E.; Ursino, M.; Cortese, M.D.; Conforti, D.; Tonin, P.; Cerasa, A. Outcome prediction in disorders of consciousness: The role of coma recovery scale revised. BMC Neurol. 2019, 19, 68.
- Lammi, M.H.; Smith, V.H.; Tate, R.L.; Taylor, C.M. The minimally conscious state and recovery potential: A follow-up study 2 to 5 years after traumatic brain injury. Arch. Phys. Med. Rehabil. 2005, 86, 746–754.
- Cabrera, L.Y.; Illes, J. Balancing ethics and care in disorders of consciousness. Lancet Neurol. 2018, 17, 112–113.
- Martens, G.; Bodien, Y.; Sheau, K.; Christoforou, A.; Giacino, J.T. Which behaviours are first to emerge during recovery of consciousness after severe brain injury? Ann. Phys. Rehabil Med. 2020, 63, 263–269.
- Cloninger, C.R. Evolution of human brain functions: The functional structure of human consciousness. Aust. N. Z. J. Psychiatry 2009, 43, 994–1006.
- Morsella, E.; Godwin, C.A.; Jantz, T.K.; Krieger, S.C.; Gazzaley, A. Homing in on consciousness in the nervous system: An action-based synthesis. Behav. Brain Sci. 2016, 39, e168.
- Edlow, B.L.; Takahashi, E.; Wu, O.; Benner, T.; Dai, G.; Bu, L.; Grant, P.E.; Greer, D.M.; Greenberg, S.M.; Kinney, H.C.; et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J. Neuropathol. Exp. Neurol. 2012, 71, 531–546.
- Schiff, N.D. Recovery of consciousness after severe brain injury: The role of arousal regulation mechanisms and some speculation on the heart-brain interface. Cleve Clin. J. Med. 2010, 77 (Suppl. 3), S27–S33.
- Schiff, N.D. Recovery of consciousness after brain injury: A mesocircuit hypothesis. Trends Neurosci. 2010, 33, 1–9.
- Cotterill, R.M. Cooperation of the basal ganglia, cerebellum, sensory cerebrum and hippocampus: Possible implications for cognition, consciousness, intelligence and creativity. Prog. Neurobiol. 2001, 64, 1–33.
- Malone, C.; Erler, K.S.; Giacino, J.T.; Hammond, F.M.; Juengst, S.B.; Locascio, J.J.; Nakase-Richardson, R.; Verduzco-Gutierrez, M.; Whyte, J.; Zasler, N.; et al. Participation Following Inpatient Rehabilitation for Traumatic Disorders of Consciousness: A TBI Model Systems Study. Front. Neurol. 2019, 10, 1314.
- Bodien, Y.G.; Giacino, J.T.; Edlow, B.L. Functional MRI Motor Imagery Tasks to Detect Command Following in Traumatic Disorders of Consciousness. Front. Neurol. 2017, 8, 688.
- Giacino, J.T.; Katz, D.I.; Whyte, J. Neurorehabilitation in disorders of consciousness. Semin. Neurol. 2013, 33, 142–156.
- Williamson, T.; Ryser, M.D.; Ubel, P.A.; Abdelgadir, J.; Spears, C.A.; Liu, B.; Komisarow, J.; Lemmon, M.E.; Elsamadicy, A.; Lad, S.P. Withdrawal of Life-supporting Treatment in Severe Traumatic Brain Injury. JAMA Surg. 2020, 155, 723–731.
- Chaturvedi, J.; Mudgal, S.K.; Venkataram, T.; Gupta, P.; Goyal, N.; Jain, G.; Sharma, A.K.; Sharma, S.K.; Bendok, B.R. Coma recovery scale: Key clinical tool ignored enough in disorders of consciousness. Surg. Neurol. Int. 2021, 12, 93.
- Alcock, S.; Batoo, D.; Ande, S.R.; Grierson, R.; Essig, M.; Martin, D.; Trivedi, A.; Sinha, N.; Leeies, M.; Zeiler, F.A.; et al. Early diagnosis of mortality using admission CT perfusion in severe traumatic brain injury patients (ACT-TBI): Protocol for a prospective cohort study. BMJ Open 2021, 11, e047305.
- Machado, C.; Perez-Nellar, J.; Estevez, M.; Gonzalez, E. Evidence-based guideline update: Determining brain death in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2011, 76, 307, author reply 308–309.
- Wijdicks, E.F.; Varelas, P.N.; Gronseth, G.S.; Greer, D.M. Evidence-based guideline update: Determining brain death in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2010, 74, 1911–1918.
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84.
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.F.; et al. Comprehensive Systematic Review Update Summary: Disorders of Consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Arch. Phys. Med. Rehabil. 2018, 99, 1710–1719.
- Thibaut, A.; Panda, R.; Annen, J.; Sanz, L.R.D.; Naccache, L.; Martial, C.; Chatelle, C.; Aubinet, C.; Bonin, E.A.C.; Barra, A.; et al. Preservation of Brain Activity in Unresponsive Patients Identifies MCS Star. Ann. Neurol. 2021, 90, 89–100.
- Thibaut, A.; Bodien, Y.G.; Laureys, S.; Giacino, J.T. Minimally conscious state “plus”: Diagnostic criteria and relation to functional recovery. J. Neurol. 2020, 267, 1245–1254.
- Bruno, M.A.; Majerus, S.; Boly, M.; Vanhaudenhuyse, A.; Schnakers, C.; Gosseries, O.; Boveroux, P.; Kirsch, M.; Demertzi, A.; Bernard, C.; et al. Functional neuroanatomy underlying the clinical subcategorization of minimally conscious state patients. J. Neurol. 2012, 259, 1087–1098.
- Sanz, L.R.D.; Thibaut, A.; Edlow, B.L.; Laureys, S.; Gosseries, O. Update on neuroimaging in disorders of consciousness. Curr. Opin. Neurol. 2021, 34, 488–496.
- Jang, S.H.; Kwon, Y.H. The relationship between consciousness and the ascending reticular activating system in patients with traumatic brain injury. BMC Neurol. 2020, 20, 375.
- Edlow, B.L.; Chatelle, C.; Spencer, C.A.; Chu, C.J.; Bodien, Y.G.; O’Connor, K.L.; Hirschberg, R.E.; Hochberg, L.R.; Giacino, J.T.; Rosenthal, E.S.; et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain 2017, 140, 2399–2414.
- Chatelle, C.; Rosenthal, E.S.; Bodien, Y.G.; Spencer-Salmon, C.A.; Giacino, J.T.; Edlow, B.L. EEG Correlates of Language Function in Traumatic Disorders of Consciousness. Neurocrit. Care 2020, 33, 449–457.
- Scarpino, M.; Lolli, F.; Hakiki, B.; Lanzo, G.; Sterpu, R.; Atzori, T.; Portaccio, E.; Draghi, F.; Amantini, A.; Grippo, A.; et al. EEG and Coma Recovery Scale-Revised prediction of neurological outcome in Disorder of Consciousness patients. Acta Neurol. Scand. 2020, 142, 221–228.
- Stender, J.; Gosseries, O.; Bruno, M.A.; Charland-Verville, V.; Vanhaudenhuyse, A.; Demertzi, A.; Chatelle, C.; Thonnard, M.; Thibaut, A.; Heine, L.; et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: A clinical validation study. Lancet 2014, 384, 514–522.
- Seel, R.T.; Sherer, M.; Whyte, J.; Katz, D.I.; Giacino, J.T.; Rosenbaum, A.M.; Hammond, F.M.; Kalmar, K.; Pape, T.L.; Zafonte, R.; et al. Assessment scales for disorders of consciousness: Evidence-based recommendations for clinical practice and research. Arch. Phys. Med. Rehabil. 2010, 91, 1795–1813.
- Sattin, D.; Minati, L.; Rossi, D.; Covelli, V.; Giovannetti, A.M.; Rosazza, C.; Bersano, A.; Nigri, A.; Leonardi, M. The Coma Recovery Scale Modified Score: A new scoring system for the Coma Recovery Scale-revised for assessment of patients with disorders of consciousness. Int. J. Rehabil. Res. 2015, 38, 350–356.
- Slomine, B.S.; Suskauer, S.J.; Nicholson, R.; Giacino, J.T. Preliminary validation of the coma recovery scale for pediatrics in typically developing young children. Brain Inj. 2019, 33, 1640–1645.
- Cortese, M.D.; Riganello, F.; Arcuri, F.; Pugliese, M.E.; Lucca, L.F.; Dolce, G.; Sannita, W.G. Coma recovery scale-r: Variability in the disorder of consciousness. BMC Neurol. 2015, 15, 186.
- Formisano, R.; Contrada, M.; Ferri, G.; Schiattone, S.; Iosa, M.; Aloisi, M. The Glasgow Outcome Scale Extended-Revised (GOSE-R) to include Minimally Conscious State in the Vegetative State/Unresponsive Wakefulness Syndrome category: A correlation with Coma Recovery Scale-Revised (CRS-R). Eur. J. Phys. Rehabil. Med. 2019, 55, 139–140.
- Aubinet, C.; Cassol, H.; Bodart, O.; Sanz, L.R.D.; Wannez, S.; Martial, C.; Thibaut, A.; Martens, G.; Carriere, M.; Gosseries, O.; et al. Simplified Evaluation of CONsciousness Disorders (SECONDs) in individuals with severe brain injury: A validation study. Ann. Phys. Rehabil. Med. 2020, S1877-0657, 30160–30163.
- Sanz, L.R.D.; Aubinet, C.; Cassol, H.; Bodart, O.; Wannez, S.; Bonin, E.A.C.; Barra, A.; Lejeune, N.; Martial, C.; Chatelle, C.; et al. SECONDs Administration Guidelines: A Fast Tool to Assess Consciousness in Brain-injured Patients. J. Vis. Exp. 2021, 168, e61968.
- Rappaport, M.; Hall, K.M.; Hopkins, K.; Belleza, T.; Cope, D.N. Disability rating scale for severe head trauma: Coma to community. Arch. Phys. Med. Rehabil. 1982, 63, 118–123.
- Varjabic, M.; Bakran, Z.; Tusek, S.; Bujisic, G. Assessment of long-term activity limitations and participation restrictions of persons with traumatic brain injury using the disability rating scale. Coll Antropol 2010, 34 (Suppl. 1), 157–164.
- Kelsen, J.; Karlsson, M.; Hansson, M.J.; Yang, Z.; Fischer, W.; Hugerth, M.; Nordstrom, C.H.; Astrand, R.; Keep, M.F.; Kilbaugh, T.; et al. Copenhagen Head Injury Ciclosporin Study: A Phase IIa Safety, Pharmacokinetics, and Biomarker Study of Ciclosporin in Severe Traumatic Brain Injury Patients. J. Neurotrauma 2019, 36, 3253–3263.
- Giacino, J.T.; Whyte, J. Amantadine to improve neurorecovery in traumatic brain injury-associated diffuse axonal injury: A pilot double-blind randomized trial. J. Head Trauma Rehabil 2003, 18, 4–5, author reply 5-6.
- Giacino, J.T.; Whyte, J.; Bagiella, E.; Kalmar, K.; Childs, N.; Khademi, A.; Eifert, B.; Long, D.; Katz, D.I.; Cho, S.; et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N. Engl. J. Med. 2012, 366, 819–826.
- Alkhachroum, A.; Eliseyev, A.; Der-Nigoghossian, C.A.; Rubinos, C.; Kromm, J.A.; Mathews, E.; Bauerschmidt, A.; Doyle, K.; Velasquez, A.; Egbebike, J.A.; et al. EEG to detect early recovery of consciousness in amantadine-treated acute brain injury patients. J. Neurol. Neurosurg. Psychiatry 2020, 91, 675–676.
- Ghalaenovi, H.; Fattahi, A.; Koohpayehzadeh, J.; Khodadost, M.; Fatahi, N.; Taheri, M.; Azimi, A.; Rohani, S.; Rahatlou, H. The effects of amantadine on traumatic brain injury outcome: A double-blind, randomized, controlled, clinical trial. Brain Inj. 2018, 32, 1050–1055.
- Hughes, S.; Colantonio, A.; Santaguida, P.L.; Paton, T. Amantadine to enhance readiness for rehabilitation following severe traumatic brain injury. Brain Inj. 2005, 19, 1197–1206.
- Fridman, E.A.; Calvar, J.; Bonetto, M.; Gamzu, E.; Krimchansky, B.Z.; Meli, F.; Leiguarda, R.C.; Zafonte, R. Fast awakening from minimally conscious state with apomorphine. Brain Inj. 2009, 23, 172–177.
- Fridman, E.A.; Krimchansky, B.Z.; Bonetto, M.; Galperin, T.; Gamzu, E.R.; Leiguarda, R.C.; Zafonte, R. Continuous subcutaneous apomorphine for severe disorders of consciousness after traumatic brain injury. Brain Inj. 2010, 24, 636–641.
- Sanz, L.R.D.; Lejeune, N.; Blandiaux, S.; Bonin, E.; Thibaut, A.; Stender, J.; Farber, N.M.; Zafonte, R.D.; Schiff, N.D.; Laureys, S.; et al. Treating Disorders of Consciousness with Apomorphine: Protocol for a Double-Blind Randomized Controlled Trial Using Multimodal Assessments. Front. Neurol. 2019, 10, 248.
- Clauss, R.P.; Guldenpfennig, W.M.; Nel, H.W.; Sathekge, M.M.; Venkannagari, R.R. Extraordinary arousal from semi-comatose state on zolpidem. A case report. S. Afr. Med. J. 2000, 90, 68–72.
- Bomalaski, M.N.; Claflin, E.S.; Townsend, W.; Peterson, M.D. Zolpidem for the Treatment of Neurologic Disorders: A Systematic Review. JAMA Neurol. 2017, 74, 1130–1139.
- Williams, S.T.; Conte, M.M.; Goldfine, A.M.; Noirhomme, Q.; Gosseries, O.; Thonnard, M.; Beattie, B.; Hersh, J.; Katz, D.I.; Victor, J.D.; et al. Common resting brain dynamics indicate a possible mechanism underlying zolpidem response in severe brain injury. eLife 2013, 2, e01157.
- Thonnard, M.; Gosseries, O.; Demertzi, A.; Lugo, Z.; Vanhaudenhuyse, A.; Bruno, M.A.; Chatelle, C.; Thibaut, A.; Charland-Verville, V.; Habbal, D.; et al. Effect of zolpidem in chronic disorders of consciousness: A prospective open-label study. Funct. Neurol. 2013, 28, 259–264.
- Sripad, P.; Rosenberg, J.; Boers, F.; Filss, C.P.; Galldiks, N.; Langen, K.J.; Clauss, R.; Shah, N.J.; Dammers, J. Effect of Zolpidem in the Aftermath of Traumatic Brain Injury: An MEG Study. Case Rep. Neurol. Med. 2020, 2020, 8597062.
- Khalili, H.; Rakhsha, A.; Ghaedian, T.; Niakan, A.; Masoudi, N. Application of Brain Perfusion SPECT in the Evaluation of Response to Zolpidem Therapy in Consciousness Disorder Due to Traumatic Brain Injury. Indian J. Nucl. Med. 2020, 35, 315–320.
- Zhang, B.; O’Brien, K.; Won, W.; Li, S. A Retrospective Analysis on Clinical Practice-Based Approaches Using Zolpidem and Lorazepam in Disorders of Consciousness. Brain Sci. 2021, 11, 726.
- Long, Y.; Li, J.; Yang, F.; Wang, J.; Wang, X. Wearable and Implantable Electroceuticals for Therapeutic Electrostimulations. Adv. Sci. 2021, 8, 2004023.
- Mishra, S. Electroceuticals in medicine—The brave new future. Indian Heart J. 2017, 69, 685–686.
- Johnson, R.L.; Wilson, C.G. A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res. 2018, 11, 203–213.
- Goyal, A.; Goetz, S.; Stanslaski, S.; Oh, Y.; Rusheen, A.E.; Klassen, B.; Miller, K.; Blaha, C.D.; Bennet, K.E.; Lee, K. The development of an implantable deep brain stimulation device with simultaneous chronic electrophysiological recording and stimulation in humans. Biosens. Bioelectron. 2021, 176, 112888.
- McLardy, T.; Ervin, F.; Mark, V.; Scoville, W.; Sweet, W. Attempted inset-electrodes-arousal from traumatic coma: Neuropathological findings. Trans. Am. Neurol. Assoc. 1968, 93, 25–30.
- Bourdillon, P.; Hermann, B.; Sitt, J.D.; Naccache, L. Electromagnetic Brain Stimulation in Patients With Disorders of Consciousness. Front. Neurosci. 2019, 13, 223.




