
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | yue luo | + 2090 word(s) | 2090 | 2021-07-21 16:31:02 | | | |
| 2 | Conner Chen | Meta information modification | 2090 | 2021-08-05 14:24:59 | | |
Video Upload Options
Flavor is one of the most important factors in attracting consumers and maximizing food quality, and the Maillard reaction (MR) is highly-involved in flavor formation. However, Maillard reaction products have a big drawback in their relatively low stability in thermal treatment and storage. Amadori rearrangement products (ARPs), MR intermediates, can alternatively act as potential flavor additives for their better stability and fresh flavor formation ability.
1. Introduction
The Maillard reaction has been one of the most important reactions in flavor generation since its first discovery by French chemist Louis-Camille Maillard in 1912 [1]. The sophisticated reaction cascades start with the formation of a Schiff base (glycosylamine) from the condensation reaction between the carbonyl groups of reducing sugars and amino groups of amino acids, peptides or proteins. The Schiff base then goes through Amadori rearrangement with a nucleophilic catalyst and forms a more stable 1-amino-1-deoxy-2-ketose, called Amadori rearrangement products (ARPs) [2]. While ARPs are specific for a Schiff base derived from aldose sugar, Heyns rearrangement products (HRPs, 2-amino-2-deoxyaldose) are derived from ketose sugar [3]. The successive breakdown of ARPs or HRPs generates abundant volatile carbocyclic and heterocyclic compounds, oligomers and polymers, thus bring various flavor and yellowish to brown colors to food products [4].
The Maillard reaction is commonly seen in thermally processed food with attractive and complex flavors, such as bread, cereal, coffee, roasted meat. These flavors mainly come from the formation of pyrazines, pyrroles, alkylpyridines, acylpyridines, furanones, furans, oxazoles, and thiophenes [5]. Each gives a unique flavor of cooked food. However, these Maillard reaction-derived flavors are of unsatisfactory stability. Food products likely go through high temperature processing like boiling, baking, frying or pasteurization and then a specific shelf life range from a few days to years before consumption. The loss of attractive flavor compounds such as acetaldehyde, furfural and butanal, has been a major concern regarding flavor quality and consumer acceptance [6]. Enormous efforts have been reported to stabilize flavor compounds, and among which nanoencapsulation is considered an efficient method [7]. Not until recently, have people started to pay attention to flavorless intermediates, ARPs. A big advantage of ARPs is better stability during storage and synchronous production of fresh and desirable flavors during thermal treatment, which makes them potential flavor enhancers and food additives. However, to our best knowledge, there is no comprehensive review on ARPs as potential food additives. Therefore, this review aims to, for the first time, elucidate aspects related to ARPs and discuss the possibility of being food additives.
For convenience, the ARP of amino acid/peptide and reducing sugar starting now is referred to as amino acid/peptide-reducing sugar-ARP. The ARP would share a similar chemical structure, named as N-(1-deoxy-d-fructos-1-yl)-amino acid/peptide (amino acid-glucose system) after the Amadori rearrangement. Some papers adopt abbreviations like fru-val for N-(1-deoxy-d-fructos-1-yl)-valine (herein after referred to as valine-glucose-ARP).
2. Occurrence in Foods
Starting from the 1950 s, ARPs have drawn attention, and an array of studies have focused on analyzing ARPs in foods since then. ARPs were first found in browned freeze-dried apricots using ion-exchange chromatography in 1958 [8]. Though the resulting spectrum is highly complicated, it proved the existence of ARPs. After that, purified glycine-, alanine- and valine-glucose-ARPs were present in beer malt and were also found in soy sauce together with isoleucine- and leucine-glucose-ARPs [9]. As shown in Table 1, seven amino acid-glucose ARPs were detected in cocoa, coffee, barley malt, wheat malt, wheat beer, bell pepper and tomato, of which tyrosine and histidine ARPs were found present for the first time in foods [10]. The drying of food accelerated ARP formation, and formed ARPs also degraded during roasting in coffee and cocoa. The highest ARP concentration is valine-glucose in unroasted cocoa and dried bell pepper at 342 and 3460 mg/kg, respectively. The results have been correlated with other studies and shown in Table 1 [11]. Eight ARPs existed in dried fruit and vegetables, milk powder, tomato juice and paste, and red peppers, and the total ranges from 1.36 to 3415 mg/100 g. The drying of tomato juice facilitated ARP formation, which was further increased under vacuum. Though amino acid-APRs are identified from foods, peptides like oligopeptides should also be a focus for their abundance in foods. Unfortunately, only a few studies paid attention and succeeded in detecting peptide-ARPs, such as carnosine-glucose-ARP from meat broth. Therefore, more attention should be paid to oligopeptide-ARP analysis in future studies to fill the gap.
Table 1. Concentration (mg/kg) of ARPs in different foods.
| Fru-Ile | Fru-Tyr | Fru-Phe | Fru-His | Fru-Met | Fru-Leu | Fru-Val | ||
| Cocoa (unroasted) | 104.00 ± 10.40 | 73.00 ± 2.92 | 104.00 ± 8.32 | 27.20 ± 1.36 | 2.33 ± 0.33 | 152.00 ± 12.16 | 342.00 ± 10.26 | |
| Cocoa (roasted) | 5.38 ± 0.32 | 3.04 ± 0.09 | 3.99 ± 0.28 | 0.62 ± 0.06 | 1.34 ± 0.12 | 6.47 ± 0.39 | 19.00 ± 0.95 | |
| Coffee (green) | 2.51 ± 0.13 | 0.18 ± 0.03 | 2.80 ± 0.81 | 0.36 ± 0.08 | 0.59 ± 0.08 | 4.65 ± 0.65 | 0.60 ± 0.03 | |
| Coffee (roasted) | 0.87 ± 0.14 | ND | 0.09 ± 0.02 | 15.33 ± 2.30 | ND | 0.81 ± 0.11 | ND | |
| Barley malt | 29.20 ± 2.04 | 11.00 ± 1.65 | 25.40 ± 3.05 | 13.10 ± 1.97 | 5.91 ± 0.95 | 33.80 ± 2.70 | 39.40 ± 4.73 | |
| Wheat malt | 27.80 ± 3.89 | 6.70 ± 0.34 | 21.10 ± 2.53 | 10.60 ± 1.06 | 4.31 ± 0.26 | 35.40 ± 5.00 | 148.00 ± 8.88 | |
| Wheat beer | 6.05 ± 0.12 | 2.91 ± 0.12 | 5.28 ± 0.05 | 2.77 ± 0.28 | ND | 11.50 ± 0.92 | 34.60 ± 0.00 | |
| Bell pepper | 0.10 ± 0.00 | ND | 0.08 ± 0.00 | 0.97 ± 0.20 | 0.10 ± 0.00 | 0.07 ± 0.01 | 0.58 ± 0.12 | |
| Bell pepper (DW) | 1.00 | ND | 0.80 | 9.70 | 1.00 | 0.70 | ND | |
| Bell pepper powder | 509.00 ± 15.27 | 210.00 ± 42.00 | 329.00 ± 26.32 | 405.00 ± 52.65 | 81.90 ± 4.10 | 592.00 ± 11.84 | 3460.00 ± 103.8 | |
| Tomato | 0.12 ± 0.01 | ND | 0.19 ± 0.03 | 0.57 ± 0.02 | ND | 0.16 ± 0.05 | ND | |
| Tomato (DW) | 2.40 | ND | 3.80 | 11.40 | ND | 3.20 | ND | |
| Tomato powder | 9.30 ± 1.67 | 7.96 ± 1.59 | 22.60 ± 3.39 | 45.00 ± 4.95 | ND | 9.60 ± 0.38 | 10.60 ± 1.70 | |
| Fru-Arg | Fru-Ala | Fru-Phe | Fru-His | Fru-Met | Fru-Leu | Fru-Val | Fru-Glu | |
| Dried strawberries (DW) | 8.4 ± 1.7 f | 30.5 ± 9.7 f | 9.0 ± 1.7 e | 12.5 ± 3.6 fg | 4.5 ± 2.2 e | 8.1 ± 1.6 e | 16.2 ± 4.5 d | 4.8 ± 2.0 d |
| Dried bananas (DW) | 2.3 ± 0.1 f | 1.6 ± 0.1 f | 0.2 ± 0.1 f | 8.2 ± 5.6 gh | 0.3 ± 0.2 e | 0.1 ± 0.1 e | 0.9 ± 0.3 d | ND |
| Dried taro (DW) | 2.3 ± 0.1 f | 74.5 ± 16.5 f | 11.4 ± 1.2 e | 16.6 ± 1.5 ef | 32.6 ± 8.1 c | 20.6 ± 8.9 e | ND | ND |
| Milk powder (DW) | 5.4 ± 0.3 f | ND | 0.2 ± 0.1 f | 0.2 ± 0.1 h | 0.7 ± 0.1 e | 0.2 ± 0.1 e | ND | ND |
| Pulled figs (DW) | 78.6 ± 3.7 e | 431.0 ± 43.8 d | 32.9 ± 2.7 d | 24.6 ± 1.4 e | 9.1 ± 0.7 de | 101.7 ± 24.1 d | 93.9 ± 20.3 c | ND |
| Tomato juice (DW) | 90.0 ± 0.8 e | ND | 10.0 ± 2.0 e | 10.0 ± 2.0 f | 16.0 ± 4.0 d | 10.0 ± 2.0 e | ND | 154.0 ± 1.5 b |
| Tomato paste (DW) | 156.0 ± 13.7 d | 295.2 ± 3.7 e | 428.7 ± 62.1 c | 186.0 ± 3.7 d | 46.3 ± 3.7 c | 262.5 ± 3.7 c | 103.3 ± 3.7 c | 1517.7 ± 3.7 a |
| Red pepper I (DW) | 2171.1 ± 103.5 b | 1291.5 ± 100.7 c | 974.3 ± 73.7 b | 454.4 ± 41.2 b | 300.0 ± 23.1 b | 1299.9 ± 90.8 b | 1341.5 ± 101.1 b | ND |
| Red pepper II (DW) | 1092.9 ± 101.1 c | 1952.2 ± 113.3 a | 836.1 ± 62.4 b | 264.5 ± 34.5 c | 240.2 ± 21.2 b | 1260.7 ± 123.1 b | 1453.9 ± 112.1 b | ND |
| Red pepper III (DW) | 24477.8 ± 212.6 a | 1479.7 ± 100.5 b | 2120.6 ± 183.7 a | 560.3 ± 49.2 a | 660.5 ± 65.2 a | 2613.6 ± 155.2 a | 2181.3 ± 143.5 a | 65.4 ± 8.7 c |
Data are mean values of triplicates (three separate work ups); Different letters (a, b, c, etc.) in the same column indicate significant (p < 0.05) differences between means. DW, dry weight; ND, not detected.
3. Stability
Stability is the most important factor affecting flavor quality, the decisive aspect of consumer acceptance. Flavor stability is affected by chemical reactivity itself, environmental factors such as light and oxygen, and influences from the food matrix, such as fat, metal ions, and radicals [12].
Maillard reaction products (MRPs) such as pyrrole and furan have been traditional key flavor compounds and food additives for decades [13]. However, one big defect of MRPs is their weak stability during storage and processing, especially the ones involved in thermal treatment. Pyranone, common in glucose- or lactose-rich food, and 3,4-dihydroxy-3-hexen-2,5-dione, a caramel odor, is significantly prone to degradation during short-term storage [14]. Furaneols, also known as strawberry furanone, are heat-labile and rapidly degrade preferably at low pH [15]. In contrast, studies have reported ARPs that were more stable during storage. Phenylalanine-xylose-ARP only reduced 6.49% after 2 months storage at room temperature while MRPs reduced more or less resulting in dramatic changes in flavor profile [16]. A consistent result is also reported for glutamic acid-glucose-ARP. In contrast to N-glycoside form rapidly degraded with acid catalyst, 13C NMR monitoring showed that glutamic acid-glucose-ARP in an aqueous solution maintained good stability for 3 days storage at pH 5 and room temperature, let alone with moderate or basic pH [17]. The opposite result for pH is shown in carnosine-glucose-ARP whose degradation rate increases with pH value increase [18]. This phenomenon depends on the amino acids/peptides’ chemical properties. Glutamic acid is acidic in aqueous solution while carnosine exists in basic form, thus are correspondingly more stable in acid and base conditions, respectively. Glycine, as the simplest amino acid, triggered more attention on its ARP investigation and model studies. As shown in Figure 1, the degradation pathways of glycine-glucose ARP in the aqueous system can be a reference for other ARP degradation studies [19].
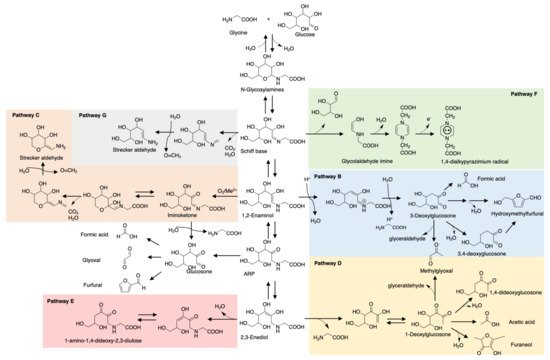
Figure 1. Degradation pathways of glycine-glucose-ARP. B. 1,2-Enolization pathway; C. Oxidative degradation pathway; D. 2,3-Enolization pathway; E. Elimination of C4-OH group of 2,3-enediol pathway; F. Cleavage of sugar moiety pathway; G. Decarboxylation pathway.
Besides the abovementioned associations with intrinsic chemical nature and pH value, the stability of ARPs is also related to temperature, water activity, and the presence of a catalyst or stabilizer. Temperature plays a leading role in ARP stability. It shows a positive correlation with ARP degradation rate as more energy providence brings in higher reactivity and instability, so is the water activity [18]. The formation and decomposition kinetics of ARPs in freeze-dried carrots were studied and found that the lower the water activity, the higher Ea of formation while the higher water content, the higher Ea of decomposition. Hence, dilute solutions of ARPs are more sensitive to changes in temperature than concentrated solutions [20]. A supporting study showed that methionine-glucose-ARP had accelerated the degradation rate under high temperature and elevated formation of 3-DG, 1-DG, glucosone, and other dicarbonyls such as glyoxal and methylglyoxal [21]. Similarly, tryptophan-glucose-ARP showed a several times faster decomposition rate under high temperature and/or high water activity [22]. However, these studies mainly focused on high temperatures over 100 °C in order to imitate the flavor generation process, seldom investigation explores the stability of ARPs under storage conditions systematically.
Based on previous studies, adding ARPs as seasoning is a promising way to prolong shelf life for low water reactivity and storage under low or room temperature. However, extensive use of ARPs in different food matrices is an ultimate goal as good flavor additives. In addition to the neutral aqueous system, various food systems should be considered, for instance, the acidic fluid environment present in juice and soft drinks, base fluids such as sparkling spring water or meat broth, emulsion systems such as milk and meat broth, solid systems such as bread, cookies, and chips, and frozen instant products such as pizza and meat products. However, few studies reported the stability of ARPs in such models, which should be a priority before heat treatment and need further research in addition to intrinsic flavor generation ability.
4. Taste
Many studies suggested that the ARPs of amino acids or peptides are correlated with umami or kokumi enhancing properties as shown in Figure 2. As glutamic acid and glucose are strongly correlated with umami taste, glutamic acid-glucose-ARP unsurprisingly showed a prominent umami-like taste at a low threshold of 1–2 mmol/L, close enough to that of monosodium glutamate (MSG) [17][23]. Accordingly, its aqueous solution exhibited distinct umami, seasoning, and bouillon-like taste similar to that containing equivalent MSG. Besides the purely chemical model, various studies have made an effort to isolate and identify umami-related compounds from food products, typically soy sauce. Only a few take a closer look at ARPs.
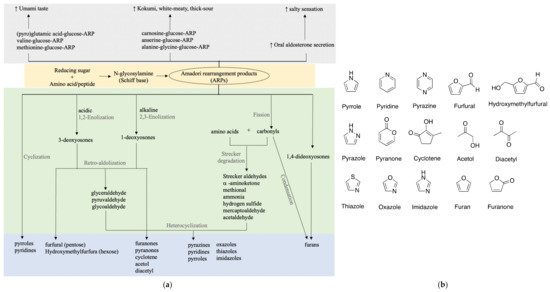
Figure 2. Taste and flavor formation of ARPs. (a) Taste enhancing properties and flavor formation of ARPs; (b) basic chemical structures of key flavors.
Pyroglutamic acid-, valine-, and methionine-glucose-ARPs were identified in a typical Japanese soy sauce named koikuchi shouyu at subthreshold concentrations, partially to contribute to the umami taste [24]. Similar umami enhancing was also reported for glutamic acid-glucose-ARP found in 25 commercial soy sauces [25]. A simultaneous quantification method of 20 ARPs in soy sauce using LC-MS was later proposed and verified in six types of soy sauce [26]. The omission of an ion-paring reagent and sample derivatization make it an applicable and propagable analytical method for ARP detection in broader food products. Besides soy sauce, umami or kokumi orosensation is always associated with meaty broths. Unsurprisingly, ARPs of dipeptide carnosine-glucose, anserine-glucose, alanine-glycine-glucose were elucidated to contribute to a white-meaty and thick-sour orosensation of chicken broth and traditional French meat-containing broth called Pot-au-Feu [27][28][29].
Roughly, ARPs exist in subthreshold concentrations individually in food products. Though not as the dominant and key taste/flavor component, they exhibit critical umami enhancing properties and the total sufficient amount and diversity affect the intensity and richness of the overall taste.
In addition to the umami taste, the salty taste is synchronously found in protein hydrolysates such as fish protein and peanut protein hydrolysates [30]. As mixtures of diverse amino acids and peptides, the protein hydrolysates may contain plenty of ARPs and correlate with a salty sensation. A supporting study was demonstrated in enzymatic hydrolysates of pea protein [31]. The concentration of Maillard reaction intermediates (MRIs) including ARPs derived from pea protein hydrolysates showed a positive correlation with umami and saltiness. Only 0.1% dosage of MRIs can attain 20% salt reduction. Further evidence was from direct evaluation of the pure ARPs. Proline-glucose-ARP is reported to provide a salty sensation as well as significant umami enhancement [32]. Electronic tongue and sensory evaluation confirmed that 0.4% of ARP addition contributed to 20% salt reduction in achieving equivalent saltiness. The mechanism comes from elevated oral aldosterone secretion stimulated by ARPs, which is strongly associated with salt sensitivity [33]. These studies illustrated that ARPs can also act as natural salt enhancers and address the call of an adult’s daily salt intake reduction to 5 g by World Health Organization.
Besides umami, salty and kokumi taste, ARPs may also have a potential association with other tastes such as sweet, bitter, and sour, because numbers of studies have illustrated correlations between these tastes and protein hydrolysates/peptides [34][35].
References
- Maillard, L.C. Action of amino acids on sugars. Formation of melanoidins in a methodical way. Compt. Rend. 1912, 154, 66–68.
- Hodge, J.E. The Amadori rearrangement. Adv. Carbohydr. Chem. 1955, 10, 169–205.
- Heyns, K.; Noack, H. The conversion of D-fructose with L-lysine and L-arginine and their relationship to non-enzymatic browning reactions. Chem. Ber. 1962, 95, 720–727.
- Xing, H.; Mossine, V.V.; Yaylayan, V. Diagnostic MS/MS fragmentation patterns for the discrimination between Schiff bases and their Amadori or Heyns rearrangement products. Carbohydr. Res. 2020, 491, 107985.
- Ruan, D.L.; Wang, H.; Cheng, F.L. The chemistry of the Maillard reaction. In The Maillard Reaction in Food Chemistry Current Technology and Applications; Parisi, S., Ed.; Springer: Berlin, Germany, 2018.
- Hansen, A.P.; Arora, D. Loss of flavor compounds from aseptically processed food products packaged in aseptic containers. In Barrier Polymers and Structures; Koros, W.J., Ed.; American Chemical Society: Boston, FL, USA, 1990; pp. 318–332.
- Ghajari, M.A.; Iman, K.; Mohammad, G.; Mahdi, J.S. Chapter 7—Nanoencapsulation of flavors. In Nanoencapsulation of Food Bioactive Ingredients; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 261–296.
- Ingles, D.L.; Reynolds, T.M. Chemistry of non-enzymic browing. IV. determination of amino acids and amino acid-deoxyfructoses in browned freeze-dried apricots. Aust. J. Chem. 1958, 11, 575–580.
- Hashiba, H. Isolation and Identification of Amadori compounds from soy sauce. Agric. Biol. Chem. 2014, 42, 763–768.
- Vinale, F.; Fogliano, V.; Schieberle, P.; Hofmann, T. Development of a stable isotope dilution assay for an accurate quantification of protein-bound N(epsilon)-(1-deoxy-D-fructos-1-yl)-L-lysine using a (13)C-labeled internal standard. J. Agric. Food Chem. 1999, 47, 5084–5092.
- Yang, C.; Zhang, S.; Shi, R.; Yu, J.; Li, S.; Tao, G.; Tsao, R.; Zhang, J.; Zhang, L. LC-MS/MS for simultaneous detection and quantification of Amadori compounds in tomato products and dry foods and factors affecting the formation and antioxidant activities. J. Food Sci. 2020, 85, 1007–1017.
- Weerawatanakorn, M.; Wu, J.C.; Pan, M.H.; Ho, C.T. Reactivity and stability of selected flavor compounds. J. Food Drug Anal. 2015, 23, 176–190.
- Deblander, J.; Van Aeken, S.; Adams, A.; De Kimpe, N.; Abbaspour Tehrani, K. New short and general synthesis of three key Maillard flavour compounds: 2-Acetyl-1-pyrroline, 6-acetyl-1,2,3,4-tetrahydropyridine and 5-acetyl-2,3-dihydro-4H-1,4-thiazine. Food Chem. 2015, 168, 327–331.
- Andrewes, P. Changes in Maillard reaction products in ghee during storage. Food Chem. 2012, 135, 921–928.
- Shu, C.K.; Mookherjee, B.D.; Ho, C.T. Volatile components of the thermal degradation of 2,5-dimethyl-4-hydroxy-3(2H)-furanone. J. Agric. Food Chem. 1985, 33, 446–448.
- Cui, H.; Yu, J.; Xia, S.; Duhoranimana, E.; Huang, Q.; Zhang, X. Improved controlled flavor formation during heat-treatment with a stable Maillard reaction intermediate derived from xylose-phenylalanine. Food Chem. 2019, 271, 47–53.
- Beksan, E.; Schieberle, P.; Robert, F.; Blank, I.; Fay, L.B.; Schlichtherle-Cerny, H.; Hofmann, T. Synthesis and sensory characterization of novel umami-tasting glutamate glycoconjugates. J. Agric. Food Chem. 2003, 51, 5428–5436.
- Kranz, M.; Hofmann, T. Food-grade synthesis of Maillard-type taste enhancers using natural deep eutectic solvents (NADES). Molecules 2018, 23, 261.
- Davidek, T.; Clety, N.; Aubin, S.; Blank, I. Degradation of the Amadori compound N-(1-deoxy-D-fructos-1-yl)glycine in aqueous model systems. J. Agric. Food Chem. 2002, 50, 5472–5479.
- Eichner, K.; Labile, R.; Wolf, W. Properties of water in foods. In North Atlantic Treaty Organization Scientific Affairs Division; Simatos, D., Multon, J., Eds.; Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1985; p. 191.
- Deng, S.; Cui, H.; Hayat, K.; Hussain, S.; Tahir, M.U.; Zhai, Y.; Zhang, Q.; Zhang, X.; Ho, C.T. Effect of methionine on the thermal degradation of N-(1-deoxy-D-fructos-1-yl)-methionine affecting browning formation. J. Agric. Food Chem. 2021, 69, 5167–5177.
- Yaylayan, V.; Forage, N.G. Determination of the kinetics and mechanism of decomposition of tryptophan Amadori rearrangement product by RP-HPLC analysis. J. Agric. Food Chem. 1991, 39, 364–369.
- Yamamoto, S.; Bamba, T.; Sano, A.; Kodama, Y.; Imamura, M.; Obata, A.; Fukusaki, E. Metabolite profiling of soy sauce using gas chromatography with time-of-flight mass spectrometry and analysis of correlation with quantitative descriptive analysis. J. Biosci. Bioeng. 2012, 114, 170–175.
- Kaneko, S.; Kumazawa, K.; Nishimura, O. Isolation and identification of the umami enhancing compounds in Japanese soy sauce. Biosci. Biotechnol. Biochem. 2011, 75, 1275–1282.
- Shiga, K.; Yamamoto, S.; Nakajima, A.; Kodama, Y.; Imamura, M.; Sato, T.; Uchida, R.; Obata, A.; Bamba, T.; Fukusaki, E. Metabolic profiling approach to explore compounds related to the umami intensity of soy sauce. J. Agric. Food Chem. 2014, 62, 7317–7322.
- Katayama, H.; Tatemichi, Y.; Nakajima, A. Simultaneous quantification of twenty Amadori products in soy sauce using liquid chromatography-tandem mass spectrometry. Food Chem. 2017, 228, 279–286.
- Kranz, M.; Viton, F.; Smarrito-Menozzi, C.; Hofmann, T. Sensomics-based molecularization of the taste of pot-au-feu, a traditional meat/vegetable broth. J. Agric. Food Chem. 2018, 66, 194–202.
- Sonntag, T.; Kunert, C.; Dunkel, A.; Hofmann, T. Sensory-guided identification of N-(1-methyl-4-oxoimidazolidin-2-ylidene)-alpha-amino acids as contributors to the thick-sour and mouth-drying orosensation of stewed beef juice. J. Agric. Food Chem. 2010, 58, 6341–6350.
- Dunkel, A.; Hofmann, T. Sensory-directed identification of beta-alanyl dipeptides as contributors to the thick-sour and white-meaty orosensation induced by chicken broth. J. Agric. Food Chem. 2009, 57, 9867–9877.
- Su, G.; Cui, C.; Zheng, L.; Yang, B.; Ren, J.; Zhao, M. Isolation and identification of two novel umami and umami-enhancing peptides from peanut hydrolysate by consecutive chromatography and MALDI-TOF/TOF MS. Food Chem. 2012, 135, 479–485.
- Zhou, X.; Cui, H.; Zhang, Q.; Hayat, K.; Yu, J.; Hussain, S.; Tahir, M.U.; Zhang, X.; Ho, C.T. Taste improvement of Maillard reaction intermediates derived from enzymatic hydrolysates of pea protein. Food Res. Int. 2021, 140, 109985.
- Wang, Y.; Cui, H.; Zhang, Q.; Hayat, K.; Yu, J.; Hussain, S.; Usman Tahir, M.; Zhang, X.; Ho, C.-T. Proline-glucose Amadori compounds: Aqueous preparation, characterization and saltiness enhancement. Food Res. Int. 2021, 144, 110319.
- Oconnor, T. Food flavours part C: The flavour of fruits. Trends Food Sci. Technol. 1991, 2, 232.
- Fu, Y.; Zhang, Y.; Soladoye, O.P.; Aluko, R.E. Maillard reaction products derived from food protein-derived peptides: Insights into flavor and bioactivity. Crit. Rev. Food Sci. Nutr. 2020, 60, 3429–3442.
- Temussi, P.A. The good taste of peptides. J. Pept. Sci. 2012, 18, 73–82.





