
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Miroslav Koóš | + 1092 word(s) | 1092 | 2021-07-07 08:36:57 | | | |
| 2 | Enzi Gong | Meta information modification | 1092 | 2021-07-28 03:12:31 | | |
Video Upload Options
The Bucherer–Bergs reaction is one of the most convenient general methods for the preparation of 5-substituted and 5,5-disubstituted hydantoins (imidazolidine-2,4-diones, 2,4-dioxoimidazolidines). Generally, in this multicomponent reaction, the aldehyde or ketone in aqueous ethanol is heated at 60–70° with potassium (or sodium) cyanide and ammonium carbonate to produce directly hydantoins 1.
1. Overview
Hydantoins and their hybrids with other molecules represent a very important group of heterocycles because they exhibit diverse biological and pharmacological activities in medicinal and agrochemical applications. They also serve as key precursors in the chemical or enzymatic synthesis of significant nonnatural α-amino acids and their conjugates with medical potential. This review provides a comprehensive treatment of the synthesis of hydantoins via the Bucherer–Bergs reaction including the Hoyer modification but limited to free carbonyl compounds or carbonyl compounds protected as acetals (ketals) and cyanohydrins used as starting reaction components. In this respect, the Bucherer–Bergs reaction provides an efficient and simple method in the synthesis of important natural products as well as for the preparation of new organic compounds applicable as potential therapeutics. The scope and limitations, as well as a comparison with some other methods for preparing hydantoins, are also discussed.
2. Bucherer–Bergs Reaction
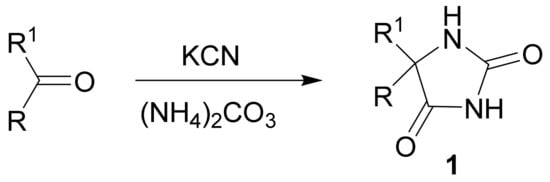
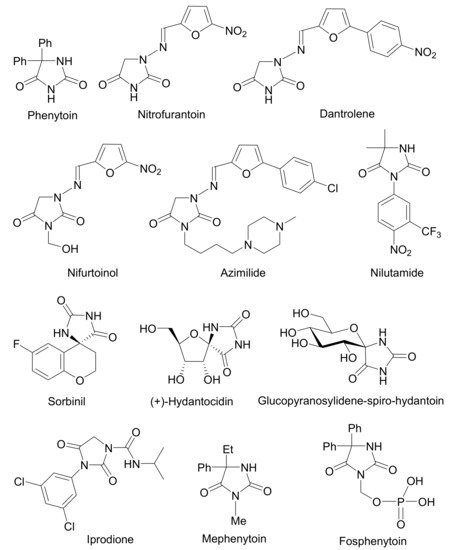
3. Conclusions
References
- Bergs, H. Verfahren zur Darstellung von Hydantoinen. German Patent DE566094, 14 December 1932.
- Ciamician, G.; Silber, P. Chemische Lichtwirkungen. Ber. Dtsch. Chem. Ges. 1905, 38, 1671–1675.
- Bucherer, H.T.; Steiner, W. Syntheses of hydantoins. I. On reactions of α-hydroxy and α-amino nitriles. J. Prakt. Chem. 1934, 140, 291–316.
- Bucherer, H.T.; Fischbeck, H.T. Hexahydrodiphenylamine and its derivatives. J. Prakt. Chem. 1934, 140, 69–89.
- Bucherer, H.T.; Lieb, V.A. Syntheses of hydantoins. II. Formation of substituted hydantoins from aldehydes and ketones. J. Prakt. Chem. 1934, 141, 5–43.
- Henze, H.R.; Long, L.M. Researches on phenylhydantoins. J. Am. Chem. Soc. 1941, 63, 1936–1938.
- Henze, H.R.; Long, L.M. 5-(4-Biphenylyl)-5-R-hydantoins and bis-5-[(4-phenyl)-5-R-hydantoin]s. J. Am. Chem. Soc. 1941, 63, 1941–1943.
- Li, J.; Li, L.; Li, T.; Li, H.; Liu, J. An efficient and convenient procedure for the synthesis of 5,5-disubstituted hydantoins under ultrasound. Ultrason. Sonochem. 1996, 3, S141–S143.
- Hoyer, H.L. Über das camphan-2-spiro-hydantoin. Chem. Ber. 1950, 83, 491–500.
- Henze, H.R.; Speer, R.J. Identification of carbonyl compounds through conversion into hydantoins. J. Am. Chem. Soc. 1942, 64, 522–523.
- Oh, C.-H.; Kim, H.J.; Hong, S.-Y.; Lee, Y.-H.; Cho, J.K.; Cho, J.-H. New 1β-methylcarbapenems having a hydantoin moiety. Neue 1β-methylcarbapeneme mit hydantoin-substitution. Arch. Pharm. 1995, 328, 385–387.
- Marchand-Brynaert, J.; Arnadei, E.; Ghosez, L. Functionalized hydantoins as potential antibiotics. Bull. Soc. Chim. Belg. 1994, 103, 213–218.
- Oliveira, S.M.; Silva, J.B.P.; Hernandes, M.Z.; Lima, M.C.A.; Galdino, S.L.; Pitta, I.R. Structure, reactivity, and biological properties of hidantoines. Quim. Nova 2008, 31, 614–622.
- Ali, O.M.; Amer, H.H.; Mosaad, A.A.; Abdel-Rahman, A.A.-H. Synthesis and antimicrobial activity of new phenytoin derivatives and their acyclic nucleoside analogs. Chem. Heterocycl. Compd. 2012, 48, 1043–1049.
- Ali, O.M.; El-Sayed, W.A.; Eid, S.A.; Abdelwahed, N.A.M.; Abdel-Rahman, A.A.-H. Antimicrobial activity of new synthesized [(oxadiazolyl)methyl]phenytoin derivatives. Acta Polon. Pharm. 2012, 69, 657–667.
- Kim, D.; Wang, L.; Caldwell, C.G.; Chen, P.; Finke, P.E.; Oates, B.; MacCoss, M.; Mills, S.G.; Malkowitz, L.; Gould, S.L.; et al. Discovery of human CCR5 antagonists containing hydantoins for the treatment of HIV-1 infection. Bioorg. Med. Chem. Lett. 2001, 11, 3099–3102.
- Verlinden, Y.; Cuconati, A.; Wimmer, E.; Rombaut, B. The antiviral compound 5-(3,4-dichlorophenyl) methylhydantoin inhibits the post-synthetic cleavages and the assembly of poliovirus in a cell-free system. Antivir. Res. 2000, 48, 61–69.
- El-Barbary, A.A.; Khodair, A.I.; Pedersen, E.B.; Nielsen, C. S-Glucosylated hydantoins as new antiviral agents. J. Med. Chem. 1994, 37, 73–77.
- Anderson, J. The role of antiandrogen monotherapy in the treatment of prostate cancer. BJU Int. 2003, 91, 455–461.
- Kassouf, W.; Tanguay, S.; Aprikian, A.G. Nilutamide as second line hormone therapy for prostate cancer after androgen ablation fails. J. Urol. 2003, 169, 1742–1744.
- Struck, R.F.; Kirk, M.C.; Rice, L.S.; Suling, W.J. Isolation, synthesis and antitumor evaluation of spirohydantoin aziridine, a mutagenic metabolite of spirohydantoin mustard. J. Med. Chem. 1986, 29, 1319–1321.
- Nakabayashi, M.; Regan, M.M.; Lifsey, D.; Kantoff, P.W.; Taplin, M.-E.; Sartor, O.; Oh, W.K. Efficacy of nilutamide as secondary hormonal therapy in androgen-independent prostate cancer. BJU Int. 2005, 96, 783–786.
- Ciechanowicz-Rutkowska, M.; Stadnicka, K.; Kiec-Kononowicz, K.; Byrtus, H.; Filipek, B.; Zygmunt, M.; Maciag, D. Structure-activity relationship of some new anti-arrhythmic phenytoin derivatives. Arch. Pharm. 2000, 333, 357–364.
- Kieć-Kononowicz, K.; Stadnicka, K.; Mitka, A.; Pekala, E.; Filipek, B.; Sapa, J.; Zygmunt, M. Synthesis, structure and antiarrhythmic properties evaluation of new basic derivatives of 5,5-diphenylhydantoin. Eur. J. Med. Chem. 2003, 38, 555–566.
- Knabe, J.; Baldauf, J.; Ahlhem, A. Racemates and enantiomers of basic substituted 5-phenylhydantoins. Syntheses and antiarrhythmic activity. (Razemate und enantiomere basisch substituierter 5-phenylhydantoine, synthese und antiarrhythmische wirkung). Pharmazie 1997, 52, 912–919.
- Matsukura, M.; Daiku, Y.; Ueda, K.; Tanaka, S.; Igarashi, T.; Minami, N. Synthesis and antiarrhythmic activity of 2,2-dialkyl-1′-(N-substituted aminoalkyl)-spiro-[chroman-4,4′-imidazolidine]-2′,5′-diones. Chem. Pharm. Bull. 1992, 40, 1823–1827.
- Thenmozhiyal, J.C.; Wong, P.T.-H.; Chui, W.-K. Anticonvulsant activity of phenylmethylenehydantoins: A structure–activity relationship study. J. Med. Chem. 2004, 47, 1527–1535.
- LeTiran, J.; Stables, J.P.; Kohn, H. Functionalized amino acid anticonvulsants: Synthesis and pharmacological evaluation of conformationally restricted analogues. Bioorg. Med. Chem. 2001, 9, 2693–2708.
- Anger, T.; Madge, D.J.; Mulla, M.; Riddall, D. Medicinal chemistry of neuronal voltage-gated sodium channel blockers. J. Med. Chem. 2001, 44, 115–137.
- Scholl, S.; Koch, A.; Henning, D.; Kempter, G.; Kleinpeter, E. The influence of structure and lipophilicity of hydantoin derivatives on anticonvulsant activity. Struct. Chem. 1999, 10, 355–366.
- Brouillette, W.J.; Jestkov, V.P.; Brown, M.L.; Akhtar, M.S.; DeLorey, T.M.; Brown, G.B. Bicyclic hydantoins with a bridgehead nitrogen. Comparison of anticonvulsant activities with binding to the neuronal voltage-dependent sodium channel. J. Med. Chem. 1994, 37, 3289–3293.
- Kwon, C.H.; Iqbal, M.T.; Wurpel, J.N.D. Synthesis and anticonvulsant activity of 2-iminohydantoins. J. Med. Chem. 1991, 34, 1845–1849.
- Botros, S.; Khalil, N.A.; Naguib, B.H.; El-Dash, Y. Synthesis and anticonvulsant activity of new phenytoin derivatives. Eur. J. Med. Chem. 2013, 60, 57–63.
- Deodhar, M.; Sable, P.; Bhosale, A.; Juvale, K.; Dumbare, R.; Sakpal, P. Synthesis and evaluation of phenytoin derivatives as anticonvulsant agents. Turk. J. Chem. 2009, 33, 367–373.
- Edmunds, J.J.; Klutchko, S.; Hamby, J.M.; Bunker, A.M.; Connolly, C.J.C.; Winters, R.T.; Quin III, J.; Sircar, I.; Hodges, J.C.; Panek, R.L.; et al. Derivatives of 5-[[1-4(4-carboxybenzyl)imidazolyl]methylidene]hydantoins as orally active angiotensin II receptor antagonists. J. Med. Chem. 1995, 38, 3759–3771.
- Somsák, L.; Kovács, L.; Tóth, M.; Ösz, E.; Szilágyi, L.; Györgydeák, Z.; Dinya, Z.; Docsa, T.; Tóth, B.; Gergely, P. Synthesis of and a comparative study on the inhibition of muscle and liver glycogen phosphorylases by epimeric pairs of D-gluco- and D-xylopyranosylidene-spiro-(thio)hydantoins and N-(D-glucopyranosyl) amides. J. Med. Chem. 2001, 44, 2843–2848.
- Oka, M.; Matsumoto, Y.; Sugiyama, S.; Tsuruta, N.; Matsushima, M. A potent aldose reductase inhibitor, (2S,4S)-6-fluoro-2′,5′-dioxospiro[chroman-4,4′-imidazolidine]-2-carboxamide (Fidarestat): Its absolute configuration and interactions with the aldose reductase by X-ray crystallography. J. Med. Chem. 2000, 43, 2479–2483.
- Murakami, N.; Ohta, M.; Kato, K.; Nakayama, K.; Mizota, M.; Miwa, I.; Okuda, J. Effects of 1-(3-bromobenzofuran-2-ylsulfonyl)hydantoin on human aldose reductase examined by a new application of HPLC system for measuring tissue polyol. Arzneimittelforschung/Drug Res. 1997, 47, 1222–1225.
- Sarges, R.; Oates, P.J. Aldose reductase inhibitors: Recent developments. Prog. Drug Res. 1993, 40, 99–161.
- Haruyama, H.; Takayama, T.; Kinoshita, T.; Kondo, M.; Nakajima, M.; Haneishi, T. Structural elucidation and solution conformation of the novel herbicide hydantocidin. J. Chem. Soc. Perkin Trans. 1 1991, 1637–1640.
- Siehl, D.L.; Subramanian, M.V.; Walters, E.W.; Lee, S.F.; Anderson, R.J.; Toschi, A.G. Adenylosuccinate synthetase: Site of action of hydantocidin, a microbial phytotoxin. Plant. Physiol. 1996, 110, 753–758.
- Heim, D.R.; Gerwick, B.C.; Murdoch, M.G.; Green, S.B. Hydantocidin: A possible proherbicide inhibiting purine biosynthesis at the site of adenylosuccinate synthetase. Pest. Biochem. Physiol. 1995, 53, 138–145.
- Mizuno, T.; Kino, T.; Takatoshi, I.; Miyata, T. Synthesis of aromatic urea herbicides by the selenium-assisted carbonylation using carbon monoxide with sulfur. Synth. Commun. 2000, 30, 1675–1688.
- Fischer, H.-P.; Buser, H.-P.; Chemla, P.; Huxley, P.; Lutz, W.; Mirza, S.; Tombo, G.M.R.; van Lommen, G.; Sipido, V. Synthesis and chirality of novel heterocyclic compounds designed for crop protection. Bull. Soc. Chim. Belg. 1994, 103, 565–581.
- Sano, H.; Sugai, S. Synthesis of (±)-carbocyclic analogue of spirohydantoin nucleoside. Tetrahedron 1995, 51, 4635–4646.
- Bazil, C.W. Sleep, sleep apnea, and epilepsy. Curr. Treat. Options Neurol. 2004, 6, 339–345.
- Bosch, J.; Roca, T.; Domènech, J.; Suriol, M. Synthesis of water-soluble phenytoin prodrugs. Bioorg. Med. Chem. Lett. 1999, 9, 1859–1862.
- Bac, P.; Maurois, P.; Dupont, C.; Pages, N.; Stables, J.P.; Gressens, P.; Evrard, P. Magnesium deficiency-dependent audiogenic seizures (MDDASs) in adult mice: A nutritional model for discriminatory screening of anticonvulsant drugs and original assessment of neuroprotection properties. J. Neurosci. 1998, 18, 4363–4373.
- Krall, R.L.; Penry, J.K.; White, B.G.; Kupferberg, H.J.; Swinyard, E.A. Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia 1978, 19, 409–428.
- Reagan, L.P.; McKittrick, C.R.; McEwen, B.S. Corticosterone and phenytoin reduce neuronal nitric oxide synthase messenger RNA expression in rat hippocampus. Neuroscience 1999, 91, 211–219.
- Taylor, C.P. Voltage-gated Na+ channels as targets for anticonvulsant, analgesic and neuroprotective drugs. Curr. Pharm. Des. 1996, 2, 375–388.
- Eadie, M.J. Phenytoin. In The Treatment of Epilepsy, 2nd ed.; Shorvon, S., Perucca, E., Fish, D., Dodson, E., Eds.; Blackwell Publishing: Oxford, UK, 2004; pp. 475–488.
- Brendstrup, L.; Hjelt, K.; Petersen, K.E.; Petersen, S.; Andersen, E.A.; Daugbjerg, P.S.; Stagegaard, B.R.; Nielsen, O.H.; Vejlsgaard, R.; Schou, G.; et al. Nitrofurantoin versus trimethoprim prophylaxis in recurrent urinary tract infection in children. A randomized, double-blind study. Acta Paediatr. Scand. 1990, 79, 1225–1234.
- D’Arcy, P.F. Nitrofuratoin. Drug Intell. Clin. Pharm. 1985, 19, 540–547.
- Richards, W.A.; Riss, E.; Kass, E.H.; Finland, M. Nitrofurantoin: Clinical and laboratory studies in urinary tract infections. AMA Arch. Intern. Med. 1955, 96, 437–450.
- Sarges, R.; Howard, H.R.; Kelbaugh, P.R. Synthesis of optically active spirohydantoins by asymmetric induction. Hydantoin formation from amino nitriles and chlorosulfonyl isocyanate. J. Org. Chem. 1982, 47, 4081–4085.
- Cohen, R.A.; Hennekens, C.H.; Christen, W.G.; Krolewski, A.; Nathan, D.M.; Peterson, M.J.; LaMotte, F.; Manson, J.E. Determinants of retinopathy progression in type 1 diabetes mellitus. Am. J. Med. 1999, 107, 45–51.
- Schmidt, R.E.; Plurad, S.B.; Coleman, B.D.; Williamson, J.R.; Tilton, R.G. Effects of sorbinil, dietary myo-inositol supplementation, and insulin on resolution of neuroaxonal dystrophy in mesenteric nerves of streptozocin-induced diabetic rats. Diabetes 1991, 40, 574–582.
- Krause, T.; Gerbershagen, M.U.; Fiege, M.; Weisshorn, R.; Wappler, F. Dantrolene—A review of its pharmacology, therapeutic use and new developments. Anaesthesia 2004, 59, 364–373.
- Dorian, P.; Borggrefe, M.; Al-Khalidi, H.R.; Hohnloser, S.H.; Brum, J.M.; Tatla, D.S.; Brachmann, J.; Myerburg, R.J.; Cannom, D.S.; van der Laan, M.; et al. Placebo-controlled, randomized clinical trial of azimilide for prevention of ventricular tachyarrhythmias in patients with an implantable cardioverter defibrillator. Circulation 2004, 110, 3646–3654.
- Lacroix, L.; Laurent, M.; Buys, M. Iprodione. In Analytical Methods for Pesticides and Plant Growth Regulators: Vol. II.; Zweig, G., Sherma, J., Eds.; Academic Press: London, UK, 1980; pp. 247–261.
- Shiozaki, M. Synthesis of hydantocidin and C-2-thioxo-hydantocidin. Carbohydr. Res. 2001, 335, 147–150.
- Shiozaki, M. Syntheses of hydantocidin and C-2-thioxohydantocidin. Carbohydr. Res. 2002, 337, 2077–2088.
- Renard, A.; Lhomme, J.; Kotera, M. Synthesis and properties of spiro nucleosides containing the barbituric acid moiety. J. Org. Chem. 2002, 67, 1302–1307.
- Walter, M.W. Structure-based design of agrochemicals. Nat. Prod. Rep. 2002, 19, 278–291.
- Nakajima, M.; Itoi, K.; Takamatsu, Y.; Kinoshita, T.; Okazaki, T.; Kawakubo, K.; Shindo, M.; Honma, T.; Tohjigamori, M.; Haneishi, T. Hydantocidin: A new compound with herbicidal activity from Streptomyces hygroscopicus. J. Antibiot. 1991, 44, 293–300.
- Bichard, C.J.F.; Mitchel, E.P.; Wormald, M.R.; Watson, K.A.; Johnson, L.N.; Zographos, S.E.; Koutra, D.D.; Oikonomakos, N.G.; Fleet, G.W.J. Potent inhibition of glycogen phosphorylase by a spirohydantoin of glucopyranose: First pyranose analogues of hydantocidin. Tetrahedron Lett. 1995, 36, 2145–2148.
- Ösz, E.; Somsák, L.; Szilágyi, L.; Kovács, L.; Docsa, T.; Tóth, B.; Gergely, P. Efficient inhibition of muscle and liver glycogen phosphorylases by a new glucopyranosylidene-spiro-thiohydantoin. Bioorg. Med. Chem. Lett. 1999, 9, 1385–1390.
- Ware, E. The chemistry of the hydantoins. Chem. Rev. 1950, 46, 403–470.
- Bateman, J.H. Hydantoin and derivatives. In Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed.; Grayson, M., Eckroth, D., Eds.; Wiley-Interscience: New York, NY, USA, 1980; Volume 12, pp. 692–711.
- López, C.A.; Trigo, G.G. The chemistry of hydantoins. Adv. Heterocycl. Chem. 1985, 38, 177–228.
- Meusel, M.; Gütschow, M. Recent developments in hydantoin chemistry: A review. Org. Prep. Proced. Int. 2004, 36, 391–443.
- Konnert, L.; Lamaty, F.; Martinez, J.; Colacino, E. Recent advances in the synthesis of hydantoins: The state of the art of a valuable scaffold. Chem. Rev. 2017, 117, 13757–13809.
- Marqués-López, E.; Herrera, R.P. Bucherer–Bergs and Strecker multicomponent reactions. In Multicomponent Reactions: Concepts and Applications for Design and Synthesis; Herrera, R.P., Marqués-López, E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 331–357.




