
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yong Chool Boo | + 2725 word(s) | 2725 | 2021-07-28 11:13:14 | | | |
| 2 | Vivi Li | -7 word(s) | 2718 | 2021-07-29 05:08:40 | | |
Video Upload Options
Resveratrol is a polyphenol compound found in many edible plants such as Vitis vinifera, and its inhibitory effects on the catalytic activity, gene expression, and posttranslational modifications of tyrosinase, a key enzyme in the melanin biosynthetic pathway, provide a mechanistic basis for its antimelanogenic effects seen in melanocytic cells, three-dimensionally reconstituted skin models, and in vivo animal models. Recent clinical studies have supported the efficacy of resveratrol and its analogs, such as resveratryl triacetate (RTA) and resveratryl triglycolate (RTG), in human skin lightening. These findings suggest that resveratrol and its analogs are potentially useful as skin lightening agents in cosmetics.
1. Introduction
In human skin, melanin is produced in a specialized organelle called “melanosome” in the melanocytes, which localizes in the basal layer of the skin epidermis [1]. Mature melanosomes filled with melanin are transferred from a single melanocyte, via dendrites, to several keratinocytes in the outer proximity, distributing melanin throughout the epidermis [2]. Melanin is an effective absorbent of UV, reducing the risk of photoaging and photocarcinogenesis [3], and is a key player in maintaining skin homeostasis [4].
Unwanted abnormal skin pigmentations are clinically and aesthetically significant conditions that can cause mental stress and lower the quality of life [5]. Various approaches are used to control hyper- and hypo-pigmentation in dermatology and cosmetology. A variety of natural and synthetic compounds that inhibit the catalytic activity of tyrosinase, which is a key enzyme in the melanin biosynthesis, have previously been reported in the literature [6][7], but their clinical efficacies are largely unknown.
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a polyphenolic compound found in various plants, including grapes, berries, and peanuts [8][9][10][11]. It is believed to act as a phytoalexin in several plants, providing defense against attack by insects and pathogens [12][13].
Resveratrol can act as an antioxidant and can modulate cell functions, signal transduction, and gene expression [14]. Resveratrol activates the nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway by a phosphoinositide 3-kinases/Akt (protein kinase B)-dependent mechanism [15][16][17]. It induces nuclear accumulation of Nrf2 and gene expression of reduced nicotinamide adenine dinucleotide phosphate (NADPH) quinone dehydrogenase 1, glutathione peroxidase 2, and the catalytic and modulatory subunits of glutamate-cysteine ligase, in the primary culture of normal human keratinocytes [18]. Resveratrol directly or indirectly activates sirtuin 1, a NAD-dependent deacetylase, that is involved in metabolic regulation, stress response, and aging processes [19][20].
Evidence supporting its antimelanogenic activity has accumulated in the last decade [21][22]. As an approach to enhance the stability and efficacy of resveratrol, our research team developed its analogs, resveratryl triacetate (RTA) and resveratryl triglycolate (RTG), and undertook human trials to evaluate their skin lightening efficacy [23][24][25]. Herein, we scrutinize recent literature on the anti-melanogenic activities and skin lightening efficacies of resveratrol and its analogs to examine their potential as active ingredients for skin lightening in the cosmetics industry.
2. Resveratrol as a Tyrosinase Inhibitor
Various stilbenoids, including resveratrol, inhibit mushroom tyrosinase activity [26][27][28]. Oxyresveratrol has been shown to exhibit more potent inhibition of L-tyrosine oxidation catalyzed by murine tyrosinase (IC50, 52.7 μM) than resveratrol (IC50 > 100 μM). Piceatannol has been shown to be a very potent inhibitor of mushroom tyrosinase (IC50, 1.53 μM), compared to kojic acid (IC50, 50.1 μM) and resveratrol (IC50, 63.2 μM) [29]. Oxyresveratrol is found in many plants, such as Morus alba, and shows antioxidant activity mitigating oxidative stress and inflammatory reactions [30][31].
Gnetin C, a resveratrol dimer isolated from melinjo (Gnetum gnemon) has been shown to be as effective as resveratrol with regard to its inhibitory activity against mushroom tyrosinase, but the former has a much weaker inhibitory activity against murine tyrosinase than the latter [32]. Vitis vinifera extracts containing gallic acid, chlorogenic acid, epicatechin, rutin, and resveratrol show competitive inhibition against mushroom tyrosinase activity [33]. Collectively, these studies suggest that resveratrol is a modest, and not a very potent inhibitor of mushroom tyrosinase.
Resveratrol has been shown to be an active component of Vitis vinifera extracts that inhibit human tyrosinase activity [34]. Resveratrol inhibited human tyrosinase activity more strongly (IC50, 0.39 µg mL−1) than p-coumaric acid (IC50, 0.66 µg mL−1) and arbutin (IC50 > 100 µg mL−1). Resveratrol had a much lower effect on mushroom tyrosinase activity than on human tyrosinase activity.
Resveratrol can be biotransformed by mushroom tyrosinase to its oxidized form, which is a more powerful inhibitor of mushroom tyrosinase than resveratrol itself [35][36][37]. The reaction products of resveratrol by tyrosinase were more toxic than resveratrol itself [38]. Oxyresveratrol is also a substrate of mushroom tyrosinase [39]. Thus, it is necessary to study whether the same mechanism also applies to human tyrosinase.
3. Antimelanogenic Mechanisms of Resveratrol and Its Analogs
Although resveratrol inhibits tyrosinase activity less effectively than oxyresveratrol in vitro, the former inhibits cellular melanogenesis more effectively than the latter [40]. Resveratrol and 4-n-butyl resorcinol synergistically inhibit tyrosinase activity and tyrosinase gene expression [41][42]. Various chemical modifications have been attempted to enhance the therapeutic potential of resveratrol [43][44]. Some chemically synthesized resveratrol analogs show more potent inhibition of tyrosinase activity, tyrosinase gene expression, and/or cellular melanin synthesis than resveratrol demonstrated [45][46][47][48]. Semi-synthetic derivatives from resveratrol show altered inhibition against tyrosinase activity and cellular melanin synthesis [40][49][50][51].
Resveratrol inhibits MITF promoter activity induced by UV or forskolin in B16 cells [52]. Resveratrol, resveratryl triacetate (RTA), and resveratryl triglycolate (RTG) lower the mRNA and protein levels of tyrosinase, DCT, and MITF in human epidermal melanocytes [40][50]. Resveratrol and its trimethyl ether decrease the tyrosinase protein level and tyrosinase activity in B16 cells stimulated by α-MSH [51]. Therefore, resveratrol and its analogs are assumed to reduce the gene expression of MITF and downstream melanogenic enzymes by inhibiting the cAMP-dependent pathway.
Resveratrol activates sirtuin 1, which in turn activates transcription factors p53 and forkhead box O (FOXO) [53]. Resveratrol can confer antimelanogenic activity through a FOXO3a-dependent mechanism [54]. Resveratrol is also known as a potent inducer of autophagy [55], which is a lysosome-dependent mechanism for removing misfolded or damaged proteins or unnecessary organelles [56]. Resveratrol increased expression levels of autophagy-related gene 5 (ATG5) while decreasing MITF, tyrosinase, and TYRP1 in Melan-A cells stimulated by α-MSH [57]. Small interfering RNA-mediated depletion of ATG5 rescued the expression of MITF, tyrosinase, and TYRP1 in the presence of resveratrol, indicating that autophagy is associated with the antimelanogenic effects of resveratrol.
Post-translational modifications of tyrosinase and other melanogenic enzymes are required for full activation [58][59]. Normal human melanocytes contain mainly the mature, Golgi-processed form of tyrosinase, but the cells treated with resveratrol contain mostly endoplasmic reticulum (ER)-retained, immature tyrosinase. This indicates that resveratrol can disrupt the trafficking of tyrosinase from the ER to the Golgi and the maturation of tyrosinase [60]. Thus, resveratrol and its analogs are considered to regulate cellular melanin synthesis by multiple mechanisms, including the inhibition of catalytic activity, gene expression, and posttranslational maturation of tyrosinase in melanocytes. The potential anti-melanogenic action mechanism of resveratrol is shown in Figure 1.
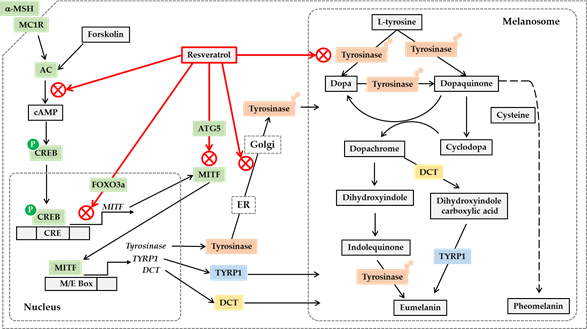
Figure 1. Potential mechanisms for the antimelanogenic action of resveratrol. On the binding of a-melanocyte stimulating hormone (a-MSH) to the melanocortin 1 receptor (MC1R) and subsequent activation of cyclic AMP (cAMP)-producing adenylate cyclase (AC) leads to the phosphorylation of cAMP-responsive element-binding protein (CREB) by protein kinase A. Phosphorylated CREB enters the nucleus and binds to cAMP response elements (CRE) on the promoter of its target genes including microphthalmia-associated transcription factor (MITF), activating their gene expression. Resveratrol can inhibit the gene expression of MITF, tyrosinase, tyrosinase-related protein 1 (TYRP1) and dopachrome tautomerase (DCT) stimulated by a-MSH or forskolin, a director activator of AC, which is mediated by a cAMP-dependent mechanism. Resveratrol can suppress MITF activation by a FOXO3a-dependent mechanism. Resveratrol can also stimulate autophagy-related gene 5 (ATG5) expression inducing autophagy, and reduce the protein levels of MITF and tyrosinase. The antimelanogenic enzymes such as tyrosinase undergo posttranslational modifications in the endoplasmic reticulum (ER) and Golgi, and resveratrol can inhibit these processes. Resveratrol can also inhibit enzyme reactions of tyrosinase involved in the synthesis of eumelanin and pheomelanin in the melanosomes. Although the synthetic route of pheomelanin is simply drawn here, it is very complicated and involves many enzymes and metabolites. There are many other pathways that are involved in the regulation of cellular melanin synthesis but are not covered in this figure.
4. Hypopigmentation Effect of Resveratrol
In vivo experiments and human tests on the skin lightening and antiaging activity of resveratrol and its analogs are listed in Table 1. In dark-skinned Yucatan swine, topical treatment with 1% resveratrol twice a day, 5 days per week, for 8 weeks resulted in visible skin lightening without signs of irritation or other undesired effects [52]. In another experiment using light-skinned Yucatan swine, skin tanning was induced by exposing them to one minimal erythema dose (MED) of UVB, once per day, on three alternate days. Topical treatment with 1% resveratrol once daily for 2 weeks, immediately after each UVB exposure and on non-UVB exposure days, reduced the UVB-induced pigment deposition in Yucatan swine.
Lee et al. have tested the hypopigmentation effect of resveratrol in brownish guinea pigs [21][61][62]. In one study [21], pigmentation was induced by exposing the dorsal skin of guinea pigs to UVB (lmax, 310 nm) at 390 mJ cm−2 thrice per week, for two weeks, and thereafter, 1% resveratrol solution was topically applied every day to these animals for 2 weeks. As a result, UVB exposure increased the pigment index from 40.7 ± 1.6 in the baseline group to 62.6 ± 2.3 in the vehicle control group and 53.4 ± 1.0 in the 1% resveratrol treatment group, indicating a hypopigmentation effect of resveratrol. Histological data suggested that resveratrol reduced melanin synthesis by decreasing DCT among the melanogenic enzymes. In subsequent studies, resveratrol-enriched rice extract and the same extract encapsulated in nanoparticles were shown to exhibit hypopigmentation effects in guinea pigs [61][62].
Table 1. In vivo and clinical studies on the skin lightening efficacy of resveratrol and its analogs.
|
Literature |
Tests |
Models |
Treatments |
Assessments |
|
Lin et al., 2002 [52] |
Yucatan swine |
Natural pigmentation |
1% Resveratrol |
Visual Evaluation |
|
UV-induced tanning |
||||
|
Lee et al., 2014 [21] |
Guinea pigs |
UV-induced tanning |
1% Resveratrol |
Instrumental methods |
|
Visual Evaluation |
||||
|
Wu et a., 2013 [22] |
Humans |
UV-induced tanning |
1% Resveratrol |
Instrumental methods |
|
Ryu et al., 2015 [23] |
Humans |
UV-induced tanning |
0.4% RTA |
Instrumental methods |
|
Natural pigmentation |
||||
|
Boo, 2016 [24] |
Humans |
UV-induced tanning |
0.8% RTA |
Instrumental methods |
|
Visual Evaluation |
||||
|
Ryu et al., 2018 [63] |
Humans |
Natural pigmentation |
0.8% RTA |
Instrumental methods |
|
Ryu et al., 2018 [25] |
Humans |
UV-induced tanning |
0.4% RTG |
Instrumental methods |
|
Visual Evaluation |
Abbreviations: RTA, resveratryl triacetate; RTG, resveratryl triglycolate.
5. Human Skin Lightening Efficacy of Resveratrol
The effects of resveratrol against skin pigmentation and sunburn caused by repetitive UV irradiation were examined in a human trial employing 15 healthy volunteers [22]. Six sites on the non-exposed dorsal skin of each volunteer were exposed to solar simulating UV at a dosage of 1.5 MED for 4 consecutive days, and different test materials were topically applied immediately after each UV exposure.
The skin color can be expressed using the Commission Internationale de l’Eclairage Lab color space composed of the degree of lightness (L*), degree of green to red (a*), and degree of yellow to blue (b*) [64]. In this study, the skin color parameters, L*, a*, and b were measured using Spectrophotometer® CM-2500d (Minolta, Tokyo, Japan) [64].
Four days after UV irradiation, L* values decreased from 63.89 to 55.91 in the control group, and from 64.20 to 59.3 in the 1% resveratrol treatment group, indicating reduced tanning in the treatment group. The a* values increased from 7.62 to 16.29 in the control group, and from 7.51 to 13.43 in the treatment group, indicating that sunburn was reduced in the treatment group. The histological analysis supported that UV-induced sunburn and suntan were reduced by resveratrol treatment.
6. Human Skin Lightening Efficacy of Resveratryl Triacetate (RTA)
As an approach to improve the stability of resveratrol as an active ingredient in cosmetics, resveratrol was acetylated to RTA as a “prodrug” form [40][65]. RTA showed higher stability in solutions, lower cytotoxicity, and a similar inhibitory effect on melanin synthesis in cultured melanocytes, as compared to resveratrol itself [40]. It is assumed that the acetylated compound may be converted to resveratrol by the esterase enzymes in cells.
The safety and skin lightening efficacy of RTA were investigated in human studies [23][24]. The primary skin irritation potentials of resveratrol and RTA were assessed at 0.1% and 0.5% concentrations in thirty-three healthy women [23], via a closed patch testing method [66][67]. On testing, resveratrol was observed to induce weak skin irritation at 0.5%, whereas RTA did not induce any adverse skin reactions [23].
The human skin lightening efficacy of RTA was evaluated [23] using the artificial tanning and natural hyperpigmentation models [68][69]. In all, 22 women with Fitzpatrick skin types III or IV were enrolled in the test using the artificial tanning model. The volunteers were randomly divided into two groups, and depending on the group, each of the two test sites in each volunteer received either the test product containing 0.4% RTA or the control product, twice daily, for 8 weeks. The skin color was represented by the individual typology angles (ITA°) which were calculated using the equation: ITA° = (arc tangent [(L* − 50)/b*]) 180/3.14159. [70]. The higher the ITA° value, the lighter the skin color.
As tanned skin underwent the depigmentation process, the ITA° increased continuously for 8 weeks, in both test and control groups. The application of the test and control products for 8 weeks increased the ITA° by 17.60% and 13.81%, respectively, and the difference was statistically significant (p < 0.05).
In another study using the natural hyperpigmentation model, 21 women were enrolled. The volunteers were divided into two groups and depending on the group, the right or left sides of the face of each volunteer received either the test product containing 0.4% RTA or the control product, twice daily, for 8 weeks. The pigmentation intensity of the highly pigmented area decreased by 2.67% and 1.46% in the test and control groups, respectively, and the difference was statistically significant (p < 0.05). These studies supported the human skin lightening efficacy of topically applied 0.4 % RTA.
The human skin lighting efficacy of 0.8% RTA was further examined using the artificial UV-induced tanning model in a separate study [24]. The intergroup difference was statistically significant (p < 0.05). Therefore, 0.8% RTA-containing cosmetic products can confer skin lightening efficacy in humans.
7. Human Skin Lightening Efficacy of Resveratryl Triglycolate (RTG)
RTG is a new hybrid compound between resveratrol and glycolic acid [50]. The resveratryl moiety of RTG was expected to reduce the production of new melanin and the glycolic moiety was expected to remove the keratin that previously accumulated melanin. RTG inhibited tyrosinase activity in vitro and MITF and tyrosine gene expression, suppressing cellular melanin synthesis as effectively as, or slightly more effectively than, resveratrol and RTA [50].
The primary skin irritation potential of RTG was tested in a human study where 30 healthy women participated [25]. The test product contained 0.4% RTG and the control product comprised the same formula without RTG. In patch testing, neither the control product nor the test product containing 0.4% RTG induced any adverse skin reactions in any participant.
The depigmenting efficacy of RTG was tested in a human trial using a UV-induced artificial tanning model [25]. The melanin index decreased from 205 to 163 in the test group and to 172 in the control group, and the difference between these two groups was statistically significant (p < 0.05). Analysis of ITA° as a skin color index showed that the test product containing 0.4% RTG and the control product increased ITA° by 24.42% and 17.81% in 6 weeks and by 28.96% and 22.06% in 8 weeks, respectively. The intergroup differences at each time point were statistically significant (p < 0.05). The pigmentation degrees visually assessed by two experienced examiners were also supportive of the clinical efficacy of RTG. The test product containing 0.4% RTG and the control product decreased the pigmentation degree by 31.9% (from 7.05 to 4.98) and 29.4% (from 7.11 to 4.84) in 8 weeks, respectively. The intergroup difference was statistically significant (p < 0.05).
8. Conclusions
In conclusion, the results from in vivo studies or clinical studies are supportive of the human skin lightening and/or antiaging efficacies of resveratrol and its analogs. In animal studies and in clinical trials, 1% resveratrol has been shown to reduce pigmentation induced by UV when it is applied topically to the skin. Resveratrol is considered to attenuate cellular melanin synthesis through inhibition of tyrosinase catalytic activity, and inhibition of processes such as tyrosinase gene expression, tyrosinase protein maturation, and autophagy. Although there is a lack of direct evidence, resveratrol might interfere with melanosome biogenesis because it reduced the activity of MITF, which is a key regulator of melanosome biogenesis as well as melanogenesis.
The resveratrol analogs, RTA and RTG, also showed human skin lightening effects in clinical trials at the tested concentrations (0.4% and 0.8% RTA, and 0.4 % RTG). Comparison of separate clinical studies with regard to the skin lightening efficacies of RTA and RTG indirectly suggests that the efficacy of RTG is slightly superior to that of RTA. RTA and RTG might act as “prodrugs” of resveratrol, and their skin lightening efficacies would depend on their penetration through the skin and biotransformation to resveratrol in cells.
References
- Schiaffino, M.V. Signaling pathways in melanosome biogenesis and pathology. Int J Biochem Cell Biol 2010, 42, 1094-1104.
- Cardinali, G.; Ceccarelli, S.; Kovacs, D.; Aspite, N.; Lotti, L.V.; Torrisi, M.R.; Picardo, M. Keratinocyte growth factor promotes melanosome transfer to keratinocytes. J Invest Dermatol 2005, 125, 1190-1199.
- Yamaguchi, Y.; Beer, J.Z.; Hearing, V.J. Melanin mediated apoptosis of epidermal cells damaged by ultraviolet radiation: factors influencing the incidence of skin cancer. Arch Dermatol Res 2008, 300 Suppl 1, S43-50.
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol 2012, 212, v, vii, 1-115.
- Maymone, M.B.C.; Neamah, H.H.; Secemsky, E.A.; Vashi, N.A. Correlating the Dermatology Life Quality Index and Skin Discoloration Impact Evaluation Questionnaire tools in disorders of hyperpigmentation. J Dermatol 2018, 45, 361-362.
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: medicinal chemistry perspective of tyrosinase inhibitors. J Enzyme Inhib Med Chem 2017, 32, 403-425.
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J Enzyme Inhib Med Chem 2019, 34, 279-309.
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct 2017, 8, 4284-4305.
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity 2009, 2, 270-278.
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive compounds. Phytother Res 2009, 23, 1197-1204.
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci Nutr 2018, 6, 2473-2490.
- Gu, J.; Hu, W.; Zhang, D.D. Resveratrol, a polyphenol phytoalexin, protects against doxorubicin-induced cardiotoxicity. J Cell Mol Med 2015, 19, 2324-2328.
- Pervaiz, S. Chemotherapeutic potential of the chemopreventive phytoalexin resveratrol. Drug Resist Updat 2004, 7, 333-344.
- Kovacic, P.; Somanathan, R. Multifaceted approach to resveratrol bioactivity: Focus on antioxidant action, cell signaling and safety. Oxid Med Cell Longev 2010, 3, 86-100.
- Yang, H.; Tang, C.Y.; Luo, C.; He, H.X.; Zhou, Y.D.; Yu, W.H. Resveratrol Attenuates the Cytotoxicity Induced by Amyloid-beta(1-42) in PC12 Cells by Upregulating Heme Oxygenase-1 via the PI3K/Akt/Nrf2 Pathway. Neurochemical Research 2018, 43, 297-305.
- Ungvari, Z.; Bagi, Z.; Feher, A.; Recchia, F.A.; Sonntag, W.E.; Pearson, K.; de Cabo, R.; Csiszar, A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 2010, 299, H18-24.
- Li, X.N.; Ma, L.Y.; Ji, H.; Qin, Y.H.; Jin, S.S.; Xu, L.X. Resveratrol protects against oxidative stress by activating the Keap-1/Nrf2 antioxidant defense system in obese-asthmatic rats. Exp Ther Med 2018, 16, 4339-4348.
- Soeur, J.; Eilstein, J.; Lereaux, G.; Jones, C.; Marrot, L. Skin resistance to oxidative stress induced by resveratrol: from Nrf2 activation to GSH biosynthesis. Free Radic Biol Med 2015, 78, 213-223.
- Gracia-Sancho, J.; Villarreal, G., Jr.; Zhang, Y.; Garcia-Cardena, G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc Res 2010, 85, 514-519.
- Gertz, M.; Giang, T.T.N.; Fischer, F.; Suenkel, B.; Schlicker, C.; Franzel, B.; Tomaschewski, J.; Aladini, F.; Becker, C.; Wolters, D.; Steegborn, C. A Molecular Mechanism for Direct Sirtuin Activation by Resveratrol. Plos One 2012, 7, e49761.
- Lee, T.H.; Seo, J.O.; Baek, S.H.; Kim, S.Y. Inhibitory effects of resveratrol on melanin synthesis in ultraviolet B-induced pigmentation in Guinea pig skin. Biomol Ther (Seoul) 2014, 22, 35-40.
- Wu, Y.; Jia, L.L.; Zheng, Y.N.; Xu, X.G.; Luo, Y.J.; Wang, B.; Chen, J.Z.; Gao, X.H.; Chen, H.D.; Matsui, M.; Li, Y.H. Resveratrate protects human skin from damage due to repetitive ultraviolet irradiation. J Eur Acad Dermatol Venereol 2013, 27, 345-350.
- Ryu, J.H.; Seok, J.K.; An, S.M.; Baek, J.H.; Koh, J.S.; Boo, Y.C. A study of the human skin-whitening effects of resveratryl triacetate. Arch Dermatol Res 2015, 307, 239-247.
- Boo, Y.C. Clinical evaluation of skin whitening effect of a cream containing resveratryl triacetate. Fragrance J Korea 2016, 2016, 72-79.
- Jo, D.J.; Seok, J.K.; Kim, S.Y.; Park, W.; Baek, J.H.; Kim, Y.M.; Boo, Y.C. Human skin-depigmenting effects of resveratryl triglycolate, a hybrid compound of resveratrol and glycolic acid. Int J Cosmet Sci 2018, 40, 256-262.
- Kim, Y.M.; Yun, J.; Lee, C.K.; Lee, H.; Min, K.R.; Kim, Y. Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechanism of action. J Biol Chem 2002, 277, 16340-16344.
- Shin, N.H.; Ryu, S.Y.; Choi, E.J.; Kang, S.H.; Chang, I.M.; Min, K.R.; Kim, Y. Oxyresveratrol as the potent inhibitor on dopa oxidase activity of mushroom tyrosinase. Biochem Biophys Res Commun 1998, 243, 801-803.
- Zheng, Z.P.; Tan, H.Y.; Wang, M. Tyrosinase inhibition constituents from the roots of Morus australis. Fitoterapia 2012, 83, 1008-1013.
- Yokozawa, T.; Kim, Y.J. Piceatannol inhibits melanogenesis by its antioxidative actions. Biol Pharm Bull 2007, 30, 2007-2011.
- Lee, H.S.; Kim, D.H.; Hong, J.E.; Lee, J.Y.; Kim, E.J. Oxyresveratrol suppresses lipopolysaccharide-induced inflammatory responses in murine macrophages. Hum Exp Toxicol 2015, 34, 808-818.
- Choi, H.Y.; Lee, J.H.; Jegal, K.H.; Cho, I.J.; Kim, Y.W.; Kim, S.C. Oxyresveratrol abrogates oxidative stress by activating ERK-Nrf2 pathway in the liver. Chem Biol Interact 2016, 245, 110-121.
- Yanagihara, M.; Yoshimatsu, M.; Inoue, A.; Kanno, T.; Tatefuji, T.; Hashimoto, K. Inhibitory effect of gnetin C, a resveratrol dimer from melinjo (Gnetum gnemon), on tyrosinase activity and melanin biosynthesis. Biol Pharm Bull 2012, 35, 993-996.
- Lin, Y.S.; Chen, H.J.; Huang, J.P.; Lee, P.C.; Tsai, C.R.; Hsu, T.F.; Huang, W.Y. Kinetics of Tyrosinase Inhibitory Activity Using Vitis vinifera Leaf Extracts. Biomed Research International 2017,
- Park, J.; Boo, Y.C. Isolation of resveratrol from vitis viniferae caulis and its potent inhibition of human tyrosinase. Evid Based Complement Alternat Med 2013, 2013, 645257.
- Bernard, P.; Berthon, J.Y. Resveratrol: an original mechanism on tyrosinase inhibition. Int J Cosmet Sci 2000, 22, 219-226.
- Gonzalvez, A.G.; Gonzalez Urena, A.; Lewis, R.J.; van der Zwan, G. Spectroscopy and kinetics of tyrosinase catalyzed trans-resveratrol oxidation. J Phys Chem B 2012, 116, 2553-2560.
- Satooka, H.; Kubo, I. Resveratrol as a kcat type inhibitor for tyrosinase: potentiated melanogenesis inhibitor. Bioorg Med Chem 2012, 20, 1090-1099.
- Ito, S.; Fujiki, Y.; Matsui, N.; Ojika, M.; Wakamatsu, K. Tyrosinase-catalyzed oxidation of resveratrol produces a highly reactive ortho-quinone: Implications for melanocyte toxicity. Pigment Cell Melanoma Res 2019,
- Ortiz-Ruiz, C.V.; Ballesta de Los Santos, M.; Berna, J.; Fenoll, J.; Garcia-Ruiz, P.A.; Tudela, J.; Garcia-Canovas, F. Kinetic characterization of oxyresveratrol as a tyrosinase substrate. IUBMB Life 2015, 67, 828-836.
- Park, J.; Park, J.H.; Suh, H.J.; Lee, I.C.; Koh, J.; Boo, Y.C. Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch Dermatol Res 2014, 306, 475-487.
- Kim, S.Y.; Park, K.C.; Kwon, S.B.; Kim, D.S. Hypopigmentary effects of 4-n-butylresorcinol and resveratrol in combination. Pharmazie 2012, 67, 542-546.
- Wang, Y.; Hao, M.M.; Sun, Y.; Wang, L.F.; Wang, H.; Zhang, Y.J.; Li, H.Y.; Zhuang, P.W.; Yang, Z. Synergistic Promotion on Tyrosinase Inhibition by Antioxidants. Molecules 2018, 23, 106.
- Ogas, T.; Kondratyuk, T.P.; Pezzuto, J.M. Resveratrol analogs: promising chemopreventive agents. Ann N Y Acad Sci 2013, 1290, 21-29.
- Pezzuto, J.M.; Kondratyuk, T.P.; Ogas, T. Resveratrol derivatives: a patent review (2009 - 2012). Expert Opin Ther Pat 2013, 23, 1529-1546.
- Choi, J.; Bae, S.J.; Ha, Y.M.; No, J.K.; Lee, E.K.; Lee, J.S.; Song, S.; Lee, H.; Suh, H.; Yu, B.P.; Chung, H.Y. A newly synthesized, potent tyrosinase inhibitor: 5-(6-hydroxy-2-naphthyl)-1,2,3-benzenetriol. Bioorg Med Chem Lett 2010, 20, 4882-4884.
- Franco, D.C.; de Carvalho, G.S.; Rocha, P.R.; da Silva Teixeira, R.; da Silva, A.D.; Raposo, N.R. Inhibitory effects of resveratrol analogs on mushroom tyrosinase activity. Molecules 2012, 17, 11816-11825.
- Liu, Q.; Kim, C.; Jo, Y.H.; Kim, S.B.; Hwang, B.Y.; Lee, M.K. Synthesis and Biological Evaluation of Resveratrol Derivatives as Melanogenesis Inhibitors. Molecules 2015, 20, 16933-16945.
- Zhu, Y.; Pan, W.H.; Ku, C.F.; Zhang, H.J.; Tsang, S.W. Design, synthesis and evaluation of novel dihydrostilbene derivatives as potential anti-melanogenic skin-protecting agents. European Journal of Medicinal Chemistry 2018, 143, 1254-1260.
- Fais, A.; Corda, M.; Era, B.; Fadda, M.B.; Matos, M.J.; Quezada, E.; Santana, L.; Picciau, C.; Podda, G.; Delogu, G. Tyrosinase inhibitor activity of coumarin-resveratrol hybrids. Molecules 2009, 14, 2514-2520.
- Park, S.; Seok, J.K.; Kwak, J.Y.; Choi, Y.H.; Hong, S.S.; Suh, H.J.; Park, W.; Boo, Y.C. Anti-melanogenic effects of resveratryl triglycolate, a novel hybrid compound derived by esterification of resveratrol with glycolic acid. Arch Dermatol Res 2016, 308, 325-334.
- Yoon, H.S.; Hyun, C.G.; Lee, N.H.; Park, S.S.; Shin, D.B. Comparative Depigmentation Effects of Resveratrol and Its Two Methyl Analogues in alpha-Melanocyte Stimulating Hormone-Triggered B16/F10 Murine Melanoma Cells. Prev Nutr Food Sci 2016, 21, 155-159.
- Lin, C.B.; Babiarz, L.; Liebel, F.; Roydon Price, E.; Kizoulis, M.; Gendimenico, G.J.; Fisher, D.E.; Seiberg, M. Modulation of microphthalmia-associated transcription factor gene expression alters skin pigmentation. J Invest Dermatol 2002, 119, 1330-1340.
- Hori, Y.S.; Kuno, A.; Hosoda, R.; Horio, Y. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS One 2013, 8, e73875.
- Kwon, S.H.; Choi, H.R.; Kang, Y.A.; Park, K.C. Depigmenting Effect of Resveratrol Is Dependent on FOXO3a Activation without SIRT1 Activation. International Journal of Molecular Sciences 2017, 18, 1213.
- Delmas, D.; Solary, E.; Latruffe, N. Resveratrol, a phytochemical inducer of multiple cell death pathways: apoptosis, autophagy and mitotic catastrophe. Curr Med Chem 2011, 18, 1100-1121.
- Khandia, R.; Dadar, M.; Munjal, A.; Dhama, K.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Iqbal, H.M.N.; Singh, K.P.; Joshi, S.K.; Chaicumpa, W. A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy. Cells 2019, 8, 64.
- Kim, E.S.; Chang, H.; Choi, H.; Shin, J.H.; Park, S.J.; Jo, Y.K.; Choi, E.S.; Baek, S.Y.; Kim, B.G.; Chang, J.W.; Kim, J.C.; Cho, D.H. Autophagy induced by resveratrol suppresses alpha-MSH-induced melanogenesis. Exp Dermatol 2014, 23, 204-206.
- White, R.; Hanson, G.C.; Hu, F. Tyrosinase maturation and pigment expression in B16 melanoma: relation to theophylline treatment and intracellular cyclic AMP. J Cell Physiol 1979, 99, 441-450.
- Halaban, R.; Pomerantz, S.H.; Marshall, S.; Lambert, D.T.; Lerner, A.B. Regulation of tyrosinase in human melanocytes grown in culture. J Cell Biol 1983, 97, 480-488.
- Newton, R.A.; Cook, A.L.; Roberts, D.W.; Leonard, J.H.; Sturm, R.A. Post-transcriptional regulation of melanin biosynthetic enzymes by cAMP and resveratrol in human melanocytes. J Invest Dermatol 2007, 127, 2216-2227.
- Lee, T.H.; Seo, J.O.; Do, M.H.; Ji, E.; Baek, S.H.; Kim, S.Y. Resveratrol-Enriched Rice Down-Regulates Melanin Synthesis in UVB-Induced Guinea Pigs Epidermal Skin Tissue. Biomol Ther (Seoul) 2014, 22, 431-437.
- Lee, T.H.; Kang, J.H.; Seo, J.O.; Baek, S.H.; Moh, S.H.; Chae, J.K.; Park, Y.U.; Ko, Y.T.; Kim, S.Y. Anti-Melanogenic Potentials of Nanoparticles from Calli of Resveratrol-Enriched Rice against UVB-Induced Hyperpigmentation in Guinea Pig Skin. Biomol Ther (Seoul) 2016, 24, 85-93.
- Choi, G.W.; Jeong, H.J.; Sek, J.K.; Baek, J.H.; Kim, Y.M.; Boo, Y.C. Skin Anti-aging Effects of a Cream Containing Resveratryl Triacetate (RTA). J Soc Cosmet Scientists Korea 2018, 44, 161-170.
- Pierard, G.E. EEMCO guidance for the assessment of skin colour. J Eur Acad Dermatol Venereol 1998, 10, 1-11.
- Hsieh, T.C.; Huang, Y.C.; Wu, J.M. Control of prostate cell growth, DNA damage and repair and gene expression by resveratrol analogues, in vitro. Carcinogenesis 2011, 32, 93-101.
- Wattanakrai, P.; Suwanachote, S.; Kulkollakarn, S.; Rajatanavin, N. The study of human skin irritation of a novel herbal skin care product and ingredients by human single closed patch testing. J Med Assoc Thai 2007, 90, 1116-1122.
- Loffler, H.; Pirker, C.; Aramaki, J.; Frosch, P.J.; Happle, R.; Effendy, I. Evaluation of skin susceptibility to irritancy by routine patch testing with sodium lauryl sulfate. Eur J Dermatol 2001, 11, 416-419.
- Makino, E.T.; Mehta, R.C.; Banga, A.; Jain, P.; Sigler, M.L.; Sonti, S. Evaluation of a hydroquinone-free skin brightening product using in vitro inhibition of melanogenesis and clinical reduction of ultraviolet-induced hyperpigmentation. J Drugs Dermatol 2013, 12, s16-20.
- Watanabe, F.; Hashizume, E.; Chan, G.P.; Kamimura, A. Skin-whitening and skin-condition-improving effects of topical oxidized glutathione: a double-blind and placebo-controlled clinical trial in healthy women. Clin Cosmet Investig Dermatol 2014, 7, 267-274.
- Wilkes, M.; Wright, C.Y.; du Plessis, J.L.; Reeder, A. Fitzpatrick Skin Type, Individual Typology Angle, and Melanin Index in an African Population: Steps Toward Universally Applicable Skin Photosensitivity Assessments. JAMA Dermatol 2015, 151, 902-903.




