
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Subbaiah Chalivendra | + 2197 word(s) | 2197 | 2021-07-22 12:09:55 | | | |
| 2 | Lindsay Dong | Meta information modification | 2197 | 2021-08-04 05:16:07 | | |
Video Upload Options
Invertebrate pests, such as insects and nematodes, not only cause or transmit human and livestock diseases but also impose serious crop losses by direct injury as well as vectoring pathogenic microbes. This is amply demonstrated by the successful and widespread use of Bacillus thuringiensis (Bt) to control mosquitos and many plant pests, the latter by the transgenic expression of Bt-insecticidal proteins in crop plants. Identifying as well as characterizing the molecular nature and regulation of the biocidal activity has led to the enormous success of Bt as a biocontrol agent, which serves as a great model for advancing nascent biocontrol agents into commercial products.
1. Introduction
Insects constitute the largest and most diverse group of animals (~2.5 million species) on Earth [1]. With the exception of a few beneficial insects such as pollinators, they form the costliest animal group to human society by spreading devastating infectious diseases in humans, livestock and crops, ravaging food stocks, damaging forests, destroying infrastructure and weakening the resilience of ecosystems [1]. The Nematoda (also called Nemathelminthes), with an estimated 500,000 species, is the second largest phylum in the animal kingdom [2]. Most species of nematodes are either innocuous or play beneficial ecological and agronomic roles, by nutrient recycling and controlling insects and other harmful nematodes [3]. At the same time, some nematodes cause serious diseases in plants, humans and other animals.
Biocontrol is the means of controlling pests and pathogens through the use of other organisms, which can be natural enemies, such as predators, parasitoids, pathogens and competitors. It is an environmentally safe, low-cost and effective approach and occurs in natural communities. Much before the introduction of chemical pesticides, biocontrol has been in practice to control agricultural pests, with the first recorded report in 304 AD from China [4].
2. Pore-Forming Toxins
Pore-forming toxins (PFTs) are the largest class of proteinaceous bacterial toxins and important virulence factors, such as colicins of E. coli and anthacin of anthrax. All PFTs are synthesized as water-soluble proteins but subsequently become membrane bound. They recognize host cells based on specific cell surface receptors, which can be proteins, lipids or sugars. The binding allows a rapid increase in the local concentration of a PFT and its oligomerization, which is followed by insertion and pore formation in the host cell membrane. The changes in the permeability of the membrane due to pore formation depends on the toxin, ranging from the loss of small ions, e.g., K+ and Ca2+, to macromolecules such as proteins [12].
B. thuringiensis (Bt, hereafter) was first isolated in Japan in 1902 from dead silkworm (Bombyx mori) larvae and soon recognized as an entomopathogenic bacterium. Bt was one of the first prokaryotic BCAs used as a commercial insecticide in France in 1938 [13]. The insect- and nematode-specific pathogenicity of Cry toxins is due to their oral toxicity, and these toxins are active against a wide range of insects from Lepidoptera, Coleoptera, Hymenoptera and Diptera, as well as nematodes [14][15].
3. Insect Ion Channel Modulators
Ion channel modulators have clear advantages as pesticidal targets in that they act quickly and allow the rapid reduction of insect pressure. Five ion channels within the insect nervous system have been the primary targets for the development of small molecule insecticides and also serve as effective targets for biopesticides. These are the γ-aminobutyric acid (GABA) receptor, the glutamate-gated chloride channel, the nicotinic acetylcholine receptor or nAChR, the voltage-gated sodium channel and the ryanodine receptor [19].
Nodulisporic acid A, a natural insecticidal indole terpene isolated from an endophytic fungus, Nodulisporium sp., also inhibits only invertebrate-specific glutamate-gated chloride (Glu-Cl) channels [20]. Based on its safety, Merck & Co. introduced synthetic versions of the compound as a potent oral formulation to control fleas and ticks in dogs and cats [21]. Ryanodine receptors (RyRs) belong to a group of ligand-gated calcium channels initially reported from vertebrates. They are located on the endoplasmic reticulum of muscle cells and neurons and play a critical role in muscle contraction. In contrast to mammals, which have three types of RyRs, insects have only a single RyR, which is a major target for modern insecticides. Ryanodine, a plant alkaloid and an important ligand of RyR, has served as a natural botanical insecticide. Attempts to generate synthetic commercial analogs of ryanodine have, so far, not succeeded. Despite the popularity of diamide insecticides due to their specificity, several agricultural pests have become resistant to the chemical insecticides due to mutations in a transmembrane region of their RyRs [22], and there is a need for alternative RyR agonists. Cyclodepsipeptides are a large family of peptide-related natural products with an α-hydroxy acid and 5–10 amino acids linked by amide and ester bonds [23]. Verticilide, a cyclooctadepsipeptide produced by Verticillium sp. FKI-1033, has been shown to bind selectively to the insect ryanodine receptor in the low micromolar range [24].
4. Innate Immunity Busters
Insects, like all invertebrates, express both innate and humoral immunity to infection. Biological control strategies that are targeted to these pathways have a great potential for success.
Bacteria belonging to 24 species of Xenorhabdus and five of Photorhabdus are known worldwide for their entomopathogenic potential. Some species (e.g., P. luminescens and X. nematophila) have mutualistic associations with nematodes and share their entomophagous lifestyle. The soil-dwelling entomopathogenic nematode larvae find and penetrate the insect larvae through natural openings and release symbiotic bacteria into the insect hemocoel. The bacteria replicate in the insect body and release immuno-suppressive toxins. At least seven secondary metabolites that inhibit PLA2, namely, benzylideneacetone (BZA), proline-tyrosine, acetylated phenylalanine-glycine-valine, indole, oxindole, cis-cyclo-PY and p-hydroxyphenyl propionic acid, have been reported. They also show significant inhibitory activities against other immune responses, such as phenoloxidase activity (PO) and hemocytic nodulation, with BZA being the most effective [25]. An isocyanide-containing compound rhabducin produced by Xenorhabdus can also inhibit phenoloxidase and thereby melanization [26]. In addition, phurealipids (urea compounds) made by these bacteria can prevent the expression of antimicrobial peptide genes in the insect hosts, a part of the humoral component of immunity [27].
5. Cyclic Lipopeptide Surfactants
Cyclic lipopeptides (CLPs) are amphiphilic molecules composed of a cyclic oligopeptide lactone ring coupled to a fatty-acid tail. Their structural diversity and cyclic configuration confer them with broad-spectrum and environmentally stable antibiosis activity against bacteria, fungi, insects, protozoa and even human tumor cell lines [28][29]. CLPs are produced mostly by Pseudomonas, Bacillus and Streptomyces spp. CLPs come in three families, namely surfactin, iturin and fengycin, and a single strain can make one or more of them contribute to varying biocidal activities. Pseudomonad spp. (e.g., P. chlororaphis, P. fluorescens, P. protegens, P. putida, P. mosselli and P. entomophila) are particularly pathogenic toward insects and nematodes.
6. Psychoactive Compounds
Many microbial parasites manipulate host insect behavior for the enhanced dispersal of their inoculum and thereby spread disease. A few of them, such as baculoviruses, hijack host genes involved in insect physiology [30], whereas others make their own [31]. Many entomopathogenic fungi cause “summit disease” (SD) behavior, an extended phenotype where parasitized insects ascend and affix to elevated substrates prior to death. For example, the fungal pathogen Entomophthora muscae infects wild Drosophila and manipulates host behavior [56]. This facilitates a wider dissemination of fungal spores from the mummified insect carcasses. It is speculated that E. muscae may produce and secrete eicosanoid-like compounds to induce behavioral changes in the dying host [32]. More details are available about the bizarre behavior in cicadas induced by the pathogenic fungi Massospora and Strongwellsea. M. cicadina, M. platypediae, M. levispora, S. tigrinae and S. acerosa keep their insect hosts alive while sporulating, which enhances dispersal via sexual transmission.
More research is needed to validate the findings from -omics analyses to unequivocally implicate any of these putative chemosignaling compounds in insect behavioral manipulation. Nevertheless, this concept has been successfully exploited in the management of some pests, e.g., codling moth (Cydia pomonella, a key insect pest of apple), using host endogenous molecules, especially pheromones [33].
7. Ribotoxins
8. Sterol Homeostasis Disruptors
9. Uncouplers and Electron Transport Inhibitors
References
- Bradshaw, C.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986.
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251.
- Ferris, H. Contribution of nematodes to the structure and function of the soil food web. J. Nematol. 2010, 42, 63–67.
- Peng, S.J. Biological Control—One Of The Fine Traditions Of Ancient Chinese Agricultural Techniques. Sci. Agric. Sin. 1983, 1, 92–98.
- Roderick, G.K.; Hufbauer, R.; Navajas, M. Evolution and biological control. Evol. Appl. 2012, 5, 419–423.
- Redmond, C.T.; Potter, D.A. Lack of efficacy of in vivo- and putatively in vitro-produced Bacillus popilliae against field populations of Japanese beetle (Coleoptera: Scarabaeidae) grubs in Kentucky. J. Econ. Entomol. 1995, 88, 846–854.
- Patel, R.; Piper, K.; Cockerill, F.R., 3rd; Steckelberg, J.M.; Yousten, A.A. The biopesticide Paenibacillus popilliae has a vancomycin resistance gene cluster homologous to the enterococcal VanA vancomycin resistance gene cluster. Antimicrob. Agents Chemother. 2000, 44, 705–709.
- Barratt, B.I.P.; Moran, V.C.; Bigler, F.; van Lenteren, J.C. The status of biological control and recommendations for improving uptake for the future. BioControl 2018, 63, 155–167.
- Carzoli, A.K.; Aboobucker, S.I.; Sandall, L.L.; Lübberstedt, T.T.; Suza, W.P. Risks and opportunities of GM crops: Bt maize example. Glob. Food Secur. 2018, 19, 84–91.
- Available online: https://www.ers.usda.gov/webdocs/charts/58020/biotechcrops_d.html?v=8259.7 (accessed on 8 July 2021).
- Perry, E.D.; Ciliberto, F.; Hennessy, D.A.; Moschini, G. Genetically engineered crops and pesticide use in U.S. maize and soybeans. Sci. Adv. 2016, 2, e1600850.
- Dal Peraro, M.; van der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016, 14, 77–92.
- Milner, R.J. History of Bacillus thuringiensis. Agric. Ecosyst. Environ. 1994, 49, 9–13.
- Angus, T.A. Association of toxicity with protein-crystalline inclusions of Bacillus sotto Ishiwata. Can. J. Microbiol. 1956, 2, 122–131.
- Hofte, H.; Whitely, H.P. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Mol. Biol. Rev. 1989, 53, 242–255.
- Panevska, A.; Hodnik, V.; Skočaj, M.; Novak, M.; Modic, Š.; Pavlic, I.; Podržaj, S.; Zarić, M.; Resnik, N.; Maček, P. Pore-forming protein complexes from Pleurotus mushrooms kill western corn rootworm and Colorado potato beetle through targeting membrane ceramide phosphoethanolamine. Sci. Rep. 2019, 9, 5073.
- Szczesny, P.; Iacovache, I.; Muszewska, A.; Ginalski, K.; van der Goot, F.G.; Grynberg, M. Extending the aerolysin family: From bacteria to vertebrates. PLoS ONE 2011, 6, e20349.
- Xiang, Y.; Yan, C.; Guo, X.; Zhou, K.; Li, S.; Gao, Q.; Wang, X.; Zhao, F.; Liu, J.; Lee, W.H.; et al. Host-derived, pore-forming toxin-like protein and trefoil factor complex protects the host against microbial infection. Proc. Natl. Acad. Sci. USA 2014, 111, 6702–6707.
- Ffrench-Constant, R.H.; Williamson, M.S.; Emyr Davies, T.G.; Bass, C. Ion channels as insecticide targets. J. Neurogenet. 2016, 30, 163–177.
- Kane, N.S.; Hirschberg, B.; Qian, S.; Hunt, D.; Thomas, B.; Brochu, R.; Ludmerer, S.W.; Zheng, Y.; Smith, M.; Arena, J.P.; et al. Drug-resistant Drosophila indicate glutamate-gated chloride channels are targets for the antiparasitics nodulisporic acid and ivermectin. Proc. Natl. Acad. Sci. USA 2000, 97, 13949–13954.
- Meinke, P.T.; Colletti, S.L.; Fisher, M.H.; Wyvratt, M.J.; Shih, T.L.; Ayer, M.B.; Li, C.; Lim, J.; Ok, D.; Salva, S.; et al. Discovery of the development candidate N-tert-butyl nodulisporamide: A safe and efficacious once monthly oral agent for the control of fleas and ticks on companion animals. J. Med. Chem. 2009, 52, 350515.
- Samurkas, A.; Fan, X.; Ma, D.; Sundarraj, R.; Lin, L.; Yao, L.; Ma, R.; Jiang, H.; Cao, P.; Gao, Q.; et al. Discovery of Potential Species-Specific Green Insecticides Targeting the Lepidopteran Ryanodine Receptor. J. Agric. Food Chem. 2020, 68, 4528–4537.
- Wang, X.; Gong, X.; Li, P.; Lai, D.; Zhou, L. Structural Diversity and Biological Activities of Cyclic Depsipeptides from Fungi. Molecules 2018, 23, 169.
- Shiomi, K.; Matsui, R.; Kakei, A.; Yamaguchi, Y.; Masuma, R.; Hatano, H.; Arai, N.; Isozaki, M.; Tanaka, H.; Kobayashi, S.; et al. Verticilide, a new ryanodine-binding inhibitor, produced by Verticilium sp. FKI-1033. J. Antibiot. 2010, 63, 77–82.
- Seo, S.; Lee, S.; Hong, Y.; Kim, Y. Phospholipase A2 inhibitors synthesized by two entomopathogenic bacteria, Xenorhabdus nematophila and Photorhabdus temperata subsp. temperata. Appl. Environ. Microbiol. 2012, 78, 3816–3823.
- Crawford, J.M.; Portmann, C.; Zhang, X.; Roeffaers, M.B.J.; Clardy, J. Small molecule perimeter defense in entomopathogenic bacteria. Proc. Natl Acad. Sci. USA 2012, 109, 10821–10826.
- Mollah, M.; Roy, M.C.; Choi, D.Y.; Hasan, M.A.; Al Baki, M.A.; Yeom, H.S.; Kim, Y. Variations of Indole Metabolites and NRPS-PKS Loci in Two Different Virulent Strains of Xenorhabdus hominickii. Front. Microbiol. 2020, 11, 583594.
- Raaijmakers, J.M.; de Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062.
- Chalivendra, S.; Ham, J.H. Bacilli in the Biocontrol of Mycotoxins. In Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol; Islam, M., Rahman, M., Pandey, P., Boehme, M., Haesaert, G., Eds.; Springer: Cham, Switzerland, 2019; Volume 2, Chapter 3; pp. 49–62.
- Wang, M.; Hu, Z. Crosstalking between baculoviruses and host insects towards a successful infection. Phil. Trans. R. Soc. B 2019, 374, 20180324.
- Boyce, G.R.; Gluck-Thaler, E.; Slot, J.C.; Stajich, J.E.; Davis, W.J.; James, T.Y.; Cooley, J.R.; Panaccione, D.G.; Eilenberg, J.; De Fine Licht, H.H.; et al. Psychoactive Plant- and Mushroom-Associated Alkaloids from Two Behavior Modifying Cicada Pathogens. Fungal Ecol. 2019, 41, 147–164.
- Gryganskyi, A.P.; Mullens, B.A.; Gajdeczka, M.T.; Rehner, S.A.; Vilgalys, R.; Hajek, A.E. Hijacked: Co-option of host behavior by entomophthoralean fungi. PLoS Pathog. 2017, 13, e1006274.
- Witzgall, P.; Stelinski, L.; Gut, L.; Thomson, D. Codling moth management and chemical ecology. Annu. Rev. Entomol. 2008, 53, 503–522.
- Chandler, D.; Davidson, G.; Pell, J.D.; Ball, B.V.; Shaw, K.; Sunderland, K.D. Fungal biocontrol of Acari. Bio. Sci. Technol. 2000, 10, 357–384.
- Lacadena, J.; García, E.A.; Sangrà, N.C.; Galán, E.H.; Cebollada, J.A.; Ortega, L.G.; Oñaderra, M.; Gavilanes, J.G.; del Pozo, A.M. Fungal ribotoxins: Molecular dissection of a family of natural killers. FEMS Microbiol. Rev. 2007, 31, 212–237.
- Jaffee, B.A. Augmentation of Soil with the Nematophagous Fungi Hirsutella rhossiliensis and Arthrobotrys haptotyla. Phytopathology 2000, 90, 498–504.
- Jing, X.; Behmer, S.T. Insect Sterol Nutrition: Physiological Mechanisms, Ecology, and Applications. Annu. Rev. Entomol. 2020, 65, 251–271.
- Purcell, J.; Greenplate, J.; Jennings, M.; Ryerse, J.; Pershing, J.C.; Sims, S.; Prinsen, M.; Corbin, D.; Tran, M.; Sammons, R. Cholesterol oxidase: A potent insecticidal protein active against boll weevil larvae. Biochem. Biophys. Res. Commun. 1993, 196, 1406–1413.
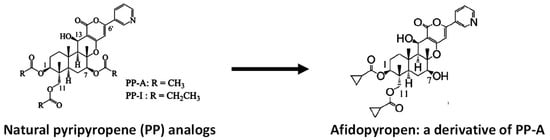
- Goto, K.; Horikoshi, R.; Mitomi, M.; Oyama, K.; Hirose, T.; Sunazuka, T.; Ōmura, S. Synthesis and insecticidal efficacy of pyripyropene derivatives. Part II—Invention of afidopyropen. J. Antibiot. 2019, 72, 661–681.
- Available online: https://www.who.int/ipcs/publications/pesticides_hazard_rev_3.pdf. (accessed on 8 July 2021).
- Pastor, F.J.; Guarro, J. Clinical manifestations, treatment and outcome of Paecilomyces lilacinus infections. Clin. Microbiol. Infect. 2006, 12, 948–960.
- Park, J.O.; Hargreaves, J.R.; McConville, E.J.; Stirling, G.R.; Ghisalberti, E.L. Production of leucinostatins and nematicidal activity of Australian isolates of Paecilomyces lilacinus (Thom) samson. Lett. Appl. Microbiol. 2004, 38, 271–276.
- Sharma, A.; Sharma, S.; Mittal, A.; Naik, S.N. Evidence for the involvement of nematocidal toxins of Purpureocillium lilacinum 6029 cultured on Karanja deoiled cake liquid medium. World J. Microbiol. Biotechnol. 2016, 32, 82.




