Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mirza Hasanuzzaman | + 8212 word(s) | 8212 | 2021-07-28 09:01:25 | | | |
| 2 | Vivi Li | Meta information modification | 8212 | 2021-07-29 04:48:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hasanuzzaman, M.; Bhuyan, M.B. Ascorbate-Glutathione Pathway. Encyclopedia. Available online: https://encyclopedia.pub/entry/12549 (accessed on 08 February 2026).
Hasanuzzaman M, Bhuyan MB. Ascorbate-Glutathione Pathway. Encyclopedia. Available at: https://encyclopedia.pub/entry/12549. Accessed February 08, 2026.
Hasanuzzaman, Mirza, Mhm Borhannuddin Bhuyan. "Ascorbate-Glutathione Pathway" Encyclopedia, https://encyclopedia.pub/entry/12549 (accessed February 08, 2026).
Hasanuzzaman, M., & Bhuyan, M.B. (2021, July 28). Ascorbate-Glutathione Pathway. In Encyclopedia. https://encyclopedia.pub/entry/12549
Hasanuzzaman, Mirza and Mhm Borhannuddin Bhuyan. "Ascorbate-Glutathione Pathway." Encyclopedia. Web. 28 July, 2021.
Copy Citation
The Ascorbate-Glutathione (AsA-GSH) pathway, also known as Asada–Halliwell pathway comprises of AsA, GSH, and four enzymes viz. ascorbate peroxidase, monodehydroascorbate reductase, dehydroascorbate reductase, and glutathione reductase, play a vital role in detoxifying ROS. Apart from ROS detoxification, they also interact with other defense systems in plants and protect the plants from various abiotic stress-induced damages. Several plant studies revealed that the upregulation or overexpression of AsA-GSH pathway enzymes and the enhancement of the AsA and GSH levels conferred plants better tolerance to abiotic stresses by reducing the ROS.
antioxidant defense
free radicals
glyoxalase system
hydrogen peroxide
plant abiotic stress
reactive oxygen species
redox biology
stress signaling
1. Introduction
Plants have an antioxidant defense system having non-enzymatic and enzymatic antioxidants in cellular organelles, which scavenges different ROS up to a certain level. If the ROS generation is higher than the scavenging ability of the antioxidant system, then oxidative damage occurs. The antioxidant defense system comprises ascorbate (AsA), glutathione (GSH), carotenoids, tocopherols, flavonoids, etc., which are some commonly known non-enzymatic antioxidants [1]. Ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione reductase (GR), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione S-transferase (GST), and peroxiredoxin (PRX) are well known enzymatic antioxidant components [2][3]. Among all of these, AsA, GSH, APX, MDHAR, DHAR, and GR comprise the AsA-GSH cycle.
Ascorbate is one of the most powerful substrates for scavenging H2O2. Ascorbate maintains the reduced state of α-tocopherol. Ascorbate is supposed to be concerned in zeaxanthin biosynthesis dissipating excess light energy in the thylakoid membranes of chloroplast and prevents oxidative stress. Ascorbate sustains reduce the state of prosthetic metal ions and maintain the activity of antioxidant enzymes [4]. Glutathione regulates various metabolic functions; it acts as an antioxidant. Glutathione peroxidase and GST utilize GSH as substrate; GPX is responsible for ROS detoxification, whereas GST is liable for xenobiotic detoxification [5]. The glyoxalase system consisting of glyoxalase I (Gly I) and glyoxalase II (Gly II) enzymes detoxifies cytotoxic and oxidative stress creator methylglyoxal (MG), where Gly I uses GSH and after finishing MG detoxification, GSH is recycled [6]. The positive role of AsA-GSH cycle components has been documented in many plants that are affected by abiotic stresses [5][6]. Participation of the GSH/glutathione disulfide (GSSG, the oxidized form of GSH) redox in maintaining a favorable cellular environment and in stress signal and adaptation were discussed in some previous findings. Glutathione participates in signal transduction, the proper pathway, of which remains unrevealed. The presence of AsA and GSH has been reported to improve osmoregulation, plant water status and nutrient status, water use efficiency, photosynthetic performance, and the overall productivity of plants. Exogenous AsA and GSH applications have been reported to enhance the antioxidant defense as well as the overall tolerance of plants against abiotic stresses. Accordingly, the enzymatic antioxidants of AsA-GSH cycle participate in scavenging ROS, whereas AsA and GSH not only directly scavenge a range of ROS but also perform many other functions to maintain a favorable state in cytosol and other cellular organelles to enhance antioxidant capacity and to reduce oxidative stress, which is induced by different abiotic stresses; AsA and GSH also improve the physiological performance of plants. Since the discovery of the AsA-GSH cycle, its most discussed topics are related to antioxidative protection.
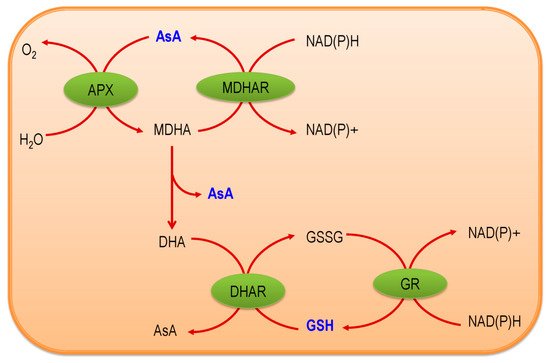
2. Ascorbate-Glutathione Pathway—An Overview
Ascorbate-Glutathione pathway (also called as Asada–Halliwell pathway) is the major pathway of antioxidant defense, which mainly detoxify the H2O2 in a plant cell. Apart from AsA and GSH, its enzymes—APX, MDHAR, DHAR, and GR [4]—have significant roles. Both AsA and GSH are found in the cytosol, nucleus, chloroplast, mitochondria, and peroxisome, where they operate the functions assisted by four enzymes and, therefore, each enzyme has several isoforms that are based on the cellular localization [7]. Both AsA and GSH are present in cellular organelles in a millimolar range, for instance, in Arabidopsis thaliana, AsA concentration is the highest (22.8 mM) in the peroxisome, where GSH is highest (14.9 mM) in mitochondria [8][9]. AsA and GSH both have high redox potentials and, therefore, interact with many components and pathways towards the maintenance of a generally reduced state. There are few steps, by which AsA and GSH work coordinately to detoxify H2O2, and at the same time, both AsA and GSH are regenerated. First, the enzyme APX converts H2O2 into water with the help of AsA as an electron donor, which is also converted into monodehydroascorbate (MDHA). This MDHA again regenerates AsA by the activity of MDHAR and a part of this is spontaneously converted into dehydroascorbate (DHA). Later, DHA is reduced to AsA again by using GSH, which results in its oxidation to produce GSSG. Finally, this GSSG regenerates GSH by the activity of GR using NADPH as the electron donor (Figure 1). Both AsA and GSH are strong antioxidants, but the maintenance of their redox state is important in conferring stress tolerance in plants, which largely depends on the activities of the four enzymes that are associated with the AsA-GSH cycle [4][10]. In the next sections, we have described all of the components of the AsA-GSH pathway.

Figure 1. Ascorbate-Glutathione (AsA-GSH) (Ascorbate-Glutathione) pathway [ascorbate, AsA; ascorbate peroxidase, APX; monodehydroascorbate, MDHA; monodehydroascorbate reductase, MDHAR; dehydroascorbate, DHA; dehydroascorbate reductase, DHAR; glutathione, GSH; oxidized glutathione, GSSG; glutathione reductase, GR; Nicotinamide adenine dinucleotide phosphate (reduced form), NAD(P)H; Nicotinamide adenine dinucleotide phosphate (oxidized form), NAD(P)+].
3. Role of AsA-GSH in Regulating Oxidative Stress under Abiotic Stresses
Abiotic stress-induced excess ROS causes oxidative stress in plants followed by cellular damage, even death. Hence, the plant itself defends against this higher ROS accumulation by their defense mechanism. Plant significantly activates the AsA-GSH pathway for ROS detoxification. In this section, we will discuss the involvement of AsA-GSH cycle for alleviating oxidative stress upon various abiotic stresses reviewing recently published articles (Table 1, Table 2, and Table 3).
Table 1. Role of AsA-GSH in regulating oxidative stress under salinity and drought.
| Plant Species | Stress Levels | Status of AsA-GSH Component(s) | ROS Regulation | References |
|---|---|---|---|---|
| Triticum aestivum L. | 100 mM NaCl | GSH content increased by 15%; Stimulated APX and GR activities by 78% and 56%, respectively | Increased H2O2 content about 79% | [11] |
| T. aestivum L. cv. BARI Gom-21 | 12% PEG for 48 and 72 h | Decreased AsA content at 48 h, but after 72 h, AsA content again enhanced; Increased GSH and GSSG content where GSH/GSSG ratio decreased time-dependently; Enhanced the activities of APX, MDHAR, and GR | Enhanced the H2O2 content by 62% and increased O2− accumulation | [12] |
| T. aestivum L. | 10% PEG | Reduced AsA/DHA and GSH/GSSG redox; Increased enzymatic antioxidants actions of AsA-GSH cycle | Increased H2O2 production | [13] |
| T. aestivum L. | 35–40% field capacity (FC) water | Increased GSH/GSSG by 64% while decreased AsA/DHA by 52% respective with a duration of stress; Enhanced APX, MDHAR, DHAR and GR activities | Increased H2O2 along with stress duration | [14] |
| T. aestivum cv. Pradip | 150 and 300 mM NaCl | Reduced AsA content upto 52%; Increased reduced and oxidized GSH accumulation by 55% and 18%, respectively with 32% higher GSH/GSSG ratio; Increased APX activity with 29% reduction of GR activity; Slightly increased MDHAR and DHAR activity | Enhanced H2O2 generation by 60% | [15] |
| Oryza sativa L. cv. BRRI dhan47 | 150 mM NaCl | Increased GSH accumulation while reduced AsA content by 49% Increased GSH content and lowered the redox status of both AsA/DHA and GSH/GSSG; Upregulated the activity of APX, MDHAR, DHAR, and GR |
Increased the production of O2− with 82% higher H2O2 accumulation | [16] |
| O. sativa L. cv. BRRI dhan49 | 300 mM NaCl | Reduced AsA and GSH accumulation by 51% and 57%, respectively; Decrease GSH/GSSG redox by 87%; Showed lowered APX (27%), MDHAR (24%), DHAR and GR (25%) activities | Increased H2O2 content upto 69% | [17] |
| O. sativa L. cv. BRRI dhan54 | 300 mM NaCl | Improved AsA content by 51% with higher GSH content; Decreased GSH/GSSG ratio by 53%; Showed higher APX (27%) and DHAR activities while decreased both GR (23%) and MDHAR activities | Accumulated 63% higher H2O2 content | [17] |
| Brassica napus L. cv. BinaSharisha-3 | 100 and mM NaCl | Reduced the AsA content by 22%; Increased GSH content by 72% and GSSG content by 88%; Unaltered the GSH/GSSG ratio; Amplified APX activity by 32%, decreased DHAR activity by 17%; Slightly increased GR activity | Accumulated higher H2O2 content by 76% | [18] |
| B. napus L. cv. Binasharisha-3 | 200 mM NaCl | Reduced the AsA content (40%) along with increased GSH (43%) and GSSG (136%) contents; Decreased the GSH/GSSG ratio (40%); Amplified the APX activity (39%) and reduced the MDHAR (29%) and DHAR (35%) activities; Improved GR activity (18%) | Showed 90% more H2O2 content | [18] |
| B. napus L. | 15% PEG | The AsA accumulation remained unaltered and reduced the AsA/DHA ratio; Enhanced GSH content by 19% and GSSG by 67% and decreased GSH/GSSG ratio; Increased APX, MDHAR, DHAR and GR activities | Higher accumulation of H2O2 by 55% | [19] |
| B. campestris L. | 15% PEG | Decreased AsA content by 27% with a decrease of AsA/DHA ratio; Increased GSH content by 33% with higher GSSG content by 79% and lowered GSH/GSSG ratio; Decreased DHAR activity | Higher accumulation of H2O2 about 109% | [19] |
| B. juncea L. | 15% PEG | Increased the AsA content and did not affect the AsA/DHA ratio; Increased GSH content by 48% and GSSG by 83% and decreased GSH/GSSG ratio; Increased APX, MDHAR, DHAR and GR activities | Accumulation of 37% higher H2O2 | [19] |
| B. juncea L. cv. BARI Sharisha-11 | 10% PEG | Reduced AsA content (14%) while increased both GSH (32%) and GSSG (48%) contents; Enhanced APX activity (24%); Decreased MDHAR and DHAR (33%) activities along with 31% increased GR activity | Acute generation of H2O2 (41%) | [20] |
| B. juncea L. cv. BARI Sharisha-11 | 20% PEG | Decreased AsA content by 34% while increased the content of GSH by 25% and GSSG by 101%; Up-regulated APX activity by 33%; Decreased activity of MDHAR and DHAR (30%) | Extreme generation of H2O2 by 95% | [20] |
| B. napus L. cv. BinaSarisha-3 | 10% PEG | Increased AsA (21%), GSH (55%) and GSSG contents while decreased GSH/GSSG ratio Unaltered the activities of APX, and increased the activity of MDHAR, DHAR, and GR (26%) | Elevated the H2O2 production | [8] |
| B. napus L. cv. BinaSarisha-3 | 20% PEG | Unaltered AsA content along with higher content of GSH (46%) and GSSG and reduced GSH/GSSG ratio; Reduced the APX and MDHAR activities along with the higher activity of DHAR and GR (23%) | Showed higher H2O2 production | [8] |
| B. napus L. cv. BinaSharisha-3 | 10% PEG | Increased AsA, GSH (31%) and GSSG (83%) accumulation with lowered GSH/GSSG ratio; Increased APX activity while reduced MDHAR and DHAR activities, but GR activity remained unaltered | Increased H2O2 content by 53% | [21] |
| B. napus L. cv. BinaSharisha- 3 | 20% PEG | Slightly increased AsA content with 26% and 225% increase of GSH and GSSG content, respectively; Reduced GSH/GSSG ratio; Increased APX activity while decreased the activity of MDHAR, DHAR, and GR (30%) | Increased about 93% H2O2 content | [21] |
| B. rapa L. cv. BARI Sharisha-15 | 20% PEG | Slightly increased AsA content with 72% and 178% increase of GSH and GSSG content, respectively; Reduced GSH/GSSG ratio by 38%; Increased APX, MDHAR, DHAR, and GR activity | Increased about 131% H2O2 content | [22] |
| Cucumis melo L. cv. Yipintianxia No. 208 | 50 mM of NaCl:Na2SO4:NaHCO3:Na2CO3 (1:9:9:1 M) | Improved AsA, GSSG and DHA contents; Lowered GSH content; Reduced the ratio of AsA/DHA and GSH/GSSG; Stimulated the activity of APX by 96% and DHAR by 38% while reducing the activity of MDHAR and GR by 48% and 34%, respectively | Increased H2O2 accumulation | [23] |
| Solanum lycopersicum L., var. Lakshmi | 0.3 and 0.5 g NaCl kg−1 soil | Reduced AsA and AsA/DHA ratio; Lowered GSH and GSSG accumulation with decreased GSH/GSSG redox; Increased APX activity by 28%, DHAR activity by 28% and GR activity by 14% | Enhanced H2O2 and O2− accumulation | [24] |
| S. lycopersicum L.cv. Boludo | 60 mM NaCl, 30 days | Reduced the activities of APX, DHAR, and GR; Increased MDHAR activity | Higher H2O2 generation | [25] |
| S. lycopersicum L. var. Pusa Ruby | 150 mM NaCl | Decreased AsA and GSH content with a higher content of DHA and GSSG; Increased APX, MDHAR, DHAr and GR activities | Higher generation of H2O2 and O2− | [26] |
| S. lycopersicum L. var. Pusa Rohini | 150 mM NaCl | Reduced AsA content by 42%; Increased both GSH and GSSG accumulation; Enhanced the activity of APX and GR by 86% and 29%, respectively with reduction of the activity of MDHAR and DHAR by 38% and 32%, respectively | Accumulated about 3 fold higher H2O2 content | [27] |
| S. lycopersicon L. cv.K-21 | 150 mM NaCl | Reduced AsA content by 40% with 50% higher GSH content; Lowered GSSG content by 23% while increased GSH/GSSG ratio by 112%; Increased APX (86%) and GR (92%) activity along with the lowered activity of MDHAR (32%) and DHAR (30%) | Elevated H2O2 content about 175% | [28] |
| Nitraria Tangutorum Bobr. | 100,200, 300 and 400 mM NaCl | Increased AsA, DHA, GSH and GSSG accumulation decreased their redox status; Enhanced the activity of APX and GR; Unvaried the activity of DHAR and MDHAR but increased DHAR activity only at 300 mM NaCl | Increased O2−and H2O2 content by 38–98 and 49–102% respectively | [29] |
| Camellia sinensis (L.) O.Kuntze | 300 mM NaCl | Enhanced the AsA and GSH content; Increased APX activity | Elevated H2O2 and O2− content | [30] |
| Phaseolus vulgaris L. cv. Nebraska | 2.5 and 5.0 dS m–1 prepared from a mixture of NaCl, CaCl2, and MgSO4 | Increased AsA, GSH, DHA and GSSG accumulations; Enhanced AsA/DHA and GSH/GSSG status; Stimulated the enzymatic activity of APX, MDHAR, DHAR and GR activities | Accumulated higher H2O2 content | [31] |
| Vigna radiate L. cv. Binamoog-1 | 25% PEG | Reduced AsA content along with higher GSH content of 92%; Increased GSSG content by 236% and reduced GSH/GSSG ratio; Amplified the activity of APX (21%) and GR while reduced MDHAR and DHAR activities | Elevated H2O2 content by 114% with higher O2− generation | [32] |
| V. radiata L. | 200 mM NaCl | Reduced AsA content; Increased GSSG and GSH accumulation and lowered GSH/GSSG ratio; Amplified the activity of APX, MDHAR, DHAR, and GR | Increased H2O2 content by 80% and O2− generation by 86% | [33] |
| V. radiata L. cv. BARI Mung-2 | 5% PEG | Reduced AsA content where decreased AsA/DHA ratio by 54%; Increased GSSG content; Upregulated the activity of APX and GR (42%) while downregulated the MDHAR (26%) and DHAR activities | Elevated H2O2 and O2− accumulation | [34] |
| Lens culinaris Medik cv. BARI Lentil-7 | 20% PEG | Lowered AsA content with higher total GSH content; Unaltered the APX and GR activities while the increased activity of MDHAR and DHAR (64%) | Accumulated higher H2O2 content | [35] |
| L. culinaris Medik cv. BARI Lentil-7 | 100 mM NaCl | Reduced AsA content by 87% while increased total GSH content by 260%; Improved the activity of APX, MDHAR, DHAR (286%) and GR (162%) | Increased H2O2 content by 15% | [35] |
| Anacardium occidentale L. | 21-day water withdrawal | Enhanced total AsA and GSH content; Increased APX activity | Reduced H2O2 generation | [36] |
| Arabidopsis | 12-day water withhold | Showed higher GSH and GSSG accumulation; Reduced GSH/GSSG ratio; Increased GR activity | Increased H2O2 accumulation rate | [37] |
| Cajanus cajan L. | Complete water withholding for 3, 6 and 9 days | Decreased GSH/GSSG ratio; Increased the activity of APX, DHAR, and GR | Higher H2O2 content | [38] |
| Amaranthus tricolor L.cv. VA13 | 30% FC | Increased AsA and GSH contents by 286% and 98%, respectively; Improved APX, MDHAR, DHAR, and GR activity by 371%, 379%, 375%, and 375%, respectively | No increment of H2O2 content | [39] |
| A. tricolor L.cv. VA15 | 30% FC | Increased AsA and GSH contents along with higher redox status of AsA/total AsA and GSH/total GSH; Enhanced the activity of APX, MDHAR, DHAR, and GR by 37%, 45%, 40%, and 2%, respectively | Accumulated higher H2O2 content by 137% | [39] |
| C. sinensis (L.) O. Kuntze | 20% PEG | Higher contents of both AsA and GSH; Enhanced the APX activity | Higher accumulation of H2O2 and O2− | [30] |
Table 2. Status of AsA-GSH in regulating oxidative stress under metal/metalloid stress.
| Plant Species | Stress Levels | Status of AsA-GSH Component(s) | ROS Regulation | References |
|---|---|---|---|---|
| Brassica napus L. cv. BinaSharisha-3 | Cd (0.5 mM and 1.0 mM CdCl2), 48 h | Reduced AsA content by 20% under 0.5 mM and 32% under 1.0 mM CdCl2 treatment; Increased GSH content only under 0.5 mM CdCl2 stress but enhanced level of GSSG by 34% under 0.5 mM and 65% under 1.0 mM CdCl2 treatment; Increased function of APX by 39% and 43% under 0.5 mM and 1.0 mM CdCl2 treatment but MDHAR and DHAR activity were diminished in dose dependant fashion; GR activity increased by 66% due to 0.5 mM CdCl2 treatment but reduced by 24% due to 1.0 mM CdCl2 treatment | Enhanced H2O2 content by 37% under 0.5 mM and 60% under 1.0 mM CdCl2 treatment | [40] |
| Gossypium spp. (genotype MNH 886) | Pb [50 and 100 μM Pb(NO3)2], 6 weeks | Increased APX activity | Increased H2O2 content | [41] |
| T. aestivum L. cv. Pradip | As (0.25 and 0.5 mM Na2HAsO47H2O), 72 h | Reduced AsA content by 14% under 0.25 and 34% underd 0.5 mM Na2HAsO4·7H2O treatment; Increased GSH content by 46% and 34%, GSSG content by 50 and 101% under 0.25 and 0.5 mM Na2HAsO4·7H2O stress; Enhanced APX function by 39% and 43% but decreased DHAR function by 33% and 30% under 0.25 and 0.5 mM Na2HAsO4·7H2O treatment; Increased GR function by 31% under 0.25 mM | Increased H2O2 content by 41% under 0.25 and 95% under 0.5 mM Na2HAsO4·7H2O treatment | [42] |
| B. napus L. viz. ZS 758, Zheda619, ZY 50 and Zheda 622 | Cr (400 µM), 15 days | Increased GSH and GSSG content; Increased APX activity | Increased H2O2 content | [43] |
| Oryza sativa L. cv. BRRI dhan29 | As (0.5 mM and 1 mM Na2HAsO4), 5 days | Decreased AsA content by 33 and 51% and increased DHA content by 27% and 40% under 0.5mM and 1mM Na2HAsO4 treatment, respectively; Decreased ratio of AsA/DHA; Enhanced GSH content by 48 and 82% under 0.5mM and 1mM Na2HAsO4 treatment, respectively; Enhanced GSSG content whereas lessened GSH/GSSG ratio by 25% under 0.5mM and 41% under 1mM Na2HAsO4 treatment; Augmented the function of APX, MDHAR, and GR, however, reduced the activity of DHAR | Increased H2O2 content by 65% and 89% under 0.5mM and 1mM Na2HAsO4 treatment, respectively | [44] |
| O. sativa L. cv. Disang (tolerant) | 100 µM AlCl3, 48 h | Increased AsA content in both roots and shoots; Enhanced the GSH content in shoots; Higher activities of APX, MDHAR, DHAR, and GR, | Elevated the generation of H2O2 and O2− | [45] |
| O. sativa L. cv. Joymati (sensitive) | 100 µM AlCl3, 48 h | Higher accumulation of AsA in both roots and shoots; Reduced the GSH content in roots while shoots content was unaltered; Increased APX, MDHAR, DHAR activities; Slightly increased GR activities | Higher accumulation of H2O2 and O2− | [45] |
| V. radiata L. cv. BARI Mung-2 | Cd (mild: 1.0 mM CdCl2, severer: 1.5 mM CdCl2), 48 h | Declined AsA content by 31% due to mild and 41% due to severe stress; Enhanced DHA level and reduced AsA/DHA ratio; GSH content did not change due to mild stress but enhanced owing to stress severity; GSSG level enhanced, and GSH/GSSG ratio decreased in a dose-dependent manner; Increased function of APX but lessened MDHAR and DHAR function due to both level of stress; GR activity increased only due to severe stress | H2O2 level and O2− generation rate was augmented by 73% and 127% due to mild and 69% and 120% due to severe Cd stresses | [46] |
| V. radiata L. cv. BARI Mung-2 | Cd (1.5 mM CdCl2), 48 h | AsA content decreased by 27%, and the ratio of AsA/DHA reduced by 80% whereas DHA content increased considerably; Augmented the function of APX and GR however lessened function of MDHAR and DHAR | Increased H2O2 level and O2− generation rate | [47] |
| O. sativa L. cv. BRRI dhan29 | Cd (0.25 mM and 0.5 mM CdCl2), 3 days | AsA content and AsA/DHA ratio reduced by 37% and 57% due to 0.25 mM CdCl2 and reduced by 51% and 68% due to 0.5 mM CdCl2, respectively; DHA content increased significantly; GSH content enhanced due to 0.25 mM CdCl2 stress, but reduced due to 0.5 mM CdCl2 stress; GSSG content enhanced by 76% under 0.25 mM and 108% under 0.5 mM CdCl2 stress; Reduced ratio of GSH/GSSG in dose dependant manner; Enhanced APX, MDHAR and GR activity | Enhenced H2O2 by 46% under 0.25 mM CdCl2 and 84% under 0.5 mM CdCl2 treatmen whereas O2− generation rate increased in dose dependant manner | [48] |
| O. sativa L. cv. BRRI dhan29 | Cd (0.3 mM CdCl2), 3 days | Lessened level of AsA and AsA/DHA ratio but enhanced DHA level; Enhanced the level of GSH and GSSG however lessened GSH/GSSG ratio; Enhanced the action of APX, MDHAR, and GR whereas declined DHAR function | Overproduced ROS (H2O2 and O2−) | [49] |
| O. sativa L. Zhunliangyou 608 | Cd (5 μM Cd(NO3)2·4H2O), 6 days | Reduced AsA content; Increased GSH content; Slightly reduced the APX activity | H2O2 content increased by 22.73% | [50] |
| Abelmoschus esculentus L. Moench | Pb (100 mg L−1), 21 days | Increased AsA content | Enhanced H2O2 content | [51] |
| B. juncea L. cv. BARI Sharisha-11 | Cr (mild: 0.15 mM K2CrO4, severe: 0.3 mM K2CrO4), 5 days | AsA content lessened by 19% due to mild and 32% due to severe stress whereas DHA level enhanced by 83% due to mild and 133% due to severe stress as well as AsA/DHA ratio lessened by 47% due to mild and 82% due to severe stress; GSH content did not change considerably but GSSG content enhanced by 42% due to mild and 67% due to severe stress as well as GSH/GSSG ratio lessened by 26% due to mild and 41% due to severe stress; The function of APX enhanced by 21% due to mild and 28% due to severe stress; The activity of MDHAR and DHAR reduced by 25 and 32% under mild and 31 and 50%, under severe stress, respectively; Mild stress increased the activity of GR by 19% while severe stress increased by 16% | H2O2 level enhanced by 24% and 46% due to mild and severe stress. Similarly, O2− generation rate also raised in a dose-dependent manner | [52] |
| B. campestris L. cv. BARISharisha 9, B. napus L. cv. BARI Sharisha-13 and B. juncea L. cv. BARI Sharisha-16 | Cd (mild: 0.25 mM CdCl2, severer: 0.5 mM CdCl2), 3 days | Decreased level ofAsA, augmented level of DHA as well as decreased AsA/DHA ratio in all studied cultivars; GSH and GSSG level enhanced, but GSH/GSSG ratio lessened in all studied cultivars; APX and GR activities of all species increased significantly under both levels of Cd toxicity | Enhanced H2O2 level and O2− production rate in all tested cultivars in a concentration-dependent fashion | [53] |
| B. juncea L. BARI Sharisha-11 | Cd (mild: 0.5 mM CdCl2, severer: 1.0 mM CdCl2), 3 days | Reduced AsA content with higher DHA content and thus decreased AsA/DHA ratio; Increased GSH and GSSG levels as well as declined GSH/GSSG ratio; APX activity increased where GR increased at mild stress but remained unaltered at severe stress; Decreased MDHAR and DHAR activities | Enhanced the H2O2 and O2−level | [54] |
| V. radiata L. cv. BARI Mung-2 | Al (AlCl3, 0.5 mM), 48 and 72 h | Enhanced DHA content but reduced AsA level and AsA/DHA ratio; Increased level of GSH and GSSG but the diminished ratio of GSH/GSSG; Augmented APX activity but decreased MDHAR and DHAR activity | Enhanced H2O2 level by 83% and O2− generation rate by 110% | [34] |
| T. aestivum L. cv. Pradip | Pb [mild: 0.5 mM Pb(NO3)2, severer: 1.0 mM Pb(NO3)2], 2 days | AsA decreased in a dose-dependent manner; Mild stress improved the GSH level, but severe stress reduced it; Increased GSSG content; Increased APX activity; Diminished activity of MDHAR and DHAR in a concentration-dependent fashion; Mild stress improved GR activity but severe stress reduced it | Mild stress increased H2O2 levels by 41%, but severe stress enhanced it by 95% while O2− generation rate also increased in a dose-dependent manner | [55] |
| B. juncea L. cv. BARI Sharisha-11 | Cd (mild: 0.5 mM CdCl2, severer: 1.0 mM CdCl2), 3 days | AsA content decreased by 24% due to mild and 42% due to severe stress whereas DHA level enhanced by 79% due mild and 200% due to severe stress; Decreased AsA/DHA ratio in dose-dependent manner; GSH and GSSG content enhanced by 19% and 44%, respectively, due to mild stress, while only GSSG content enhanced due to severe stress by 72%; The ratio of GSH/GSSG declined by 17% due to mild and 43% due to severe stress; Enhanced APX by 15% due to mild and 24% due to severe stress; The activity of MDHAR and DHAR reduced by 12% and 14% due to mild stress whereas 17% and 24%, due to severe stress, respectively; The activity of GR enhanced under mild stress by 16% and lessened under severe stress by 9% | Level of H2O2 enhanced by 43% due to mild and 54% due to severe stress. Augmented O2− generation rate in a dose-dependent manner | [56] |
| B. juncea L. cv. varuna | Ni, (150 μM NiCl2.6H2O), 1 week | AsA content decreased by 61% whereas GSH and GSSG content increased by 75% and 151%, respectively; Enhanced function of APX by 60% and GR by 72%; DHAR and MDHAR activities were decreased by 62% and 65%, respectively | Increased H2O2 by 3.23-fold | [57] |
| Pisum sativum L. cv. Corne de Bélier | Pb (500 mg PbCl2 kg−1), 28 days | Increased APX and GR activity | Increased H2O2 content | [58] |
| O. sativa L. cv. BRRI dhan54 | Ni (0.25 mMand 0.5 mM NiSO4·7H2O) | Diminished content of AsA and enhanced content of DHA as well as the lessened ratio of AsA/DHA by 73% and 92% under 0.25 mM and 0.5 mM NiSO4·7H2O stress; GSH and GSSG level enhanced in a dose-dependent manner. However, the GSH/GSSG ratio reduced only under 0.5 mM NiSO4·7H2O treatment; Increased APX, MDHAR, DHAR and GR activity by 70%, 61%, 19% and 37% under 0.25 mM NiSO4·7H2O and 114%, 115%, 31% and 104% under 0.5 mM NiSO4·7H2O treatment, respectively | Increased H2O2 content by 28% and 35% due to 0.25 mM and 0.5 mM NiSO4·7H2O treatment | [59] |
| Capsicum annuum L.cv. Semerkand | Cd (0.1 mM CdCl2), 3 weeks | Enhanced AsA and GSH content | Increased H2O2 content | [60] |
| C. annuum L. cv. Semerkand | Pb (0.1 mM PbCl2), 3 weeks | Enhanced AsA and GSH content | Increased H2O2 content | [60] |
| Zea mays L. cv. Run Nong 35 and Wan Dan 13 | Cd (50 mg 3CdSO4·8H2O kg−1 soil), 6 months | Decreased GSH content | Increased accumulation of H2O2 | [61] |
Table 3. Role of AsA-GSH in regulating oxidative stress under extreme temperature, flooding, and atmospheric pollutant.
| Plant Species | Stress Levels | Status of AsA-GSH Component(s) | ROS Mitigation | References |
|---|---|---|---|---|
| Actinidia deliciosa | 45 °C, 8 h | Increased content of AsA; Higher activity of APX, MDHAR, DHAR, and GR | Increased H2O2 content | [62] |
| Zea mays L. cv. Ludan No. 8 | 46 °C, 16 h | Decreased GSH, and GSSG content, but interestingly GSH/(GSH + GSSG) ratio increased; Reduced GR activity | - | [63] |
| Cinnamonum camphora | 40 °C, 2 days | Reduced AsA content with higher DHA content; Increased GSH and GSSG content; Enhanced the activities of APX, MDHAr, DHAR, and GR | Higher content of H2O2 and O2− | [64] |
| S. lycopersicum L. cv. Ailsa Craig | 40 °C, 9 h | Higher APX and GR activities by 74% and 45%, respectively | H2O2 content increased by 49% | [65] |
| S. lycopersicum L.cv. Boludo | 35 °C, 30 days | Increased the APX, DHAR and GR activities; Reduced the MDHAR activity | Increased H2O2 content | [25] |
| Vicia faba L. cv. C5 | 42 °C, 48 h | Enhanced the AsA, GSH ans GSSG content significantly; The enzymatic activity of APX and GR also enhanced | Extreme accumulation of O2− and H2O2 | [66] |
| V.radiata L. cv. BARI Mung-2 | 40 °C, 48 h | Decreased 64% in AsA/DHA ratio; GSSG pool increased; Higher APX (42%) and GR (50%) activities but declined activities of MDHAR (17%) and DHAR | Higher H2O2 content and O2−production rate | [34] |
| Z. mays cv. CML-32 and LM-11 | 40 °C, 72 h | Increased AsA content in both shoot and root of tolerant (CML-32) one, but unaffected in the susceptible (LM-11) one; Both APX and GR activity increased in roots of CML-32 but reduced in the shoot | Higher H2O2 accumulation, especially in shoots | [67] |
| L. esculentum Mill. cv. Puhong 968 | 38/28 °C day/night, 7 days | AsA+DHA and DHA increased by 220% and 99% respectively; AsA/DHA ratio decreased by 33%.; Higher GSSG (25%), but reduced GSH content (23.4%) and GSH/GSSG ratio (39%); APX, MDHAR, DHAR and GR activities declined | Enhanced O2− generation rate and H2O2 content by 129% and 33% respectively | [68] |
| Nicotiana tabacum cv. BY-2 | 35 °C, 7 days | Total GSH and AsA contents rose after 7 days heat stress; Increased MDHAR. DHAR and GR activities up to 72 h | The increasing trend of H2O2 generation was observed up to 72 h, and then a sharp decline occurred | [69] |
| Ficus concinna var. subsessilis | 35 °C and 40 °C, 48 h | AsA content reduced at 40 °C but GSH content similar to control at both 35 and 40 °C; DHA content enhanced by 49% at 35 °C and by 70% at 40 °C; APX activity increased by 51% and 30% at 35 °C and 40 °C; Activities of MDHAR, DHAR, and GR increased at 35 °C, but GR activity decreased by 34% at 40 °C | At 35 °C, 103% higher H2O2 content and 58% higher O2−production rate and at 40 °C those were 3.3- and 2.2-fold respectively | [70] |
| T. aestivum cv. Hindi62 and PBW343 | Heat stress environment, Late sown (Mid-January) | Higher activities of MDHAR and DHAR was observed in heat-tolerant (Hindi62) one whereas other enzyme activities seemed mostly to decline with time | The content of H2O2 was higher up to 14 DAA compared to non-stressed seedlings | [67] |
| G. hirsutum cv. Siza | Waterlogged pot for 3 days and 6 days | Increased content of AsA by 20% at 3 days and 30% at 6 days of waterlogging; Lower APX, MDHAR and GR activities | Enhanced O2− generation rate by 22 and 53% and H2O2 content by 10 and 39% at 3 and 6 days of waterlogging, respectively | [63] |
| Sesamum indicum L. cv. BARI Til-4 | Waterlogged pot by 2 cm standing water on the soil surface for 2, 4, 6 and 8 days | Reduced AsA content upto 38%; Enhanced GSH and GSSG content significantly; Increased APX and MDHAR activities; Reduced DHAR activity upto 59%; GR activity decreased upto 23% | Increased H2O2content sharply | [71] |
| Z. mays cv. Huzum-265 and Huzum-55 | Root portions waterlogged for 21 h | Reduced AsA content in both cultivars; Increased APX activity in both cultivars | - | [72] |
| Glycine max L. | Waterlogged pot for 14 days | GSH activity declined sharply in roots but shoots unaffected; Reduced GR activity in shoots but roots unaffected | - | [73] |
| Trifolium repens L. cv. Rivendel and T. pratense L. cv. Raya | 2 cm standing water on the soil surface for 14 days and 21 days | Increased contents of both oxidized and reduced AsA observed in both genotypes | Higher H2O2 generation in both genotypes | [74] |
| V. radiata L. cvs. T-44 and Pusa Baisakhi; and V. luteola | Pot filled with water to 1–2 cm height below the soil level, 8 days | Increased activities of both APX and GR in tolerant genotypes but in susceptible one, activities reduced | Reduced contents of O2−and H2O2 in susceptible (Pusa Baisakhi) cultivar | [75] |
| O. sativa L. MR219-4, MR219-9 and FR13A | Complete submergence for 4, 8 and 12 days | APX activity declined by 88% in FR13A under 4 days of submergence but decreased about 64 and 83% under 8 and 12 days of submergence; GR activity increased in FR13A and MR219-4 cultivars by 10- and 13-fold respectively after 8 days | - | [76] |
| Allium fistulosum L. cv. Erhan | Waterlogging (5 cm) at substrate surface for 10 days | Lower APX and GR activities | Increased rate of O2− generation by 240.4% and 289.8% higher H2O2 content | [77] |
| C. cajan L. genotypes ICPL 84,023 and ICP 7035 | Soil surface waterlogged (1–2 cm) for 6 days | Reduced APX and GR activities in susceptible genotype, which was higher in tolerant one | Lower accumulation of H2O2 and rate of O2− generation | [78] |
| S. melongena L. cv. EG117 and EG203 | Flooding with a water level of 5 cm, 72 h | Increased AsA content in susceptible EG117 genotype GSH content in both genotypes; Increased APX activity but decreased GR activity | - | [79] |
| S. lycopersicum cv. ASVEG and L4422 | Flooding with a water level of 5 cm, 72 h | Increase in both AsA and GSH contents; Non-significant changes in APX and GR activities | - | [79] |
| Lolium perenne | Grown in an area with high air pollution | APX and DHAR activities decreased while MDHAR and GR activities increased | A higher concentration of H2O2 in pollens | [80] |
| Populus deltoides × Populus nigra cvs. Carpaccio and Robusta | O3 treatment (120 nmol mol−1 for 13 h), 17 days | No impact on AsA and GSH contents; DHAR activity decreased while GR and MDHAR activity increased | - | [81] |
| Fragaria x anansa | High dose of carbon monoxide (CO) nitroxide (NOx) and sulfur dioxide (SO2) | The activity of both APX and GR increased upto medium dose but reduced under high dose | H2O2 content as well as O2− generation rate increased | [82] |
| O. sativa L. cvs. SY63 and WXJ14 | Continuous O3 exposure for up to 79 days | Both AsA and GSH contents are more likely to decrease; APX, MDHAR, DHAR, and GR activity increased up to 70 days of O3 exposure | Both O2− generation rate and H2O2 contents increased | [83] |
| Prosopis juliflora | Grown in the polluted industrial region | The content of AsA and APX activity increased under polluted environment | - | [84] |
| Erythrina orientalis | Grown in a polluted industrial area | Increased activities of both APX and GR enzymes recorded | - | [85] |
| T. aestivum L. cv. BARI Gom-26 | Acidic pH (4.5) of growing media | Increased AsA and GSH content; Improved redox balance of GSH/GSSG; Increased activity of APX, MDHAR, DHR, and GR | H2O2 contents increased by 209% | [86] |
3.1. Salinity
One of the most devastating abiotic stress factors—salinity by which cultivable land is becoming barren thus reduces total crop production day by day. Oxidative stress is the most dangerous event under salt inundation is imposed by salinity-induced ionic and osmotic stress [7]. Hence, these ionic and osmotic stress both disturb the photosystem, and thus cause excess ROS, such as 1O2, O2−, H2O2, and OH. Salinity-persuaded acute ROS accumulations, then bother cellular redox followed by cellular damage counting membrane dysfunction, DNA damage, collapse the enzymatic action, along with distraction of the antioxidant defense system [87][26]. At this point, the plant synthesizes cellular AsA and GSH, which act as non-enzymatic antioxidants by involving their enzymatic components to detoxify ROS up to tolerable levels (Table 1).
However, the enzymes of AsA-GSH pathway showed their differential responses intolerant and sensitive varieties due to saline toxicity. Among salt-tolerant (Pokkali) and sensitive (BRRI dhan29) rice cultivars. Pokkali responded by enhancing the enzymatic activities of the AsA-GSH cycle, where, lowered APX and higher DHAR activity along with unchanged MDHAR and GR activities were found from BRRI dhan29. Rahman et al. [87][16] reported about the well involvement of AsA-GSH cycle in salt-stressed O. sativa where ROS generation was extreme. Here, salt exposed rice enhanced the reduced and oxidized GSH content with a lesser amount of AsA by the higher APX, MDHAR, DHAR, and GR activities against overproduced ROS. Vigna radiata was grown under the saline condition [88] and where salt-induced oxidative stress was marked with extreme O2− and H2O2 overgeneration. Salt-stressed V. radiata augmented GSH and GSSG contents along with lowered AsA, whereas up-regulated the activity of all enzymatic antioxidants of AsA-GSH cycle and thus responded with elevated ROS [33]. Salt exposed Lens culinaris up-stimulated both MDHAR and DHAR activities, which resulted in a lesser amount of AsA and indicated the overproduced H2O2 detoxification [35]. Recently, Singh et al. [24] disclosed the incremental activity of enzymatic antioxidants, including APX, DHAR, and GR, with lower AsA, GSH, and GSSG contents, because of salt-induced higher ROS accumulation in Solanum lycopersicum. Similarly, 150 mM salt-treated S. lycopersicum also decreased AsA content, which might be used in H2O2 detoxification, while better GSH showed its role in lowering H2O2. Ahmad et al. [28] also observed higher APX, and GR activities, while MDHAR and DHAR activities again reduced as well as supported AsA-GSH mediated ROS regulation. Ahanger et al. [27] reported the same response of S. lycopersicum upon saline toxicity. Both activities of APX and GR were enhanced in salt-treated Triticum aestivum besides elevated H2O2 generation and resulted in higher GSH accumulation [11]. The activity of APX, MDHAR, DHAR, and GR enhanced in salt-stressed S. lycopersicum to check the excessive H2O2 generation, which resulted in lowered AsA and GSH contents [26].
The changes in AsA-GSH pathway were investigated in salt-stressed Nitraria tangutorum by applying a varied level of NaCl (100, 200, 300, and 400 mM) [29]. They noticed a gradual enhancement of AsA, DHA, GSH, and GSSG contents by keeping pace with sequential increment of salt-induced H2O2. Here, increased MDHAR and DHAR activities in stressed seedlings also contributed to increasing AsA, and higher DHAR and GR were responsible for better GSH and GSSG contents [26][89]. Talaat et al. corroborated these results with salt-exposed Phaseolus vulgaris [31]. Thus, as a part of plant antioxidant defense under salinity, AsA-GSH pathway is very efficient to regulate extra ROS for being tolerant.
3.2. Drought
Drought is another most important abiotic stress, which generates excess ROS accumulation and thus causes variation in the enzymatic activities of AsA-GSH pathway for ROS detoxification. The enzymatic responses of AsA-GSH pathways varied, depending upon plant species, plant age, drought intensity, and duration [7]. Commonly, drought up-regulated the enzymatic antioxidant activities of AsA-GSH pool [7][22]. Plant tolerance to drought stress is categorized based on stress-induced endogenous antioxidants contents along with enzymatic activities (Table 2). Dendranthema grandiflorum responded differentially according to their tolerant and sensitive varieties, where tolerant one comparatively displayed better enzyme activity of antioxidants than the sensitive ones [90]. Lou et al. [14] demonstrated how T. aestivum responded upon drought exposure. Hence, they noticed that the AsA-GSH cycle responded considerably with excess ROS generation by significant variation of GSH/GSSG and AsA/DHA redox along with the steady increment of H2O2. Their team also observed the enzymatic up-stimulation of AsA-GSH pathway to alleviate stress by scavenging excess ROS in T. aestivum spike. Thus, T. aestivum showed higher participation of AsA with higher APX activity in drought exposure for scavenging extra H2O2, as well as higher enzymatic activity to run the AsA-GSH pathway systematically [13].
Drought-stressed A. thaliana enhanced GSH and GSSG content along with the higher GR activity [37]. Hence, Arabidopsis showed the GSH dependent H2O2 detoxification to attain tolerance. Higher total AsA was accumulated in Cajanus cajan upon complete water restriction conditions for up to nine days to defend against excess H2O2 toxicity [38]. Hence, drought enhanced the enzymatic activity of APX, DHAR, and GR for decreasing GSH/GSSG, as well as controlling ROS levels.
Similarly, the tolerant genotype VA13 of Amaranthus tricolor showed comparatively better tolerance under drought stress than the sensitive one (VA15) by expressing differential responses of the enzymatic and non-enzymatic ROS detoxification pathways [39]. Hence, VA13 expressed a remarkable increment in AsA-GSH redox by accelerating the enzymatic antioxidative actions by which increased non-enzymatic antioxidants (AsA and GSH) accumulation, which are vital for ROS detoxification.
Vigna radiata responded differently regarding different drought intensities [32] to control diverse levels of ROS. Moderate drought imposed by 10% polyethylene glycol (PEG) induced comparatively lowered ROS than severe drought (by 20% PEG). Therefore, severe drought-stressed Brassica showed a larger use of AsA-GSH pathways against higher H2O2 generation than moderate stress. Here, higher stress caused a higher increase of APX activity along with the lowest MDHAR and DHAR activity, while GR activity reduced differently than lower stress exposure to rapeseeds seedlings. Additionally, Hasanuzzaman et al. [21] also observed AsA and GSH both antioxidants contents reduced under severe drought condition, but increased under moderate stress. Bhuiyan et al. [22] found increased AsA content in B. rapa under drought (20% PEG). They also observed increased APX activity in drought-stressed seedlings, which assisted in efficiently scavenging the H2O2. Another two enzymes related to AsA regeneration MDHAR and DHAR also upregulated, as a result the AsA level was increased and strongly maintained its redox balance during oxidative stress situation. Nahar et al. [32] narrated the function of AsA as ROS detoxifier under drought stress where AsA content reduced in V. radiata with the increasing of ROS generation. Here, drought-induced higher APX activity enhanced the oxidation of AsA by scavenging H2O2, and improved GR activity increased the supply of GSH for involving ROS detoxification. Anacardium occidentale also showed the active participation of AsA-GSH cycle by integrative responses of both non-enzymatic and enzymatic antioxidants for drought-induced excess ROS regulation, where the higher accumulation of AsA and GSH, along with APX activity, coordinately reduced the overproduced H2O2 [36]. Thus, the AsA-GSH pathways involve in ROS detoxification as well as ROS homeostasis by eliminating excess ROS for keeping them up to the requirement of functioning cell signals.
3.3. Toxic Metals/Metalloids
Due to the fast industrialization of the modern world and unrestrained anthropogenic activities, toxic metals/metalloids stresses have become a gargantuan problem for the plant growth and development [91]. Plants experience toxic metals/metalloids stress try to survive to some extent by using their well-established antioxidant defense system. But, the activity and performance of defense system differ with stress concentration, stress duration, plant type, and age of the plant.
The enzymes of AsA-GSH pathway confirmed their differential responses to different toxic metals/metalloids stress (Table 2). Mahmud et al. [52] confirmed that due to Cr stress, the few components of AsA-GSH pathway increased their amount or activity in B. juncea L. cv. BARI Sharisha-11. They found five days duration of 0.15 mM and 0.3 mM K2CrO4 treatment decreased the content of AsA, but did not change the GSH content. Moreover, activities of APX and GR were enhanced; however, the activities of MDHAR and DHAR were diminished. The higher APX and GR activity might play a function in scavenging excess ROS. A similar upregulation of APX and GR was also recorded in B. napus L. cv. Binasharisha-3 due to Cd treatment [92]. From two separate experiments, they also found Cd stress (0.5 mM and 1.0 mM CdCl2) for 48 h decreased the AsA content, but increased GSH content only under 0.5 mM CdCl2 treatment. Exposure of Gossypium to 50 and 100 μM Pb(NO3)2 for six weeks increased the H2O2 content and APX activity [41]. The addition of 150 μM NiCl2·6H2O in growing media of B. juncea L. for one week increased the H2O2 content. Moreover, Ni stress decreased the AsA level but augmented the content of GSH and GSSG. Nickel also diminished the function of DHAR and MDHAR, however enhanced APX and GR activity [57]. Similar differential responses of AsA-GSH pathway components were also observed under As [44] and Al [34] toxicity. It can be stated that overproduced ROS plays the signaling role to some extent and inaugurate the higher activity of AsA-GSH enzymes under metals/metalloids toxicity. The upregulation of enzymes plays a significant role in maintaining the redox balance of AsA-GSH pathway under stress condition.
3.4. Extreme Temperature
Along with the rise in average global temperature, HT stress has been turned into a topic to be concerned about among environmentalists and researchers worldwide. In general, a 5 °C temperature rise above the optimum temperature of growth is considered to be extreme temperature stress or HT stress or heat shock to any plant species [93][62]. Heat stress causes denaturation of protein and membrane lipids, enzyme inactivation, inhibited protein synthesis, and loss of membrane integrity [94], which results from the disruption of cellular homeostasis through the ROS formed in a mass amount under heat stress [62][95]. Focusing on the role of AsA-GSH pathway to scavenge these ROS, different crop species under different levels of extreme or HT stress have been studied (Table 3).
Khanna-Chopra and Chauhan [96] selected a warmer season to induce HT stress to two different cultivars of wheat (T. aestivum), which are Hindi62 (heat-tolerant) and PBW343 (heat-sensitive). They sowed the wheat seeds in mid-January and considered it as heat stress environment, while the control plants were sown in mid-November and considered as the non-stress environment. Data were collected at seven days interval up to 35 days after anthesis (DAA), and the results showed a sharp increase in H2O2 content up to 14 days, but then declined. Whereas, MDHAR and DHAR enzymes’ activity only increased in Hindi62, but APX and GR activities showed a fluctuating pattern of alteration in both cultivars [96]. Another cereal Z. mays when experimented similarly with two different cultivars; LM-11 (heat-sensitive) and CML-32 (heat-tolerant), exposed to 40 °C for 72 h, resulted in higher APX and GR activities in CML-32 roots, while a reduction occurred in the shoot. In LM-11, none of the enzyme activity or AsA content was affected [67]. Higher levels of O2− production rate and H2O2 content were observed in Ficus concinna seedlings under 48 h of HT (35 °C and 40 °C) stress condition, where AsA and GSH contents were unaffected at 35 °C, while declining AsA at 40 °C temperature [70]. The activity of APX, MDHAR, DHAR, and GR enzymes increased at 35 °C, but then again reduced at 40 °C to the level of control plants [70]. Under similar heat stress condition (40 °C, 48 h), V. radiata seedlings resulted in decreased GSH content and MDHAR-DHAR activities, but higher APX-GR activities [34]. Kiwi fruit (Actinidia deliciosa) seedlings, when exposed to 45 °C in an incubator for 8 h, resulted in higher AsA content and enhanced activity of all the AsA-GSH cycle enzymes [62]. Tomato seedlings were studied in two different aspects: short-term heat shock (40 °C, 9 h) [65] and long-term heat stress (38/28 °C day/night, seven days) [68]. In both experiments, the enhancement of O2− generation rate and H2O2 content were recorded, but enzyme (APX and GR) activity was only increased at short-term stress condition [65], while the long-term heat exposure reduced all four enzymes activities and GSH content [68]. Similar enzymatic activity was observed in Nicotiana tabacum seedlings after seven days of heat (35 °C) stress [69]. From the above discussion, it can be stated that heat stress prevailing for a longer duration is less likely to have the capability to modulate AsA-GSH pathway as compared to short-term heat stress.
3.5. Flooding
Changes in global climate result in the frequent or unexpected occurrence of heavy rainfall in different regions of the globe, which causes a sudden flood and disrupts the normal ecosystem [6]. Such changes in the ecosystem may cause the extinction of plants species and imbalance in the natural environment [6]. Flooding-induced production of ROS and subsequent cellular damage has been authenticated in many studies so far [76][73][63]. Following are the discussion regarding crop species facing flooding stresses and modulation of their AsA-GSH pathway by flooding stress (Table 3).
Pigeon pea (C. cajan) seedlings that are exposed to waterlogged condition for six days revealed that tolerant cultivar could increase APX and GR activities, but a susceptible one cannot [78]. They also observed that, unlike other cases, waterlogging caused a lower accumulation of H2O2 and O2− [78]. In another experiment with V. radiata, Sairam et al. [75] showed that waterlogging similarly reduced the H2O2 and O2−production rate in susceptible cultivar, while the tolerant ones remained unaffected. However, both APX and GR enzymes’ activity increased in tolerant genotypes, while the susceptible one got reduced [75]. The enhanced production rate of O2−and H2O2 content under flooding stress has been reported in cotton [63], Welsh onion [77], and clover [74] plants. Cotton (G. hirsutum cv. Siza) plants after three and six days of flood exposure raised the AsA content but reduced the activity of APX, MDHAR, and GR [63]. A similar reduction in APX and GR enzymes activities was also recorded in Welsh onion (Allium fistulosum L.) after 10 days of waterlogging stress [77]. When Z. mays seedlings were waterlogged for 21 h at their root portions, they resulted in reduced AsA content and increased APX activity [72]. On the other hand, under long duration (14 days) flooding stress, Glycine max L. plants showed a reduction of GSH activity in roots and GR activity in the shoot, but the GSH in shoot and GR in root were not affected [73]. In case of complete submergence of O. sativa L. plants for two, four, or eight days, elevated levels GR enzyme activity was recorded, while APX enzyme activity increased only in tolerant cultivar [76]. Accordingly, the discussion reveals that the impact of flooding stress on AsA-GSH pathway varies depending upon the plant species and duration.
3.6. Atmospheric Pollutants
Atmospheric pollutants are the substances that are assembled in the air to a level or magnitude that is dangerous for living beings. Plants that are grown under different levels of atmospheric pollution have shown their oxidative stress responses and AsA-GSH pathway regulation in different manners (Table 3).
Erythrina orientalis plants were grown in three different locations of Philippines: La Mesa (a non-polluted area); and, Makati and Quezon (highly air-polluted cities). The results revealed that plants grown in the non-polluted area had lower activities of APX and GR as compared to the ones grown in highly polluted areas [85]. A similar increase in APX activity along with higher AsA content was recorded in Prosopis juliflora plants grown under polluted industrial region [84]. In a recent experiment, Lucas et al. [80] studied Lolium perenne plants that were grown under two different areas of Spain, Madrid, and Ciudad Real, where Madrid was considered to be more polluted than Ciudad Real. The findings indicated that the pollens of L. perenne accumulated a higher concentration of H2O2 and in shoots APX and DHAR activity declined, but the activity of MDHAR and GR increased in the shoot of L. perenne plants that were grown in Madrid [80]. When rice seedlings were exposed to continuous O3 treatment, the results showed a remarkable increase in both O2− generation rate and H2O2 content. In addition, contents of AsA and GSH reduced, while APX, MDHAR, DHAR, and GR activity increased up to 70 days of O3 exposure in SY63 cultivar and up to 79 days of O3 exposure in WXJ14 cultivar [83]. Ascorbate and GSH contents were not affected by O3 exposure in the Populus seedlings, but DHAR activity was lower, while the activity of GR and MDHAR was higher after 17 days of O3 treatment [81]. Young strawberry (Fragaria x anansa) seedlings were exposed to three different levels of CO, NOx, and SO2, which are as follows: CO @ 133, 267, and 533 ppm, NOx and SO2 @ 25, 50, and 199 ppm corresponding to low, medium, and high dose, respectively. As a result of exposure to these atmospheric pollutants, H2O2 content as well as O2− generation rate increased. However, at low and medium doses of their exposure APX and GR activity increased, while at a high dose that decreased [82]. All sorts of atmospheric pollutants have a remarkable effect on AsA-GSH pathway, but further studies are required to demonstrate that those pollutants completely induced the modification of the AsA-GSH pathway.
3.7. Other Stress
Conklin et al. confirmed the positive role of AsA in protecting plants from ultraviolet (UV) radiation [72], where they found that Vit-C deficient mutant of A. thaliana was suffered by stress-induced damages than that of wild type. AsA-deficient mutants also showed sensitivity to O3 stress due to a lower biosynthesis of AsA [97]. Gao and Zhang [98] reported that vitc1 mutants of A. thaliana showed physiological disorders and greater oxidative damages than the wild type, which was due to lower activities of antioxidant enzymes. Mutant plants also showed lower GSH/GSSG and higher DHA/(AsA+DHA) ratio than the wild type. Singh et al. [99] observed a decrease in AsA-GSH cycle enzymes in UV-exposed plants, which in turn affected the plants with oxidative stress. Similar to higher plants, marine macroalga Ulva fasciata also showed a positive correlation between enhanced the functions of AsA-GSH cycle and better tolerance of plants to UV radiation [100]. In their study, the scavenging of H2O2 was regulated by AsA-GSH cycle components, especially APX and GR. Noshi et al. [101] reported that AsA-GSH redox pool provided better protection of Arabidopsis from high-light mediated oxidative stress, which was mainly attained due to the higher activities of DHAR. However, both AsA and GSH were found to be responsible for conferring high light (HL) stress [101]. Later, Zheng et al. [102] that susceptibility of Arabidopsis mutant was to HL stress was related to the deficiency of AsA and GSH. When AsA deficient A. thaliana mutant (vtc2-1) was exposed to HL, they generated a high level of H2O2 (an oxidative stress marker) than the wild type, which was highly and negatively correlated with the total AsA content. The lack of AsA also resulted in lower chlorophyll (chl) content, chl fluorescence parameters, and PSII photochemistry [102]. Recently, Choudhury et al. [103] studied the metabolomics of A. thaliana grown under HL and found that the increased biosynthesis of GSH supports the photochemistry that supports Arabidopsis better survival under HL stress.
The pivotal role of the AsA-GSH cycle was observed in low pH stress also. Bhuyan et al. [86] tested five spring wheat cultivars at different levels of low pH stress. Their observation exhibited that low-pH stress resulted in elevated O2−and H2O2 generation. A decrease in AsA content with increased DHA content was observed, although the APX activity decreased. Increased MDHAR activity was observed, but the ratio of AsA/DHA was not increased. Decreased GSH content and increased GSSG content were found where DHAR and GR activity decreased, resulting in a drop in the GSH/GSSG ratio.
References
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930.
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Exogenous silicon attenuates cadmium-induced oxidative stress in Brassica napus L. by modulating AsA-GSH pathway and glyoxalase system. Front. Plant Sci. 2017, 8, 1061.
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648.
- Hasanuzzaman, M.; Hossain, M.A.; Teixeira da Silva, J.A.; Fujita, M. Plant responses and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop Stress and its Management: Perspectives and Strategies; Bandi, V., Shanker, A.K., Shanker, C., Mandapaka, M., Eds.; Springer: Dordrecht, The Netherlands, 2012.
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. PMBP 2017, 23, 249–268.
- Hasanuzzaman, M.; Mahmud, J.A.; Nahar, K.; Anee, T.I.; Inafuku, M.; Oku, H.; Fujita, M. Responses, adaptation, and ROS metabolism in plants exposed to waterlogging stress. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Khan, M.I.R., Khan, N.A., Eds.; Springer: New York, NY, USA, 2017.
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int. J. Mol. Sci. 2017, 18, 200.
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Anee, T.I.; Parvin, K.; Fujita, M. Nitric oxide pretreatment enhances antioxidant defense and glyoxalase systems to confer PEG-induced oxidative stress in rapeseed. J. Plant Interact. 2017, 12, 323–331.
- Zechmann, B. Compartment-specific importance of glutathione during abiotic and biotic stress. Front. Plant Sci. 2014, 5, 566.
- Szarka, A.; Tomasskovics, B.; Bánhegyi, G. The ascorbate-glutathione-α-tocopherol triad in abiotic stress response. Int. J. Mol. Sci. 2012, 13, 4458–4483.
- Sehar, Z.; Masood, A.; Khan, N.A. Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ. Exp. Bot. 2019, 161, 277–289.
- Al Mahmud, J.; Biswas, P.K.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Exogenous application of gibberellic acid mitigates drought-induced damage in spring wheat. Acta Agrobot. 2019, 72, 1776.
- Shan, C.; Zhang, S.; Ou, X. The roles of H2S and H2O2 in regulating AsA-GSH cycle in the leaves of wheat seedlings under drought stress. Protoplasma 2018, 255, 1257–1262.
- Lou, L.; Li, X.; Chen, J.; Li, Y.; Tang, Y.; Lv, J. Photosynthetic and ascorbate-glutathione metabolism in the flag leaves as compared to spikes under drought stress of winter wheat (Triticum aestivum L.). PLoS ONE 2018, 13, e0194625.
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365.
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front. Plant Sci. 2016, 7, 609.
- Hasanuzzaman, M.; Alam, M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed. Res. Int. 2014, 2014, 757219.
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol. Trace Elem. Res. 2011, 143, 1704–1721.
- Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Alleviation of osmotic stress in Brassica napus, B. campestris, and B. juncea by ascorbic acid application. Biol. Plant. 2014, 58, 697–708.
- Alam, M.M.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous salicylic acid ameliorates short-term drought stress in mustard (Brassica juncea L.) seedlings by up-regulating the antioxidant defense and glyoxalase system. Aust. J. Crop Sci. 2013, 7, 1053–1063.
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Khan, M.I.R.; Fujita, M. Silicon-mediated regulation of antioxidant defense and glyoxalase systems confers drought stress tolerance in Brassica napus L. S. Afr. J. Bot. 2018, 115, 50–57.
- Bhuiyan, T.F.; Ahamed, K.U.; Nahar, K.; Al Mahmud, J.; Bhuyan, M.B.; Anee, T.I.; Fujita, M.; Hasanuzzaman, M. Mitigation of PEG-induced drought stress in rapeseed (Brassica rapa L.) by exogenous application of osmolytes. Biocatal. Agric. Biotechnol. 2019, 20, 101197.
- Jin, X.; Liu, T.; Xu, J.; Gao, Z.; Hu, X. Exogenous GABA enhances muskmelon tolerance to salinity-alkalinity stress by regulating redox balance and chlorophyll biosynthesis. BMC Plant Biol. 2019, 19, 48.
- Singh, M.; Singh, V.P.; Prasad, S.M. Nitrogen alleviates salinity toxicity in Solanum lycopersicum seedlings by regulating ROS homeostasis. Plant Physiol. Biochem. 2019, 141, 466–476.
- García-Martí, M.; Piñero, M.C.; García-Sanchez, F.; Mestre, T.C.; López-Delacalle, M.; Martínez, V.; Rivero, R.M. Amelioration of the oxidative stress generated by simple or combined abiotic stress through the K+ and Ca2+ supplementation in tomato plants. Antioxidants 2019, 8, 81.
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.; Mohsin, S.M.; Fujita, M. Quercetin mediated salt tolerance in tomato through the enhancement of plant antioxidant defense and glyoxalase systems. Plants 2019, 8, 247.
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE 2018, 13, e0202175.
- Ahmad, P.; Abd_Allah, E.F.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Siddique, K.H. Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate–glutathione cycle and secondary metabolites. Sci. Rep. 2018, 8, 13515.
- Yan, Y.; Pan, C.; Du, Y.; Li, D.; Liu, W. Exogenous salicylic acid regulates reactive oxygen species metabolism and ascorbate–glutathione cycle in Nitraria tangutorum Bobr. under salinity stress. PMBP 2018, 24, 577–589.
- Li, J.; Yang, Y.; Sun, K.; Chen, Y.; Chen, X.; Li, X. Exogenous melatonin enhances cold, salt and drought stress tolerance by improving antioxidant defense in tea plant (Camellia sinensis (L.) O. Kuntze). Molecules 2019, 24, 1826.
- Talaat, N.B. Effective microorganisms enhance the scavenging capacity of the ascorbate-glutathione cycle in common bean (Phaseolus vulgaris L.) plants grown in salty soils. Plant Physiol. Biochem. 2014, 80, 136–143.
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Glutathione-induced drought stress tolerance in mung bean: Coordinated roles of the antioxidant defence and methylglyoxal detoxification systems. AoB Plants 2015, 7, plv069.
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol. Plant. 2015, 59, 745–756.
- Nahar, K.; Hasanuzzaman, M.; Alam, M.; Rahman, A.; Mahmud, J.-A.; Suzuki, T.; Fujita, M. Insights into spermine-induced combined high temperature and drought tolerance in mung bean: Osmoregulation and roles of antioxidant and glyoxalase system. Protoplasma 2017, 254, 445–460.
- Hossain, M.S.; Alam, M.U.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Mahmud, J.A.; Fujita, M. Use of iso-osmotic solution to understand salt stress responses in lentil (Lens culinaris Medik.). S. Afr. J. Bot. 2017, 113, 346–354.
- Lima, C.S.; Ferreira-Silva, S.L.; Carvalho, F.E.L.; Neto, M.C.L.; Aragão, R.M.; Silva, E.N.; Sousa, R.M.J.; Silveira, J.A.G. Antioxidant protection and PSII regulation mitigate photo-oxidative stress induced by drought followed by high light in cashew plants. Environ. Exp. Bot. 2018, 149, 59–69.
- Nguyen, K.H.; Mostofa, M.G.; Watanabe, Y.; Tran, C.D.; Rahman, M.M.; Tran, L.S.P. Overexpression of GmNAC085 enhances drought tolerance in Arabidopsis by regulating glutathione biosynthesis, redox balance and glutathione-dependent detoxification of reactive oxygen species and methylglyoxal. Environ. Exp. Bot. 2019, 161, 242–254.
- Sreeharsha, R.V.; Mudalkar, S.; Sengupta, D.; Unnikrishnan, D.K.; Reddy, A.R. Mitigation of drought-induced oxidative damage by enhanced carbon assimilation and an efficient antioxidative metabolism under high CO2 environment in pigeonpea (Cajanus cajan L.). Photosynth. Res. 2019, 139, 425–439.
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496.
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol. Trace Elem. Res. 2012, 149, 248–261.
- Bharwana, S.A.; Ali, S.; Farooq, M.A.; Iqbal, N.; Abbas, F.; Ahmad, M.S.A. Alleviation of lead toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes suppressed lead uptake and oxidative stress in cotton. J. Bioremed. Biodeg. 2013, 4, 187.
- Hasanuzzaman, M.; Fujita, M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 2013, 22, 584–596.
- Gill, R.A.; Zang, L.; Ali, B.; Farooq, M.A.; Cui, P.; Yang, S.; Ali, S.; Zhou, W. Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 2015, 120, 154–164.
- Rahman, A.; Mostofa, M.G.; Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidant defense and glyoxalase systems and stress markers. BioMed. Res. Int. 2015, 2015, 340812.
- Awasthi, J.P.; Saha, B.; Panigrahi, J.; Yanase, E.; Koyama, H.; Panda, S.K. Redox balance, metabolic fingerprint and physiological characterization in contrasting North East Indian rice for aluminum stress tolerance. Sci. Rep. 2019, 9, 8681.
- Nahar, K.; Rahman, M.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Physiological and biochemical mechanisms of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings. Environ. Sci. Pollut. Res. Int. 2016, 23, 21206–21218.
- Nahar, K.; Rahman, M.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Polyamine and nitric oxide crosstalk: Antagonistic effects on cadmium toxicity in mungbean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol. Environ. Saf. 2016, 126, 245–255.
- Rahman, A.; Mostofa, M.G.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Exogenous calcium alleviates cadmium-induced oxidative stress in rice (Oryza sativa L.) seedlings by regulating the antioxidant defense and glyoxalase systems. Braz. J. Bot. 2016, 39, 393–407.
- Rahman, A.; Hossain, M.S.; Mahmud, J.A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Manganese-induced salt stress tolerance in rice seedlings: Regulation of ion homeostasis, antioxidant defense and glyoxalase systems. PMBP 2016, 22, 291–306.
- Wan, Y.; Wang, K.; Liu, Z.; Yu, Y.; Wang, Q.; Li, H. Effect of selenium on the subcellular distribution of cadmium and oxidative stress induced by cadmium in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2019, 26, 16220.
- Hussain, I.; Siddique, A.; Ashraf, M.A.; Rasheed, R.; Ibrahim, M.; Iqbal, M.; Akbar, S.; Imran, M. Does exogenous application of ascorbic acid modulate growth, photosynthetic pigments and oxidative defense in okra (Abelmoschus esculentus (L.) Moench) under lead stress? Acta Physiol. Plant. 2017, 39, 144.
- Mahmud, J.A.; Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Hossain, M.S.; Fujita, M. Maleic acid assisted improvement of metal chelation and antioxidant metabolism confers chromium tolerance in Brassica juncea L. Ecotoxicol. Environ. Saf. 2017, 144, 216–226.
- Mahmud, J.A.; Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Fujita, M. Relative tolerance of different species of Brassica to cadmium toxicity: Coordinated role of antioxidant defense and glyoxalase systems. Plant Omics J. 2017, 10, 107–117.
- Mahmud, J.A.; Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Fujita, M. EDTA reduces cadmium toxicity in mustard (Brassica juncea L.) by enhancing metal chelation, antioxidant defense and glyoxalase systems. Acta Agrobot. 2019, 72, 1722.
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Alharby, H.F.; Fujita, M. Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J. Plant Interact. 2018, 13, 203–212.
- Mahmud, J.A.; Hasanuzzaman, M.; Nahar, K.; Bhuyan, M.H.M.B.; Fujita, M. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: Coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol. Environ. Saf. 2018, 147, 990–1001.
- Abd_Allah, E.F.; Hashem, A.; Alam, P.; Ahmad, P. Silicon alleviates nickel-induced oxidative stress by regulating antioxidant defense and glyoxalase systems in mustard plants. J. Plant Growth Regul. 2019, 1–14.
- Dias, M.C.; Mariz-Ponte, N.; Santos, C. Lead induces oxidative stress in Pisum sativum plants and changes the levels of phytohormones with antioxidant role. Plant Physiol. Biochem. 2019, 137, 121–129.
- Hasanuzzaman, M.; Alam, M.M.; Nahar, K.; Mohsin, S.M.; Bhuyan, M.H.M.B.; Parvin, K.; Fujita, M. Silicon-induced antioxidant defense and methylglyoxal detoxification works coordinately in alleviating nickel toxicity in Oryza sativa L. Ecotoxicology 2019, 28, 261–276.
- Kaya, C.; Akram, N.A.; Sürücü, A.; Ashraf, M. Alleviating effect of nitric oxide on oxidative stress and antioxidant defence system in pepper (Capsicum annuum L.) plants exposed to cadmium and lead toxicity applied separately or in combination. Sci. Hortic. 2019, 255, 52–60.
- Zhang, K.; Wang, G.; Bao, M.; Wang, L.; Xie, X. Exogenous application of ascorbic acid mitigates cadmium toxicity and uptake in Maize (Zea mays L.). Environ. Sci. Pollut. Res. 2019, 26, 19261–19271.
- Liang, D.; Gao, F.; Ni, Z.; Lin, L.; Deng, Q.; Tang, Y.; Wang, X.; Luo, X.; Xia, H. Melatonin improves heat tolerance in kiwifruit seedlings through promoting antioxidant enzymatic activity and glutathione S-transferase transcription. Molecules 2018, 23, 584.
- Wang, H.; Chen, Y.; Hu, W.; Snider, J.L.; Zhou, Z. Short-term soil-waterlogging contributes to cotton cross tolerance to chronic elevated temperature by regulating ROS metabolism in the subtending leaf. Plant Physiol. Biochem. 2019, 139, 333–341.
- Ma, Y.; Wang, B.; Zhang, R.; Gao, Y.; Zhang, X.; Li, Y.; Zuo, Z. Initial simulated acid rain impacts reactive oxygen species metabolism and photosynthetic abilities in Cinnamonum camphora undergoing high temperature. Ind. Crops Prod. 2019, 135, 352–361.
- Ahammed, G.J.; Wen, X.; Liu, A.; Chen, S. Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ. Exp. Bot. 2018, 161, 303–311.
- Alamri, S.A.; Siddiqui, M.H.; Al-Khaishany, M.Y.; Khan, M.N.; Ali, H.M.; Alakeel, K.A. Nitric oxide-mediated cross-talk of proline and heat shock proteins induce thermotolerance in Vicia faba L. Environ. Exp. Bot. 2019, 161, 290–302.
- Khanna, P.; Kaur, K.; Gupta, A.K. Salicylic acid induces differential antioxidant response in spring maize under high temperature stress. Indian J. Exp. Biol. 2016, 54, 386–393.
- Sang, Q.Q.; Shu, S.; Shana, X.; Guo, S.R.; Sun, J. Effects of exogenous spermidine on antioxidant system of tomato seedlings exposed to high temperature stress. Russ. J. Plant Physiol. 2016, 63, 645–655.
- Sgobba, A.; Paradiso, A.; Dipierro, S.; Gara, L.B.; de Pinto, M.C. Changes in antioxidants are critical in determining cell responses to short- and long-term heat stress. Physiol. Plant. 2015, 153, 68–78.
- Jin, S.H.; Li, X.Q.; Wang, G.G.; Zhu, X.T. Brassinosteroids alleviate high-temperature injury in Ficus concinna seedlings via maintaining higher antioxidant defence and glyoxalase systems. AoB Plants 2015, 7, plv009.
- Anee, T.I.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Bhuiyan, T.F.; Alam, M.U.; Fujita, M.; Hasanuzzaman, M. Oxidative damage and antioxidant defense in Sesamum indicum after different waterlogging durations. Plants 2019, 8, 196.
- Jaiswal, A.; Srivastava, J.P. Changes in reactive oxygen scavenging system and protein profiles in maize roots in response to nitric oxide under waterlogging stress. Indian J. Biochem. Biophys. 2018, 55, 26–33.
- Kim, Y.; Seo, C.W.; Khan, A.L.; Mun, B.G.; Shahzad, R.; Ko, J.W.; Yun, B.W.; Park, S.K.; Lee, I.J. Exo-ethylene application mitigates waterlogging stress in soybean (Glycine max L.). BMC Plant Biol. 2018, 18, 254.
- Simova-Stoilova, L.; Demirevska, K.; Kingston-Smith, A.; Feller, U. Involvement of the leaf antioxidant system in the response to soil flooding in two Trifolium genotypes differing in their tolerance to waterlogging. Plant Sci. 2012, 183, 43–49.
- Sairam, R.K.; Dharmar, K.; Lekshmy, S.; Chinnusamy, V. Expression of antioxidant defense genes in mung bean (Vigna radiata L.) roots under water-logging is associated with hypoxia tolerance. Acta Physiol. Plant. 2011, 3, 735–744.
- Damanik, R.I.; Maziah, M.; Ismail, M.R.; Ahmad, S.; Zain, A.M. Responses of the antioxidative enzymes in Malaysian rice (Oryza sativa L.) cultivars under submergence condition. Acta Physiol. Plant 2010, 32, 739–747.
- Yiu, J.-C.; Liu, C.-W.; Fang, D.Y.; Lai, Y.-S. Waterlogging tolerance of Welsh onion (Allium fistulosum L.) enhanced by exogenous spermidine and spermine. Plant Physiol. Biochem. 2009, 47, 710–716.
- Sairam, R.K.; Kumutha, D.; Ezhilmathi, K.; Chinnusamy, V.; Meena, R.C. Waterlogging induced oxidative stress and antioxidant enzyme activities in pigeon pea. Biol. Plant. 2009, 53, 493–504.
- Lin, K.H.R.; Weng, C.C.; Lo, H.F.; Chen, J.T. Study of the root antioxidative system of tomatoes and eggplants under waterlogged conditions. Plant Sci. 2004, 167, 355–365.
- Lucas, J.A.; Gutierrez-Albanchez, E.; Alfaya, T.; Feo-Brito, F.; Gutiérrez-Mañero, F.J. Oxidative stress in ryegrass growing under different air pollution levels and its likely effects on pollen allergenicity. Plant Physiol. Biochem. 2019, 135, 331–340.
- Dusart, N.; Gérard, J.; Thiec, D.L.; Collignon, C.; Jolivet, Y.; Vaultier, M.N. Integrated analysis of the detoxification responses of two Euramerican poplar genotypes exposed to ozone and water deficit: Focus on the ascorbate-glutathione cycle. Sci. Total Environ. 2019, 651, 2365–2379.
- Muneer, S.; Kim, T.H.; Choi, B.C.; Lee, B.S.; Lee, J.H. Effect of CO, NOx and SO2 on ROS production, photosynthesis and ascorbate–glutathione pathway to induce Fragaria x annasa as a hyperaccumulator. Redox Biol. 2014, 2, 91–98.
- Wang, J.L.; Zeng, Q.; Zhu, J.G.; Liu, G.; Tang, H.Y. Dissimilarity of ascorbate–glutathione (AsA–GSH) cycle mechanism in two rice (Oryza sativa L.) cultivars under experimental free-air ozone exposure. Agric. Ecosyst. Environ. 2013, 165, 39–49.
- Seyyednejad, S.M.; Koochak, H.; Vaezi, J. Changes in anti-oxidative enzymes activity, protein content and ascorbic acid level in Prosopis juliflora exposed to industrial air pollution. J. Biol. Today World 2013, 2, 482–492.
- Woo, S.Y.; Lee, D.K.; Lee, Y.K. Net photosynthetic rate, ascorbate peroxidase and glutathione reductase activities of Erythrina orientalis in polluted and non-polluted areas. Photosynthetica 2007, 45, 293–295.
- Bhuyan, M.B.; Hasanuzzaman, M.; Al Mahmud, J.; Hossain, M.S.; Alam, M.U.; Fujita, M. Explicating physiological and biochemical responses of wheat cultivars under acidity stress: Insight into the antioxidant defense and glyoxalase systems. PMBP 2019, 25, 865–879.
- Rahman, A.K.; Al Mahmud, J.; Hasanuzzaman, M.; Hossain, M.S.; Fujita, M. Salt stress tolerance in rice: Emerging role of exogenous phytoprotectants. In Advances in International Rice Research; Li, Q., Ed.; InTech: Rijeka, Croatia, 2017; pp. 139–174.
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Manganese-induced cadmium stress tolerance in rice seedlings: Coordinated action of antioxidant defense, glyoxalase system and nutrient homeostasis. C. R. Biol. 2016, 339, 462–474.
- Hossain, M.S.; Hasanuzzaman, M.; Sohag, M.M.H.; Bhuyan, M.H.M.B.; Fujita, M. Acetate-induced modulation of ascorbate: Glutathione cycle and restriction of sodium accumulation in shoot confer salt tolerance in Lens culinaris Medik. PMBP Plants 2019, 25, 443–455.
- Sun, J.; Gu, J.; Zeng, J.; Han, S.; Song, A.P.; Chen, F.; Fang, W.; Jiang, J.; Chen, S. Changes in leaf morphology, antioxidant activity and photosynthesis capacity in two different drought-tolerant cultivars of chrysanthemum during and after water stress. Sci. Hortic. 2013, 161, 249–258.
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828.
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Alharby, H.F.; Razafindrabe, B.H.N.; Fujita, M. Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: An intrinsic study on antioxidant defense and glyoxalase systems. Front. Plant Sci. 2017, 8, 115.
- Rodriguez, V.M.; Soengas, P.; Alonso-Villaverde, V.; Sotelo, T.; Cartea, M.E.; Velasco, M.P. Effect of temperature stress on the early vegetative development of Brassica oleracea L. BMC Plant Biol. 2015, 15, 145.
- Gupta, N.K.; Agarwal, S.; Agarwal, V.P.; Nathawat, N.S.; Gupta, S.; Singh, G. Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol. Plant. 2013, 35, 1837–1842.
- Vasseur, F.; Pantin, F.; Vile, D. Changes in light intensity reveal a major role for carbon balance in Arabidopsis responses to high temperature. Plant Cell Environ. 2011, 34, 1563–1576.
- Khanna-Chopra, R.; Chauhan, S. Wheat cultivars differing in heat tolerance show a differential response to oxidative stress during monocarpic senescence under high temperature stress. Protoplasma 2015, 25, 1241–1251.
- Conklin, P.L.; Saracco, S.A.; Norris, S.R.; Last, R.L. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 2000, 154, 847–856.
- Gao, Q.; Zhang, L. Ultraviolet-B-induced oxidative stress and antioxidant defense system responses in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. J. Plant Physiol. 2008, 165, 138–148.
- Singh, V.P.; Srivastava, P.K.; Prasad, S.M. Differential effect of UV-B radiation on growth, oxidative stress and ascorbate-glutathione cycle in two cyanobacteria under copper toxicity. Plant Physiol. Biochem. 2012, 61, 61–70.
- Shiu, C.-T.; Lee, T.-M. Ultraviolet-B-induced oxidative stress and responses of the ascorbate–glutathione cycle in a marine macroalga Ulva fasciata. J. Exp. Bot. 2005, 56, 2851–2865.
- Noshi, M.; Hatanaka, R.; Tanabe, N.; Terai, Y.; Maruta, T.; Shigeoka, S. Redox regulation of ascorbate and glutathione by a chloroplastic dehydroascorbate reductase is required for high-light stress tolerance in Arabidopsis. Biosci. Biotechnol. Biochem. 2016, 80, 870–877.
- Zheng, L.-D.; Li, M.; Chow, W.S.; Peng, C.-L. Susceptibility of an ascorbate-deficient mutant of Arabidopsis to high-light stress. Photosynthetica 2018, 56, 427–432.
- Choudhury, F.K.; Devireddy, A.R.; Azad, R.K.; Shulaev, V.; Mittler, R. Rapid accumulation of glutathione during light stress in Arabidopsis. Plant Cell Physiol. 2018, 59, 1817–1826.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Revisions:
2 times
(View History)
Update Date:
29 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




