
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cristina Banfi | + 6194 word(s) | 6194 | 2020-06-30 05:38:49 | | | |
| 2 | Rita Xu | -2062 word(s) | 4132 | 2020-07-06 12:01:23 | | |
Video Upload Options
Recent advances in proteomic studies provided additional important information concerning the platelet biology and their response to several pathophysiological pathways. Platelets indeed are a heterogeneous small anucleate blood cell population with a central role both in physiological haemostasis and in pathological states, spanning from thrombosis to inflammation, and cancer.
Herein, a critical overview is provided on principal platelet proteomic studies focused on platelet biology from signalling to granules content, platelet proteome changes in several diseases, and the impact of drugs on platelet functions. Targeted quantification methods by means of mass spectrometry might be employed for more precise, robust and accurate quantification of selected proteins, which might be used as biomarkers for disease diagnosis, prognosis and therapy, and their strong clinical impact in the near future.
1. Definition
Platelets are a heterogeneous small anucleate blood cell population with a central role both in physiological haemostasis and in pathological states, spanning from thrombosis to inflammation, and cancer. Recent advances in proteomic studies provided additional important information concerning the platelet biology and the response of platelets to several pathophysiological pathways. Platelets circulate systemically and can be easily isolated from human samples, making proteomic application very interesting for characterizing the complexity of platelet functions in health and disease as well as for identifying and quantifying potential platelet proteins as biomarkers and novel antiplatelet therapeutic targets. To date, the highly dynamic protein content of platelets has been studied in resting and activated platelets, and several subproteomes have been characterized including platelet-derived microparticles, platelet granules, platelet releasates, platelet membrane proteins, and specific platelet post-translational modifications.
2. Introduction
2.1. Platelet biology: An Overview
Platelets are small anucleate blood cells produced by megakaryocytes in the bone marrow and lungs [1][2]. Once released by their megakaryocytic precursor, platelets enter the bloodstream and circulate for 7–10 days, after which they are cleared in the spleen and liver [3]. Platelets are highly specialized effector cells in physiological haemostasis and play a central role in pathological thrombosis [3]. In primary haemostasis, they rapidly adhere to the damaged vessel wall at the site of injury and aggregate to form a platelet plug. Failure to form an adequate plug underlies bleeding disorders, while excessive platelet reactivity leads to an increased risk of thrombosis.
Platelets are now known to play major effector activities in a number of additional functions, including inflammatory reactions and innate immune responses [4]. Instrumental to these activities is the ability of platelets to respond to signals from the endothelium, circulating cells, or other blood components [3]. Platelets are present in high numbers in the circulation (150.000 to 400.000 per microliter of whole blood in humans) and they continuously patrol their environment using cell surface receptors and adhesion molecules, including integrins, selectins, toll-like receptors, transmembrane receptors, immunoglobulin superfamily receptors, tyrosine kinase receptors, lipid receptors and others [5][6]. Moreover, platelets can alter the environment in response to various stimuli through the release of bioactive mediators from different storage granules (α-granules, dense granules and lysosomes), bioactive lipid products formed by oxidation of free fatty acids, and extracellular vesicles [3][7][8][9]. The secretion products, including coagulation factors, growth factors, chemokines, cytokines, microbicidal proteins, prostaglandins, thromboxane A2 (TXA2), eicosanoids, and RNA species, influence many physiological and pathophysiological processes beyond haemostasis [3][5][7][10].
Platelet activation includes numerous signaling pathways, through local prothrombotic factors and platelet secretion products. Platelet adhesion to the extracellular matrix involves the binding between exposed collagen and platelet glycoprotein receptors, causing the platelet shape to change and the release of their granules contents.
Platelets are not a homogeneous cell population and their morphological heterogeneity is present at rest, upon stimulation by agonists, and within the haemostatic plug. Circulating platelets are heterogeneous in size, age, and responsiveness [11]. Studies investigating the functional differences of platelet subpopulations have emerged only in the second half of the last century [3][11]. However, the causes of platelet functional heterogeneity and how structural heterogeneity relates to variation in platelet responses remain largely unknown [3].
Although platelets are anucleate, they have long been known to contain RNAs. This genomic material was not merely a remnant from the precursor megakaryocyte, but rather the result of a highly regulated sorting process by which megakaryocytes invest platelets with mRNA during thrombopoiesis [12]. Platelets display a diverse repertoire of coding and noncoding RNAs, diverse pathways for processing RNA transcripts, specialized mechanisms of translation, and the capacity to synthesize new proteins and alter the constitutive platelet proteome in response to activating signals [13]. In addition, differential transfer of RNAs from megakaryocyte to platelet, and from various circulating cells, can alter gene expression in platelets [11]. Therefore, platelets have high versatility and adaptability in structure and function, and the platelet transcriptome has a relevant role in mediating platelet function in health and disease [3][13][14]. Multiple mutations associated with defects of platelet function have been identified in genes encoding receptors, intracellular signaling proteins, cytoskeletal proteins, and proteins regulating the biogenesis of platelet granules [3][15][16].
Genomics and transcriptomics of megakaryocytes and platelets are now being extensively investigated in basic and clinical studies. Moreover, because platelets circulate systemically and are easily obtained, several recent studies have highlighted the potential use of platelet transcripts as biomarkers, even for diseases without apparent platelet etiology [12].
Over the years, proteomic studies provided additional important information concerning the platelet biology and their related diseases [17]. Recent advances helped to elucidate platelet localization, interactions, post-translational modifications (PTMs), and activation states of gene products. The highly dynamic protein content of platelets has been studied using resting and activated platelets, and various subproteomes have been characterized including releasates, granules, platelet-derived microparticles (PMPs), membrane and cytoskeletal proteins, and specific PTMs occurring in platelets.
3. Data, Model, Applications and Influences
3.1. Platelets and Proteomics
Platelet dysfunction is often attributable to alterations in protein expression and dynamic occurrence of PTMs. Therefore, particular attention must be paid to studying their proteome in order to understand better their biological mechanisms and multiple functions. Platelets can be easily isolated from the human samples and show limited levels of protein synthesis, making the proteomic application very interesting. Proteomics has emerged as a powerful tool for characterizing the complexity of platelet functions in health and disease as well as for identifying potential novel antiplatelet therapeutic targets [18]. Therefore, proteomics, in combination with the other “omic” technologies, may contribute to improving the knowledge of complex processes underlying the platelet response to several pathophysiological pathways.
Over the past twenty years, mass spectrometry (MS) and its application in proteomic studies lead to the compilation of an extensive list of proteins expressed in platelets and relevant data on protein–protein interactions and PTMs. Novel and more sensitive MS-based instruments and technologies provided the possibility to identify and quantify ever lower protein amounts. Numerous studies demonstrated the ability of proteomics to measure the differences of proteins and their isoforms quantitatively, covering about 6–7 logs of dynamic range in abundance.
Mass spectrometry ensures high-sensitivity, specific and throughput analysis of a given proteome and high-performance liquid chromatography (LC) systems before the MS analysis lead to a significant increase in the separation of highly complex samples. Furthermore, thanks to quantitative MS measurements, either based on label-free approaches or stable isotope labelling, considerable improvements have been made in the large-scale analysis of proteins and their modifications.
In this review, we provide a critical overview on the most important platelet proteomic studies, in healthy and disease conditions, dedicated to the different aspects of platelet biology from signaling to granules content and the impact of drugs on platelet functions. Recent advances in quantitative platelet proteomics are crucial for better understanding platelet activation and aggregation processes, and they will have a strong clinical impact in the near future [19].
3.2. Platelet Activation and Signaling
3.2.1. Proteins Involved in Platelet Activation and Signaling
Platelet activation is a complex biological process that includes numerous signaling pathways. Cytoskeletal and signaling proteins have an important role in platelet functions, and thus they are crucial targets for the platelet proteomic studies.
Platelet adhesion to the extracellular matrix involves the binding between exposed collagen and platelet glycoprotein receptors, causing the platelet shape to change and the release of their granules contents. Platelets contain at least three major granules types and granule exocytosis has a fundamental role in platelet activities [20]. Multiple pathways can stimulate platelet activation, through local prothrombotic factors (e.g., tissue factor (TF)) and platelet secretion products. Glycoprotein VI (GPVI) is the primary signaling receptor on platelet membranes involved in their activation on exposed collagen [21][22]. Platelet activation by the GPVI receptor is mediated by immunoreceptor tyrosine-based activation motif signaling, but also G-protein-coupled receptor-mediated signaling can influence platelet response to several soluble agonists such as adenosine diphosphate (ADP), serotonin, TXA2, prostaglandin E2 (PGE2) and thrombin [23]. These molecules increase the response to injury, promoting an extended platelet aggregation, and between them, thrombin is the most potent agonist able to activate platelets mainly through the interaction with protease-activated receptors (PAR) on their surface. Thrombin activates both PAR-1 and PAR-4 on human platelets with distinct mechanisms that have important implications for the development of PAR antagonists preventing thrombin-induced platelet activation [24]. The final event of platelet activation is the upregulation of integrin adhesion receptors, among which the most important is Glycoprotein IIb/IIIa (GPIIb/IIIa) receptor that allows the binding of fibrinogen or von Willebrand factor (vWF), contributing to platelet aggregation. Activated platelets interact with the vascular endothelium and circulating leukocytes through P-selectin, and play a central role in inflammation, thrombosis, and atherogenesis [25][26]. Moreover, activated platelets express functional CD40 ligand (CD40L) (also known as CD154), which is a transmembrane molecule involved in cell signaling in innate and adaptive immunity [27]. The CD40L also binds receptor CD40 expressed on endothelial cells to stimulate the secretion of chemokines and synthesis of adhesion molecules. The CD40L may be cleaved and released in its soluble form (sCD40L), which is produced by platelets only after activation and has a cytokine-like role, inducing the expression of E-selectin, P-selectin and vascular cell adhesion molecule 1 (VCAM-1) on vascular cells, as well as the release of matrix metalloproteinases (MMPs) and interleukin 6 and 8 (IL-6 and IL-8) [27]. The sCD40L is clearly involved in the pathophysiology of atherosclerosis and atherothrombosis [28][29].
Platelet factor 4 (PF4) or CXC motif chemokine ligand 4 is produced by platelets and it is contained in their α-granules (Figure 1). Upon platelet activation, PF4 is released into the circulation, where it covers several important roles in physiological and pathological conditions. It is involved in both inflammation and angiogenesis in wound healing through regulation of growth factor activity and platelet activation [30]. In addition, PF4 promotes reactive oxygen species (ROS) generation in vascular disorders [31], such as atherosclerosis or ischemia/reperfusion injury, and monocyte recruitment to the endothelium by means of its binding with RANTES (regulated on activation, normal T cell expressed and secreted), which is stored in high amounts in platelet α-granules and plays an active role in the atherosclerotic disease process [32]. Both PF4 and RANTES are released from α-granules upon platelet activation and hetero-aggregates of PF4 and RANTES promote monocyte adhesion in inflammation or atherosclerosis [33].
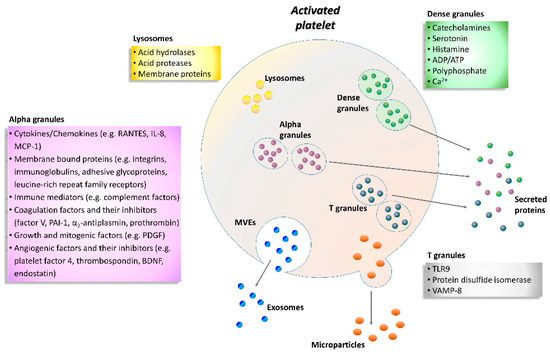
Figure 1. Schematic representation of the activated platelet showing multivesicular elements, exosomes, microparticles and specific granule releasates. Relevant proteins and factors are released following platelet activation. A plethora of biological substances including adhesion molecules, cytokines, chemokines, coagulation factors, angiogenic factors, immunologic mediators, growth factors, and lysosomal enzymes are grouped according to the type of granule. ADP, adenosine diphosphate; ATP, adenosine triphosphate; BDNF, brain-derived neurotrophic factor; IL-8, interleukin-8; MCP-1, monocyte chemotactic protein-1; MVEs, multivesicular elements; PAI-1, plasminogen activator inhibitor-1; PDGF, platelet-derived growth factor; RANTES, regulated on activation, normal T cell expressed and secreted; TLR9, toll-like receptor 9; VAMP-8, vesicle-associated membrane protein-8.
Besides their central role in haemostasis, platelets also interact with and remove pathogens from the blood stream at sites of infection and inflammation [34]. Platelets bind activated neutrophils, inducing the formation of neutrophil extracellular traps (NETs), which contain externalized DNA and DNA-associated nuclear and granular proteins, such as neutrophil elastase and myeloperoxidase. NETs can also acquire TF from the blood and can entrap platelets and fibrin. Although physiologically beneficial when released during an infection, an uncontrolled and excessive NET formation may contribute to the initiation and progression of atherosclerotic lesions and to arterial, venous, and cancer-associated thrombosis [35]. Moreover, NETs are found in a variety of other conditions such as lung injury and autoimmune diseases.
Enzyme-linked immunosorbent assay (ELISA) or Western blot are commonly used to study platelets activation markers; however, flow cytometry is a more standardized method that allows quantification of the expression of platelet activation markers and receptors and their association with other blood cells [21]. Mainly three types of granules have been found inside the platelets: α-granules, dense granules, and lysosomes (Figure 1). In addition, T granules have been described as another platelet intracellular compartment that is characterized by the coexpression of toll-like receptor 9 and protein disulfide isomerase during pro-platelet production [36]. All these granules are storage of adhesion molecules, cytokines, chemokines and coagulation or angiogenic factors (Figure 1).
In particular, α-granules are the most abundant type, about 50–80 per platelet that include both membrane-bound proteins and soluble proteins [37]. Membrane-bound proteins are then expressed on the platelet surface and comprise integrins, immunoglobulins, adhesive glycoproteins, leucine-rich repeat family receptors and other granule membrane-specific receptors. Hundreds of soluble proteins are typically released by α-granules and many of them are present in plasma with differences in structure or function. Secreted α-granule proteins are bioactive proteins involved in wide-ranging physiologic functions, among which innate immunity, inflammation, coagulation and mitogenesis, and with opposing activities (e.g., pro- and anticoagulants, proteases and inhibitors).
Dense granules have a distinct specific cargo regarding both biogenesis and function, which contains a few types of small molecules, such as catecholamines, ADP, ATP, polyphosphate and Ca2+ [38].
Instead, the third type of granule, platelet lysosome, contains primarily acid hydrolases and proteases [39]. Their secretion has a significant role in digestion of phagocytic and cytosolic components, as well as fibrinolysis and degradation of extracellular matrix (ECM) components, and vascular remodeling.
Activated platelets release numerous chemokines that control the movement of leukocytes from the vasculature towards the site of tissue damage or infection, regulate the phagocytosis and ROS production. Moreover, the high expression of adhesion molecules and ligands on the platelet surface promotes the interaction between platelets and endothelium or leukocytes, during haemostasis and inflammation. Integrins also facilitate platelets to bind to ECM molecules and other cells playing an important role in cell signaling. Since platelet surface markers have, in general, short detectability in human blood, platelet-monocyte aggregates have been detected in several studies as a more sensitive and accurate marker that describes platelet activation and a prothrombotic state in diseases, like advanced atherosclerosis, stable coronary artery disease, acute myocardial infarction, systemic inflammatory and autoimmune disorders, and neoplasms [40][41][42]. Similarly, circulating PMPs, that are the most abundant microparticles in the bloodstream (approximately 70–90% of circulating microparticles), are considered as potential biological markers for platelet activation [43]. They carry several unique proteins derived from platelets and mediate the communication between cells, promote the release of cytokines, and are involved in inflammation, angiogenesis, cancer progression and tissue regeneration [44].
Platelet microparticles have great relevance in a wide range of disease processes and high circulating levels have been observed in patients with cardiovascular diseases (CVDs), such as atherosclerosis [45], hypertension [46], thrombosis [47], and stroke [48]. Research interest in PMPs has grown past few years and focused on the development of more accurate methods for quantifying them to clarify their physiological role and their potential involvement in several clinical situations as disease biomarkers or new targets for antiplatelet drugs [44].
3.2.2. Platelet Priming
As mentioned before, within the bloodstream, platelets are subjected to the influence of a wide spectrum of activating and inactivating biomolecules and conditions [3]. In physiological states, the net result of these influences is the inhibition of spontaneous platelet adhesion and activation. In disease conditions, the threshold for platelet activation can be increased (negative platelet priming) or lowered (positive platelet priming) by systemic and local changes in the balance between activating and inactivating factors, resulting in altered responsiveness of platelets to agonists [3]. Negative platelet-priming substances include vessel wall-derived factors, such as nitric oxide, prostaglandin I2 (PGI2), adenosine, and thrombomodulin, and bioactive mediators present in plasma, such as PGE2, insulin, and polyunsaturated fatty acid products of 12-lipoxygenase. Positive priming factors include vessel wall-derived factors, such as vWF multimers and intercellular adhesion molecules, and bioactive mediators present in plasma, such as adrenaline, insulin-like growth factor 1, thrombopoietin, growth arrest-specific protein 6, sCD40L, cholesterol, PGE2 (at low-dose), and stromal cell-derived factor-1α [3].
The excitability of platelets has been shown to be altered in a number of conditions associated with high risk of developing atherosclerosis-related CVDs, such as hyperlipidemia [49], diabetes mellitus (DM) [50][51], hypertension [52], obesity [53] and cigarette-smoke exposure [54][55], as well as in other disease conditions including autoimmune diseases [56], hematological disorders and cancer [57]. Thus, modulation of platelet responsiveness due to platelet priming is likely to have important pathophysiological roles. However, whether platelet priming is a cause or consequence of the pathogenesis of diseases requires further investigation, since both acute and chronic disease conditions may themselves act as primers, either through exposure to tissue damage or through inflammatory mediators or pathogens [58].
3.3. The Role of Platelets in Disease
Developments in the field of platelet biology have led to new insights into platelet formation, function, heterogeneity, genetics, signaling and communication. The emergence of newly discovered and previously unrecognized biological capacities of platelets has led to the increasing recognition that platelets have a functional role in the pathophysiology of a wide variety of diseases, beyond the disorders of coagulation (Figure 2). The aim of this chapter is to provide a brief overview of the current understanding of platelet contribution to diseases not traditionally related to platelet number and/or function, for which platelet proteomics studies are available. Instead, this chapter will not address the role of platelets in hemostatic diseases. For an in-depth view of the recent progress in haemostatic diseases readers are referred to up-to-date publication [59].
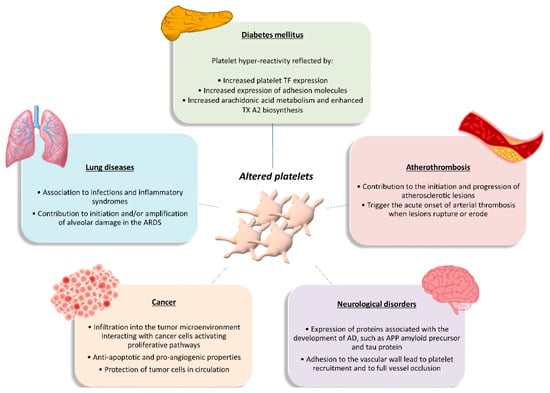
Figure 2. Functional role of altered platelets in the pathophysiology of several diseases. Brief overview of altered platelet contribution to atherothrombosis, diabetes mellitus, lung diseases, cancer, and neurological disorders. AD, Alzheimer’s disease; APP, amyloid precursor protein; ARDS, acute respiratory distress syndrome; TF, tissue factor; TXA2, thromboxane A2.
3.3.1. Platelets in Atherothrombosis
Platelets are essential for primary haemostasis and repair of the endothelium, but they also contribute to all stages of atherothrombosis [60]. Under physiological conditions, the intact, non-activated endothelium prevents platelet adhesion to the arterial wall. Under inflammatory conditions, however, platelets can adhere to the activated endothelial cell monolayer via adhesion receptors such as GPIb and GPVI and become activated [60][61]. Activated platelets release inflammatory and mitogenic mediators into the microenvironment, thereby altering the chemotactic, adhesive, and proteolytic properties of the endothelium. These platelet-induced alterations of endothelial-cell functions support leukocyte recruitment into nascent atherosclerotic plaques [60]. Moreover, platelets communicate biochemical signals to neutrophils, monocytes, and subsets of lymphocytes through adhesive molecules, such as P-selectin, and a multitude of secreted soluble mediators [62]. The initial contact is driven by the exposure of P-selectin on the activated platelets, which binds to P-selectin glycoprotein ligand 1 (PSGL-1) on the leukocyte surface, enhancing their adhesion on activated endothelial cells and inducing the production of TF by monocytes [60][62]. Signaling by P-selectin also stimulates monocytes and macrophages to produce chemoattractants or growth factors. Moreover, engagement by P-selectin of the PSGL-1 on the leukocyte surface initiates the formation of platelet-leukocyte aggregates that trigger mutual activation and release of bioactive mediators by both platelets and leukocytes, thereby modulating leukocyte function and fine-tuning immune responses [60][62][63]. Activated platelets also release chemokines that promote the differentiation of monocytes into macrophages (e.g., PF4), as well as matrix-degrading enzymes such as MMP 2 or 9 [60]. Moreover, activated platelets disseminate microparticles, which are intact vesicles that form by budding from the membrane. As with whole platelets, these microparticles interact with leukocytes and other inflammatory cells and can amplify inflammation in the arterial wall [62].
Besides contributing to the initiation and progression of atherosclerotic lesions, platelets trigger the acute onset of arterial thrombosis when these lesions rupture or undergo erosion [60]. The exposure of the thrombogenic substrates to circulating platelets challenges platelet recruitment to the injured vessel wall in a well-coordinated series of events: platelet ‘arrest’ onto the exposed subendothelium; recruitment and activation of additional platelets through the local release of major platelet agonists; and stabilization of the platelet aggregates [64]. These events ultimately result in the formation of a non-occlusive or occlusive platelet-fibrin thrombus. Acute occlusive coronary thrombus growth is most frequently the cause of acute coronary syndromes (ACS) and in some cases even of sudden coronary death [65].
3.3.2. Platelets in Diabetes Mellitus
Diabetes mellitus is a multifactorial disease closely associated with both micro- and macrovascular complications and a high risk of atherothrombotic events. Platelets play a major role in the pathophysiology of DM. Platelets of DM patients are indeed characterized by dysregulation of several signaling pathways leading to platelet activation, which represents an early event in the natural history of DM [66][67]. The detrimental metabolic state that precedes and accompanies diabetes, characterized by acute hyperglycemia, glycemic variability, and insulin resistance, is thought to be responsible for the alterations in platelet function seen in DM. These metabolic abnormalities may affect platelet transcriptome and/or posttranscriptional regulation through intermediate mediators, such as oxidative stress with isoprostane formation, inflammatory molecule production, endothelial dysfunction with circulating endothelial cells and microparticles release, and cross-talk between cells with miRNA exchange through circulating microparticles [68]. The net result of these influences is platelet hyperreactivity, as reflected by enhanced platelet TF expression and expression of TF-positive platelet-leukocyte aggregates; increased expression of adhesion molecules such as P-selectin; and increased arachidonic acid metabolism and enhanced TXA2 biosynthesis [68]. In turn, hyperactivated platelets have fundamental roles in both the development and the propagation of sustained inflammation in DM, are increasingly recognized as the culprit cells implicated in the propensity to atherothrombosis in the setting DM, and contribute to diabetes vascular complications. Thus, platelets appear as both targets and effectors in the pathophysiology of DM, carrying and transducing metabolic derangement into vascular injury [67].
Future efforts to decrease the thrombotic burden in diabetes should target specific disease-based mechanisms. In this perspective, high-throughput techniques are fundamental since they offer a unique opportunity to deciphering the molecular networks altered by the metabolic derangement associated with type 2 diabetes mellitus (T2DM), such as the platelet transcriptome and proteome composition and/or post-transcriptional regulation [68].
3.3.3. Platelets in Cancer
Tumorigenesis is a multistep process requiring concerted changes in both tumor cells and the tumor microenvironment. Experimental evidence has highlighted platelets as active players in all steps of tumorigenesis including tumor growth, tumor cell extravasation, and metastasis [69][70]. They infiltrate into the tumor microenvironment to directly interact with cancer cells and can activate the same proliferative pathways that are activated through oncogenic mutations, thus contributing to the initiation and progression of disease. Platelets also exert anti-apoptotic roles in both hematopoietic and solid tumor malignancies, thus sustaining tumor cell survival [69][70]. Moreover, platelets have the ability to deliver multiple proangiogenic factors to the tumor, including vascular endothelial growth factor, platelet-derived growth factor, fibroblast growth factors, and MMPs, as well as the ability to stimulate the expression of proangiogenic factors by the tumor cells. In that way, platelets promote the neovascularization needed to assure an adequate blood supply for delivering necessary nutrients, removing waste, and oxygenating the tumor [69]. In the circulation, platelets help circulating tumor cells to escape the deadly attack of the immune system by building a partial physical barrier toward natural killer cells, through the formation of platelet-tumor cell aggregates, and by interfering with the recognition of cancer cells by natural killer cells, through the transfer of “normal” major histocompatibility complex class I molecules onto the surface of tumor cells [69][70]. This ability of platelets to protect tumor cells in circulation from immune surveillance is likely to significantly contribute to the metastatic process. Additionally, by activating the platelet-derived transforming growth factor-β/Smad and nuclear factor kB pathways, platelet-derived transforming growth factor-β facilitates an invasive epithelial-to-mesenchymal transition phenotype in cancer cells and increase metastases [70]. In addition, platelets may contribute to metastasis, helping circulating tumor cells to attach to the endothelium, providing signals to establish a pre-metastatic niche, and facilitating extravasation at a distant site [69][70][71]. Finally, platelets even influence the sensitivity of chemotherapy and other targeted therapies in cancer patients [70]. On the other hand, tumor cells mediate platelet activation, leading to platelet aggregation and the release of platelet-derived growth and proangiogenic factors in the tumor microenvironment, which may contribute to tumor growth and angiogenesis and further magnifies the pro-coagulant milieu generated by the interaction between platelets and cancer cells [70].
Overall, increasing evidence supports the notion that in a cancerous setting, the normal hemostatic role for platelets can be hijacked to promote tumor growth, survival and metastasis, and that cancer cells and platelets maintain a complex, bidirectional communication. Based on this evidence, it is to be hoped that developing methods to specifically target platelet interaction with tumor cells without interfering with normal platelet functions could provide a significant advance in the treatment of cancer patients, especially in the metastatic setting [69][70][72].
References
- Machlus, K.R.; Italiano, J.E. 2—Megakaryocyte Development and Platelet Formation. In Platelets (Fourth Edition); Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 25–46.
- Lefrancais, E.; Ortiz-Munoz, G.; Caudrillier, A.; Mallavia, B.; Liu, F.; Sayah, D.M.; Thornton, E.E.; Headley, M.B.; David, T.; Coughlin, S.R.; et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 2017, 544, 105–109.
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179.
- Semple, J.W.; Italiano, J.E., Jr.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274.
- von Hundelshausen, P.; Weber, C. Platelets as immune cells: Bridging inflammation and cardiovascular disease. Circ. Res. 2007, 100, 27–40.
- Clemetson, K.J.; Clemetson, J.M. 9—Platelet Receptors. In Platelets (Fourth Edition); Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 169–192.
- Flaumenhaft, R.; Sharda, A. 19—Platelet Secretion. In Platelets (Fourth Edition); Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 349–370.
- O’Donnell, V.B.; Murphy, R.C.; Watson, S.P. Platelet lipidomics: Modern day perspective on lipid discovery and characterization in platelets. Circ. Res. 2014, 114, 1185–1203.
- Gasecka, A.; Nieuwland, R.; Siljander, P.R.M. 22—Platelet-Derived Extracellular Vesicles. In Platelets (Fourth Edition); Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 401–416.
- Provost, P. 6—Platelet MicroRNAs. In Platelets (Fourth Edition); Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 127–138.
- Jobe, S. Platelet Heterogeneity. In Platelets in Thrombotic and Non-Thrombotic Disorders; Gresele, P., Kleiman, N.S., Lopez, J., Page, C., Eds.; Springer: Cham, Switzerland, 2017.
- Rowley, J.W.; Manne, B.K.; Weyrich, A.S. The Platelet Transcriptome: Coding RNAs. In Platelets in Thrombotic and Non-Thrombotic Disorders; Gresele, P., Kleiman, N.S., Lopez, J., Page, C., Eds.; Springer: Cham, Switzerland, 2017.
- Rowley, J.W.; Weyrich, A.S.; Bray, P.F. 7—The Platelet Transcriptome in Health and Disease. In Platelets (Fourth Edition); Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 139–153.
- McManus, D.D.; Freedman, J.E. MicroRNAs in platelet function and cardiovascular disease. Nat. Rev. Cardiol. 2015, 12, 711–717.
- Fisher, M.H.; Di Paola, J. Genomics and transcriptomics of megakaryocytes and platelets: Implications for health and disease. Res. Pract. Thromb. Haemost. 2018, 2, 630–639.
- Nurden, A.T.; Nurden, P. Inherited disorders of platelet function: Selected updates. J. Thromb. Haemost. JTH 2015, 13 (Suppl. 1), S2–S9.
- Freson, K. 8—The Platelet Proteome. In Platelets (Fourth Edition); Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 155–167.
- Loosse, C.; Swieringa, F.; Heemskerk, J.W.M.; Sickmann, A.; Lorenz, C. Platelet proteomics: From discovery to diagnosis. Expert Rev. Proteom. 2018, 15, 467–476.
- Izquierdo, I.; Garcia, A. Platelet proteomics applied to the search for novel antiplatelet therapeutic targets. Expert Rev. Proteom. 2016, 13, 993–1006.
- Sharda, A.; Flaumenhaft, R. The life cycle of platelet granules. F1000Research 2018, 7, 236.
- Yun, S.H.; Sim, E.H.; Goh, R.Y.; Park, J.I.; Han, J.Y. Platelet Activation: The Mechanisms and Potential Biomarkers. Biomed Res. Int. 2016, 2016, 9060143.
- Ozaki, Y.; Suzuki-Inoue, K.; Inoue, O. Platelet receptors activated via mulitmerization: Glycoprotein VI, GPIb-IX-V, and CLEC-2. J. Thromb. Haemost. JTH 2013, 11 (Suppl. 1), 330–339.
- Gurbel, P.A.; Kuliopulos, A.; Tantry, U.S. G-protein-coupled receptors signaling pathways in new antiplatelet drug development. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 500–512.
- Sidhu, T.S.; French, S.L.; Hamilton, J.R. Differential signaling by protease-activated receptors: Implications for therapeutic targeting. Int. J. Mol. Sci. 2014, 15, 6169–6183.
- Thomas, M.R.; Storey, R.F. The role of platelets in inflammation. Thromb. Haemost. 2015, 114, 449–458.
- Lievens, D.; von Hundelshausen, P. Platelets in atherosclerosis. Thromb. Haemost. 2011, 106, 827–838.
- Aloui, C.; Prigent, A.; Sut, C.; Tariket, S.; Hamzeh-Cognasse, H.; Pozzetto, B.; Richard, Y.; Cognasse, F.; Laradi, S.; Garraud, O. The signaling role of CD40 ligand in platelet biology and in platelet component transfusion. Int. J. Mol. Sci. 2014, 15, 22342–22364.
- Pamukcu, B.; Lip, G.Y.; Snezhitskiy, V.; Shantsila, E. The CD40-CD40L system in cardiovascular disease. Ann. Med. 2011, 43, 331–340.
- Antoniades, C.; Bakogiannis, C.; Tousoulis, D.; Antonopoulos, A.S.; Stefanadis, C. The CD40/CD40 ligand system: Linking inflammation with atherothrombosis. J. Am. Coll. Cardiol. 2009, 54, 669–677.
- Lord, M.S.; Cheng, B.; Farrugia, B.L.; McCarthy, S.; Whitelock, J.M. Platelet Factor 4 Binds to Vascular Proteoglycans and Controls Both Growth Factor Activities and Platelet Activation. J. Biol. Chem. 2017, 292, 4054–4063.
- Woller, G.; Brandt, E.; Mittelstadt, J.; Rybakowski, C.; Petersen, F. Platelet factor 4/CXCL4-stimulated human monocytes induce apoptosis in endothelial cells by the release of oxygen radicals. J. Leukoc. Biol. 2008, 83, 936–945.
- Virani, S.S.; Nambi, V.; Hoogeveen, R.; Wasserman, B.A.; Coresh, J.; Gonzalez, F., 2nd; Chambless, L.E.; Mosley, T.H.; Boerwinkle, E.; Ballantyne, C.M. Relationship between circulating levels of RANTES (regulated on activation, normal T-cell expressed, and secreted) and carotid plaque characteristics: The Atherosclerosis Risk in Communities (ARIC) Carotid MRI Study. Eur. Heart J. 2011, 32, 459–468.
- von Hundelshausen, P.; Koenen, R.R.; Sack, M.; Mause, S.F.; Adriaens, W.; Proudfoot, A.E.; Hackeng, T.M.; Weber, C. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood 2005, 105, 924–930.
- Kim, S.J.; Jenne, C.N. Role of platelets in neutrophil extracellular trap (NET) production and tissue injury. Semin. Immunol. 2016, 28, 546–554.
- Thalin, C.; Hisada, Y.; Lundstrom, S.; Mackman, N.; Wallen, H. Neutrophil Extracellular Traps: Villains and Targets in Arterial, Venous, and Cancer-Associated Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1724–1738.
- Thon, J.N.; Peters, C.G.; Machlus, K.R.; Aslam, R.; Rowley, J.; Macleod, H.; Devine, M.T.; Fuchs, T.A.; Weyrich, A.S.; Semple, J.W.; et al. T granules in human platelets function in TLR9 organization and signaling. J. Cell Biol. 2012, 198, 561–574.
- Blair, P.; Flaumenhaft, R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189.
- Chen, Y.; Yuan, Y.; Li, W. Sorting machineries: How platelet-dense granules differ from alpha-granules. Biosci. Rep. 2018, 38.
- Heijnen, H.; van der Sluijs, P. Platelet secretory behaviour: As diverse as the granules . or not? J. Thromb. Haemost. JTH 2015, 13, 2141–2151.
- Gremmel, T.; Ay, C.; Riedl, J.; Kopp, C.W.; Eichelberger, B.; Koppensteiner, R.; Panzer, S. Platelet-specific markers are associated with monocyte-platelet aggregate formation and thrombin generation potential in advanced atherosclerosis. Thromb. Haemost. 2016, 115, 615–621.
- Czepluch, F.S.; Kuschicke, H.; Dellas, C.; Riggert, J.; Hasenfuss, G.; Schafer, K. Increased proatherogenic monocyte-platelet cross-talk in monocyte subpopulations of patients with stable coronary artery disease. J. Intern. Med. 2014, 275, 144–154.
- Loguinova, M.; Pinegina, N.; Kogan, V.; Vagida, M.; Arakelyan, A.; Shpektor, A.; Margolis, L.; Vasilieva, E. Monocytes of Different Subsets in Complexes with Platelets in Patients with Myocardial Infarction. Thromb. Haemost. 2018, 118, 1969–1981.
- Italiano, J.E., Jr.; Mairuhu, A.T.; Flaumenhaft, R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr. Opin. Hematol. 2010, 17, 578–584.
- Varon, D.; Shai, E. Platelets and their microparticles as key players in pathophysiological responses. J. Thromb. Haemost. JTH 2015, 13 (Suppl. 1), S40–S46.
- Wang, Z.T.; Wang, Z.; Hu, Y.W. Possible roles of platelet-derived microparticles in atherosclerosis. Atherosclerosis 2016, 248, 10–16.
- Bao, H.; Chen, Y.X.; Huang, K.; Zhuang, F.; Bao, M.; Han, Y.; Chen, X.H.; Shi, Q.; Yao, Q.P.; Qi, Y.X. Platelet-derived microparticles promote endothelial cell proliferation in hypertension via miR-142-3p. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 3912–3923.
- Mezouar, S.; Mege, D.; Darbousset, R.; Farge, D.; Debourdeau, P.; Dignat-George, F.; Panicot-Dubois, L.; Dubois, C. Involvement of platelet-derived microparticles in tumor progression and thrombosis. Semin. Oncol. 2014, 41, 346–358.
- El-Gamal, H.; Parray, A.S.; Mir, F.A.; Shuaib, A.; Agouni, A. Circulating microparticles as biomarkers of stroke: A focus on the value of endothelial- and platelet-derived microparticles. J. Cell. Physiol. 2019, 234, 16739–16754.
- Wang, N.; Tall, A.R. Cholesterol in platelet biogenesis and activation. Blood 2016, 127, 1949–1953.
- Vinik, A.I.; Erbas, T.; Park, T.S.; Nolan, R.; Pittenger, G.L. Platelet dysfunction in type 2 diabetes. Diabetes Care 2001, 24, 1476–1485.
- Santilli, F.; Davi, G.; Consoli, A.; Cipollone, F.; Mezzetti, A.; Falco, A.; Taraborelli, T.; Devangelio, E.; Ciabattoni, G.; Basili, S.; et al. Thromboxane-dependent CD40 ligand release in type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2006, 47, 391–397.
- Gkaliagkousi, E.; Passacquale, G.; Douma, S.; Zamboulis, C.; Ferro, A. Platelet activation in essential hypertension: Implications for antiplatelet treatment. Am. J. Hypertens. 2010, 23, 229–236.
- Badimon, L.; Bugiardini, R.; Cenko, E.; Cubedo, J.; Dorobantu, M.; Duncker, D.J.; Estruch, R.; Milicic, D.; Tousoulis, D.; Vasiljevic, Z.; et al. Position paper of the European Society of Cardiology-working group of coronary pathophysiology and microcirculation: Obesity and heart disease. Eur. Heart J. 2017, 38, 1951–1958.
- Harding, S.A.; Sarma, J.; Josephs, D.H.; Cruden, N.L.; Din, J.N.; Twomey, P.J.; Fox, K.A.; Newby, D.E. Upregulation of the CD40/CD40 ligand dyad and platelet-monocyte aggregation in cigarette smokers. Circulation 2004, 109, 1926–1929.
- Csordas, A.; Bernhard, D. The biology behind the atherothrombotic effects of cigarette smoke. Nat. Rev. Cardiol. 2013, 10, 219–230.
- Scherlinger, M.; Guillotin, V.; Truchetet, M.E.; Contin-Bordes, C.; Sisirak, V.; Duffau, P.; Lazaro, E.; Richez, C.; Blanco, P. Systemic lupus erythematosus and systemic sclerosis: All roads lead to platelets. Autoimmun. Rev. 2018, 17, 625–635.
- Baaten, C.; Ten Cate, H.; van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet populations and priming in hematological diseases. Blood Rev. 2017, 31, 389–399.
- Spronk, H.M.H.; Padro, T.; Siland, J.E.; Prochaska, J.H.; Winters, J.; van der Wal, A.C.; Posthuma, J.J.; Lowe, G.; D’Alessandro, E.; Wenzel, P.; et al. Atherothrombosis and Thromboembolism: Position Paper from the Second Maastricht Consensus Conference on Thrombosis. Thromb. Haemost. 2018, 118, 229–250.
- Michelson, A.D.; Cattaneo, M.; Frelinger, A.; Newman, P. Platelets (Fourth Edition), 4th ed.; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2019.
- Davi, G.; Patrono, C. Platelet activation and atherothrombosis. New Engl. J. Med. 2007, 357, 2482–2494.
- Gawaz, M.; Borst, O. 26—The Role of Platelets in Atherothrombosis. In Platelets (Fourth Edition); Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 459–467.
- Totani, L.; Evangelista, V. Platelet-leukocyte interactions in cardiovascular disease and beyond. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2357–2361.
- Finsterbusch, M.; Schrottmaier, W.C.; Kral-Pointner, J.B.; Salzmann, M.; Assinger, A. Measuring and interpreting platelet-leukocyte aggregates. Platelets 2018, 29, 677–685.
- Badimon, L.; Padro, T.; Vilahur, G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur. Heart J. Acute Cardiovasc. Care 2012, 1, 60–74.
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632.
- Pretorius, E. Platelets as Potent Signaling Entities in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. TEM 2019, 30, 532–545.
- Santilli, F.; Simeone, P.; Liani, R.; Davi, G. Platelets and diabetes mellitus. Prostaglandins Other Lipid Mediat. 2015, 120, 28–39.
- Santilli, F.; Simeone, P.; Liani, R.; Davì, G. Platelets and Diabetes. In Platelets in Thrombotic and Non-Thrombotic Disorders; Gresele, P., Kleiman, N.S., Lopez, J., Page, C., Eds.; Springer: Cham, Switzerland, 2017.
- Franco, A.T.; Corken, A.; Ware, J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015, 126, 582–588.
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood, A.K. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell 2018, 33, 965–983.
- Labelle, M.; Begum, S.; Hynes, R.O. Platelets guide the formation of early metastatic niches. Proc. Natl. Acad. Sci. USA 2014, 111, E3053–E3061.
- Xu, X.R.; Yousef, G.M.; Ni, H. Cancer and platelet crosstalk: Opportunities and challenges for aspirin and other antiplatelet agents. Blood 2018, 131, 1777–1789.




