The influence of dietary choline availability on cognition is currently being suggested by animal and human studies which have focused mainly on the early developmental stages. The available rodent (rats and mice) research published during the last two decades provides compelling support for the relevance of dietary choline in cognition. The beneficial effects of choline supplementation are more evident in recognition rather than in spatial memory tasks when assessing nonpathological samples whilst these effects extend to other relational memory tasks in neuropathological models.
1. Introduction
The interest for improving cognitive functions by dietary supplementation has increased over the last decades. However, the evaluation of human studies is hindered by the difficulty of controlling a number of social, educational and economic factors. It is also difficult because a wide variety of both supplemented diets with various combinations of ingredients and behavioral indexes are used
[1]. Even though animal studies can overcome some of these difficulties regarding the selection of the specific dietary intervention and the control of confounding variables, they often lack a systematic approach based on a deep knowledge of the cognitive functional architecture. In fact, cognition is a poorly defined term which includes a variety of functions such as attention, learning and memory. It has been pointed out that this makes it difficult to draw conclusions based on comparisons between different studies
[2], and it is necessary to define the processes assessed by a particular behavioral test taking into account the underlying theoretical framework
[3].
In animal studies, cognition refers to the performance in learning tasks that, in addition to memory, also require the contribution of several processes such as attention, motivation, arousal and emotion. With respect to memory, independent mechanisms have been proposed for working memory, which consists of the ability to temporally hold and manipulate a limited amount of information. In rodents, working memory is assessed in tasks relying on the information available within a testing session but not between sessions
[4], and this has been related with the prefrontal cortex function
[5], although other brain areas cannot be excluded. Regarding long-term memory, a dissociation between hippocampal-dependent declarative/relational memory versus nondeclarative forms of memory which depend on a variety of different brain systems is widely accepted (
[6], for a review). In rodents, spatial learning tasks are frequently used to assess declarative memory. To solve a navigation task requires hippocampal integrity, but other nonhippocampal-dependent strategies based on simple associative learning or habit formation may also be used if individual cues signal the target location. Context learning and other learning tasks including contextual information also involve relational memory requiring the hippocampal involvement. Likewise, the ability to remember a previously exposed item is assessed in rodents in the object recognition memory task. This task requires the integrity of brain regions adjacent to the hippocampus, such as the perirhinal cortex or even the hippocampus, if the procedure involves contextual or temporal information
[7]. Moreover, a brain region might contribute to various forms of learning. In fact, although the amygdala is critical for fear conditioning in rodents
[8], it also plays a role in declarative and nondeclarative memory, provided that emotionally arousing learning tasks are used. Therefore, the evaluation of the diet–cognition relationship in animal research needs to take into account the analysis of the learning and memory tasks used.
Choline, which is among the micronutrients included in supplemented diets, has been previously related to cognition. Choline is an essential nutrient requiring appropriate dietary levels to complement the endogenous synthesis in the liver and brain
[9]. It plays a role in maintaining cell membranes and myelination throughout the synthesis of phospholipids, as the precursor of acetylcholine affects cholinergic neurotransmission and it contributes to epigenetic changes by the methyl metabolism through betaine and the homocysteine reduction
[10]. In particular, the basal forebrain cholinergic system plays a relevant role in attentional modulation of the frontoparietal cortex, as well as in the hippocampal memory functions. In addition, cholinergic neurotransmission in the amygdala is involved in anxiety-related tasks. Figure 1 shows the main functions of dietary choline in the nervous system. In addition to its role in the maintenance of cell membranes and epigenetic processes, its influence on brain acetylcholine levels has been associated with different cognitive processes mediated by several brain areas (amygdala, hippocampus, prefrontal cortex, etc.) and brain circuits.
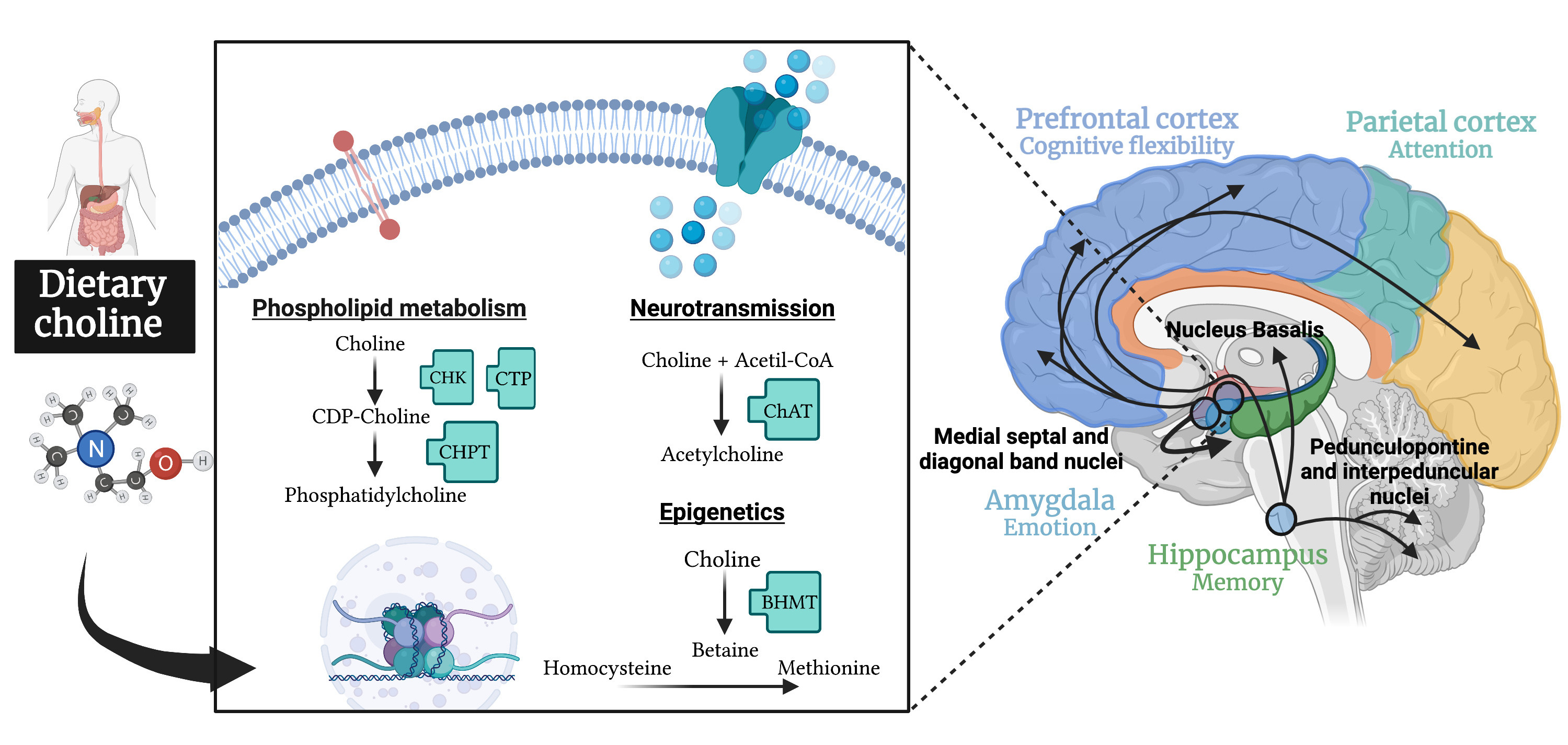
Figure 1. Schematic diagram depicting the biological pathways by which dietary choline supply can influence the brain function related to cognition. Most of the available evidence focuses on the role of choline as a precursor of the widespread neurotransmitter acetylcholine associated with the modulation of brain areas related with attention, memory, behavioral flexibility and emotion. Emerging evidence highlights the potential relevance of its contribution to maintain cell membranes through the phospholipid synthesis and to the epigenetic changes associated with cognition as a methyl donor. Abbreviations: BHMT, betaine homocysteine-methyltransferase; CDP-choline, cytidine 50-diphosphocholine; ChAT, choline acetyltransferase; CHK, choline kinase; CHPT cholinephosphotransferase; CTP, phosphocholine cytidylyltransferase.
Created with BioRender.com.
2. Dietary Choline Impact on Cognition
2.1. General Description of Data
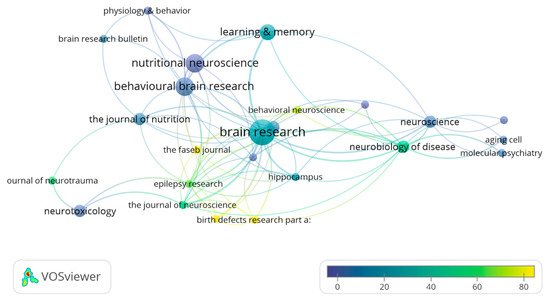
Regarding the bibliographic characteristics of the 44 articles included in this systematic review, they have been published in 25 different scientific journals. Amongst them, Brain Research with 7 papers published can be highlighted, followed by Nutritional Neuroscience (4 papers) and Behavioral Brain Research (4 papers). Moreover, the Brain Research papers received a total of 182 citations, followed by Neurotoxicology and Teratology (123 citations in 2 articles) and Birth Defects Research part A: Clinical and Molecular Teratology (106 citations in only 1 article published) (Figure 2).
Figure 2. Network data map of the scientific journals in which the articles have been published. Point size represents the number of published articles. The colors represent the number of citations of each article. The lines indicated the links between journals based on crosscitations.
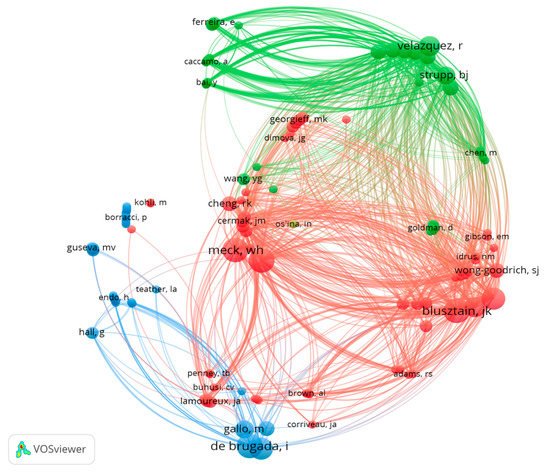
A total of 146 authors have been involved in the publication of these 44 articles. Jan Krzystof Blusztajn, Christina L. Williams, Isabel de Brugada and Warren H. Meck are the authors with the most articles published on the topic (7 each). The analysis of the number of publications per author and their co-authorship shows three large groups, represented by JK. Blusztajn, I. de Brugada and Ramón Velazquez (Figure 3).
Figure 3. Network data map of the articles’ authorship indicating research groups and collaborations. Point size represents the number published articles. The colors and lines show clusters based on coauthorship.
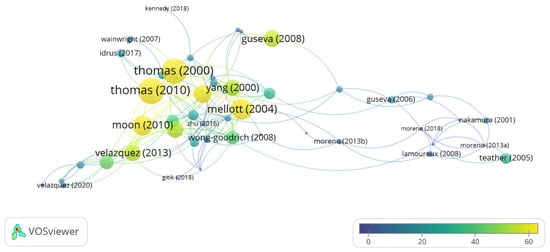
Taking into account the total number of citations, the three most cited authors are JK. Blusztain (332 citations, average of 47.4 per paper), CL. Williams (258 citations, average of 36.8 per paper) and Jennifer D. Thomas (229 citations, average of 76.3). Moreover, of the 44 articles included in our database the three most cited articles are Thomas et al. 2010 (106 citations), followed by Thomas et al. 2000 (104 citations) and Mellot et al. 2004 (79 citations) (Figure 4). A total of 1123 authors have been cited in the 44 papers selected; the three with the most citations are WH Meck (146), Steven H. Zeisel (57) and JD Thomas (40).
Figure 4. Network data map of the articles’ citations. The point size and color represent the number of citations received. The lines represent the number of co-citations.
2.2. Animals: Strain, Sex and Age
The majority of the studies (35 of the 44) used rats, mainly Sprague-Dawley (n = 23) and Wistar (n = 9). Mice models were used exclusively to explore the effect of supplementation on pathologies. All the mice studies except one, were transgenic models of different conditions such as APP/PS1 mice for Alzheimer’s disease, Ts65Dn mice for Down’s syndrome and BTBR T + Itpr3tf/J mice for autism. Gitik et al. 2018 assessed the effect of choline supplementation on C57BL/6J mice exposed to nicotine during adolescence.
Regarding sex, 8 of the 44 studies used both males and females, whilst in only two studies, all the subjects were females. The rest of the studies (n = 33) included only males, and only one did not specify the sex.
The age at which the animals were tested varied amongst the studies. This factor is of relevance when interpreting the results. Thus, although most of the results were obtained in adult animals, the age at testing varies from 2 to 24 months. The majority of these studies used young-adult 2–7 month old subjects, but subjects older than 8 months could be considered mature-adult and those older than 20 months as aged. A few studies investigated the effect of prenatal interventions on the cognitive abilities of preweaning or early postweaned and adolescent subjects about 28–42 days of age according to the strict adolescence criteria based on behavioral discontinuities.
2.3. Choline Supply: Administration Procedure and Dose
The great majority of the revised studies investigated the effect of choline supplementation supplied either in dry diet or diluted in water. Eight of them also included a choline-deficient group for comparison. The majority of the studies found delivered choline supplementation in palatable dry diets. Most of them applied choline chloride supplemented diets containing about 4.5 times the choline content of the standard diets. All of them, except who used a supplementation of 9 g/kg versus 2 g/kg in the standard diet, compared 5g/kg versus 1.1 g/kg.
2.4. Choline Intervention: Timing and Duration
Most of the studies applied choline supplementation or deprivation early during development, either prenatally or during infancy and adolescence. With regard to the prenatal interventions, several studies covered either particular gestational periods or the entire gestation period, and nine studies even continued the prenatal treatment throughout the postnatal period. Among the latter, some continued the intervention during the first postnatal week, but the rest covered the entire gestation and the postnatal period until weaning.
These long interventions prevent conclusions on the specific developmental processes involved in the results obtained. However, the studies focused on specific prenatal periods coincide in the duration and timing of the choline intervention since nearly all cover a 7 day period from the embryonic days (ED) 12–17, which coincides with the origin of the basal forebrain cholinergic neurons and critical changes in the hippocampal development.
A different picture appears with regard to postnatal interventions since they vary in duration from 14 to 270 days. In spite of this, we have identified that the most used durations are those around 30 days and 90 days, but longer durations of several months have also been applied.
2.5. Behavioral Tasks and Processes Targeted
The studies we have investigated have applied a bulk of procedures for assessing cognition and emotion in combination with other processes, such as motor performance and social interaction in rodents. Although it is difficult to dissociate the processes involved in a given procedure and the same task might be modified in order to explore different processes, there are specialized behavioral tests such as the rotarod intended for evaluation of motor coordination and balance, the open-field, the elevated plus maze and the forced swim test for emotionality/anxiety and the three-chamber test for social interaction.
Regarding cognition, most of the studies included in this review have assessed hippocampal-dependent declarative memory applying procedures to induce relational learning in the Morris water maze, the radial version of the water maze and the Barnes maze.
The novel object recognition task is also widely accepted as a test of declarative recognition memory but both the memory processes and the neural circuit involved seem to depend on the procedure applied.
With respect to nondeclarative memory, different learning tasks are included in the studies identified. Associative learning has been assessed in classical conditioning procedures of fear conditioning, active avoidance, passive avoidance, taste aversion learning, context aversion, context-dependent conditioning and extinction and visuospatial discrimination learning. Instrumental learning with varying reinforcement schedules in operant chambers has been used to assess temporal processing. Procedures to evaluate attention have been applied in both classical conditioning and operant conditioning.
Although the search terms of this review focused on those studies aimed at evaluating the effect of dietary choline availability on cognition, some of the identified studies assessed motionality/anxiety in order to explore different behavioral domains. Various tests have been used in the studies reviewed. The open-field test has been used to assess anxiety-like behaviors such as low exploratory locomotion and avoidance of the center area thus reducing the exploration to the area close to the walls, a behavioral pattern called thigmotaxis. The elevated plus maze is also a behavioral test of anxiety. Lastly, the forced swim test assesses behavioral despair as the animal is placed in a container full of water. Since it represents an inescapable situation, low latency to cease attempts to escape and immobility are considered indexes of depressive-like behavior. Other less frequent measures of anxiety are marble burying and the light–dark test.
Whilst no effect was seen in cognitive performance assessed in the Morris water maze, early supplementation decreased anxiety-like behavior in the open-field and behavioral despair in the forced swim test. Thus, adult rats supplemented either prenatally or during adolescence but not during adulthood increased exploration and time spent in the center of the open-field test while prenatal supplementation increased mobility in the forced swim test. Accordingly, prenatal choline supplementation (ED 12–17) attenuated, but did not prevent, the age-related decline in exploration of the open field. This is consistent with a beneficial effect of a 9 month adult period of choline supplementation in the open field test, the elevated plus maze and the light–dark test reported in the model of age-related dementia APP/PS1 mic.
3. Discussion
A general overview of the results reported by the rodent studies reviewed confirms a beneficial effect of choline supplementation which is more evident in those tasks requiring long-term relational memory and those conditions associated with immature or impaired cognitive functions. Interestingly, except in one case, no effect of choline supplementation on the standard Morris water-maze task has been reported in nonpathological conditions, whilst enhanced performance in recognition memory tasks was obtained in studies performed by different laboratories with different retention intervals. Nonetheless, the improvement of spatial learning and memory arises in supplemented rodent models of pathological conditions, such as the APP/PS1 mice model of Alzheimer’s disease, the Ts65Dn mice model of Down’s syndrome, traumatic brain injury, impoverished environment and seizure inducing doses of pilocarpine or kainic acid. Prenatal choline supplementation also accelerated the development of both the ability to perform the relational water maze task and to recognize a familiar object after a 24 h delay. Consistent with a selective beneficial effect of choline supplementation on learning tasks involving relational memory, in most of the cases, the improvement is also found in other relational learning tasks that include contextual and temporal information such as context-dependent extinction, contextual fear conditioning, context aversion and peak-interval timing. On the contrary, no effect has been found in appetitive associative learning and conditioned inhibition, auditory fear conditioning and active avoidance learning. This does not support our hypothesis because this is often interpreted as a selective effect of choline supplementation on learning tasks requiring hippocampal-dependent relational memory. However, the selective effect of choline supplementation on specific tasks can be explained either by the level of task difficulty so that a ceiling effect could mask the impact of supplementation in the easier tasks or by a selective effect of supplementation on specific brain systems.
The results obtained in rodents do not permit us to draw conclusions regarding the value of dietary choline interventions in humans, but they point to critical issues to be considered in human studies such as choline dosage, timing and duration of the intervention, as well as the type of cognitive assessment applied. The finding that dietary choline has been shown to be more efficient in pathological conditions suggests the possibility of easy and noninvasive interventions which deserve to be assessed in clinical trials.