
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shyam S Chaurasia | + 1186 word(s) | 1186 | 2021-07-14 11:35:23 | | | |
| 2 | Peter Tang | + 1 word(s) | 1187 | 2021-07-26 05:21:22 | | |
Video Upload Options
Small Leucine-Rich Proteoglycans (SLRPs) are key extracellular matrix proteins that play a role in many fundamental biological processes involved in the maintenance of retinal homeostasis.
1. Introduction
2. Classification of Retinal SLRPs
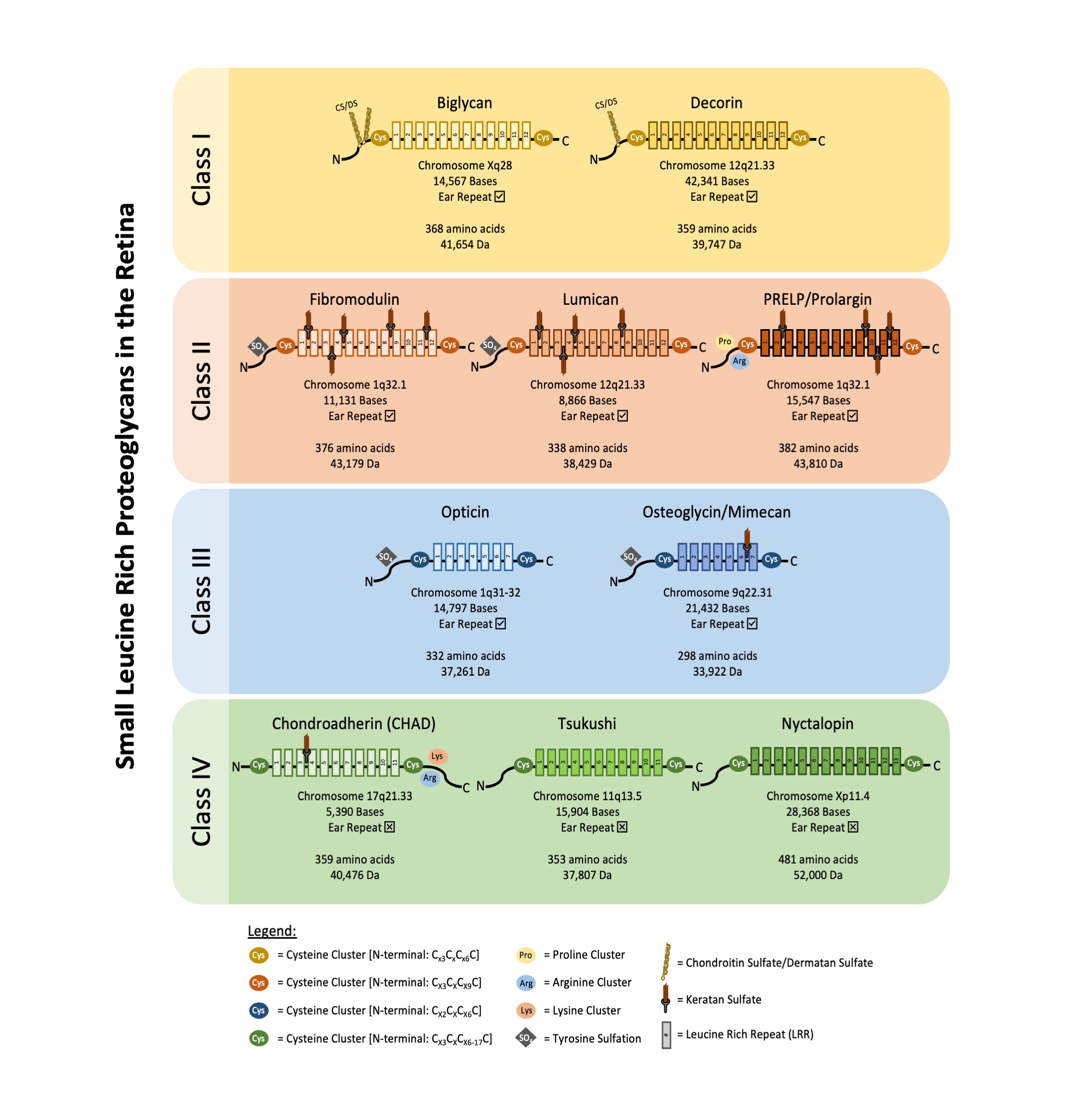
SLRPs can be broadly divided into five distinct classes based on their number of LRRs, amino acid residues at the N-terminus and their chromosomal organization. In canonical classes I–III, a capping motif, comprising of two terminal LRR and an “ear repeat”, can be found. These ear repeats maintain the protein core’s structural conformation and influences its ligand binding ability [5]. On the other hand, Class IV and V SLRPs do not have ear repeats [6]. Figure 1 summarizes the gene–protein information for each SLRP that has been found in the retina. Table 1 summarizes the distribution of known SLRPs in the retina.
|
Biglycan |
Decorin |
Fibromodulin |
Lumican |
PRELP/ Prolargin |
Opticin |
Osteoglycin/ Mimecan |
Chondro-adherin (CHAD) |
Tsukushi |
Nyctalopin |
|
|---|---|---|---|---|---|---|---|---|---|---|
|
ILM |
Human [7] |
Human [7] |
Human [7] |
Human [7] |
Human [7] |
Human [7] |
Human [7] |
|||
|
NFL |
Human [7], Mice [8] |
Human [7], Mice [8], Rat [9] |
Human [7], Mice [8] |
Human [7] |
Human [7] |
Human [7] |
Human [7] |
|||
|
GCL |
Human [7], Mice [8] |
Human [7], Mice [8], Rat [9] |
Human [7], Mice [8] |
Human [7], Mice [10] |
Human [7] |
Human [7] |
Human [7] |
Rat [12], Chick [13] |
||
|
IPL |
Human [7], Mice [8] |
Human [7], Mice [8], Rat [9] |
Human [7], Mice [8] |
Human [7], Mice [10] |
Human [7] |
Human [7] |
Human [7] |
Mice [14] |
||
|
INL |
Human [7], Mice [8] |
Human [7], Mice [8], Rat [9] |
Human [7], Mice [8] |
Human [7], Mice [10] |
Human [7] |
Human [7] |
Human [7] |
Mice [16] |
Rat [12], Chick [13] |
|
|
OPL |
Human [7], Mice [8] |
Human [7], Mice [8], Rat [9] |
Human [7], Mice [8] |
Human [7], Mice [10] |
Human [7] |
Human [7] |
Human [7] |
Mice [14] |
Mice [12], Rat [12], |
|
|
ONL |
Human [7] |
Human [7], Mice [8], Rat [9] |
Human [7], Mice [8] |
Human [7] |
Human [7], Mice [17] |
Human [7] |
Human [7] |
|||
|
PRL |
Human [7] |
Human [7], Mice [8], Rat [9] |
Human [7], Mice [8] |
Human [7] |
Human [7] |
Human [7] |
Human [7] |
Mice [14] |
Human [11] |
|
|
IPM |
Rat [18] |
|||||||||
|
RPE |
Human [7] |
Mice [17] |
Human [7], Canine [19] |
ILM inner limiting membrane; NFL nerve fiber layer; GCL ganglion cell layer; IPL inner plexiform layer; INL inner nuclear layer; OPL outer plexiform layer; ONL outer nuclear layer; PRL photoreceptor layer; IPM interphotoreceptor matrix; RPE retinal pigmented epithelium.
3. Class I Retinal SLRPs
4. Class II Retinal SLRPs
Fibromodulin, lumican and proline/arginine-rich end leucine-rich repeat protein (PRELP) are class II SLRPs.
5. Class III Retinal SLRPs
Opticin and osteoglycin/mimecan are class III SLRPs.
Opticin may play a role in vitreoretinal adhesion by modulating macromolecular surface coatings of collagen fibrils in the inner limiting membrane [35]. It also prevents pathological angiogenesis by acting as a competitive inhibitor to prevent the adhesion of endothelial cells to collagen [36]. This is supported by another study whereby opticin deficient OIR mice depicted a significant increase in preretinal neovascularization [37]. Opticin may also serve as a potential biomarker for neovascular AMD [38]. Additionally, it has been shown to bind and regulate growth factors in the chick retina [39].
Osteoglycin/mimecan has been found to interact with the leucine-rich protein, B7, which is expressed in the human cornea, iris, retina and sclera [40]. However, little is known about B7’s role in modulating retinal integrity.
6. Class IV Retinal SLRPs
Chondroadherin, tsukushi and nyctalopin are class IV SLRPs.
Chondroadherin (CHAD) has been detected in both human [7] and mouse [14] retina. However, its functional roles have yet to be determined.
Tsukushi is expressed in mice inner nuclear layer [16] and suggested to be involved in the regulation and proliferation of Müller glia. However, further evaluation will be required to determine its role in the retina.
Nyctalopin has been found to be associated with transient receptor potential melastatin 1 (TRPM1) [41] and mGluR6 [42]. They form a complex that enables fast signal transmissions for the ON-bipolar cell (BC) response. Another protein, LRIT3 has also been reported to interact with nyctalopin and is required for the localization of nyctalopin to ON BC dendritic tips [43]. Nyctalopin also regulates retinal activity required for the maintenance of axon terminal segregation in the dorsal–lateral geniculate nucleus [44]. Mutations in the nyctalopin gene can result in congenital stationary night blindness [45] and myopia [46].
7. Other SLRPs Unknown to the Retina
Apart from the 10 SLRPs discussed above, there are seven other SLRPs yet to be studied in the retina, namely, extracellular matrix 2 (ECM2) and asporin from class I; osteoadherin and keratocan from class II; epiphycan from class III; and podocan and podocan-like-protein-1 (Podnl1) from class V. Further evaluation to determine the role of SLRPs in the retina can potentially expand our understanding of complex retinal diseases and provide new opportunities for the treatment for the vision loss.
References
- Jonas, J.B.; Cheung, C.M.G.; Panda-Jonas, S. Updates on the epidemiology of age-related macular degeneration. Asia Pac. J. Ophthalmol. 2017, 6, 493–497.
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564.
- Retinopathy of Prematurity | National Eye Institute. Available online: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/retinopathy-prematurity (accessed on 6 April 2021).
- Holmström, G.; Tornqvist, K.; Al-Hawasi, A.; Nilsson, Å.; Wallin, A.; Hellström, A. Increased frequency of retinopathy of prematurity over the last decade and significant regional differences. Acta Ophthalmol. 2018, 96, 142–148.
- Park, H.; Huxley-Jones, J.; Boot-Handford, R.P.; Bishop, P.N.; Attwood, T.K.; Bella, J. LRRCE: A leucine-rich repeat cysteine capping motif unique to the chordate lineage. BMC Genomics 2008, 9, 599.
- Dellett, M.; Hu, W.; Papadaki, V.; Ohnuma, S. Small leucine rich proteoglycan family regulates multiple signalling pathways in neural development and maintenance. Dev. Growth Differ. 2012, 54, 327–340.
- Keenan, T.D.L.; Clark, S.J.; Unwin, R.D.; Ridge, L.A.; Day, A.J.; Bishop, P.N. Mapping the differential distribution of proteoglycan core proteins in the adult human retina, choroid, and sclera. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7528–7538.
- Ali, S.A.M.; Hosaka, Y.Z.; Masoto, U. Expression of small leucine-rich proteoglycans in the developing retina and kainic acid-induced retinopathy in ICR mice. J. Vet. Med. Sci. 2011, 1852, 1833–1845.
- Inatani, M.; Tanihara, H.; Honjo, M.; Hangai, M.; Kresse, H.; Honda, Y. Expression of proteoglycan decorin in neural retina. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1783–1791.
- Liu, L.; Wu, J.; Zhou, X.; Chen, Z.; Zhou, G. The impact of visible light on the immature retina: A model of early light exposure in neonatal mice. Brain Res. Bull. 2012, 87, 534–539.
- Bech-Hansen, N.T.; Naylor, M.J.; Maybaum, T.A.; Sparkes, R.L.; Koop, B.; Birch, D.G.; Bergen, A.A.B.; Prinsen, C.F.M.; Polomeno, R.C.; Gal, A.; et al. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat. Genet. 2000, 26, 319–323.
- Pesch, K.; Zeitz, C.; Fries, J.E.; Münscher, S.; Pusch, C.M.; Kohler, K.; Berger, W.; Wissinger, B. Isolation of the mouse nyctalopin gene Nyx and expression studies in mouse and rat retina. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2260–2266.
- Bech-Hansen, N.T.; Cockfield, J.; Liu, D.; Logan, C.C. Isolation and characterization of the leucine-rich proteoglycan nyctalopin gene (cNyx) from chick. Mamm. Genome 2005, 16, 815–824.
- Tasheva, E.S.; Ke, A.; Conrad, G.W. Analysis of the expression of chondroadherin in mouse ocular and non-ocular tissues. Mol. Vis. 2004, 10, 544–554.
- Morgans, C.W.; Ren, G.; Akileswaran, L. Localization of nyctalopin in the mammalian retina. Eur. J. Neurosci. 2006, 23, 1163–1171.
- Ohta, K.; Ito, A.; Tanaka, H. Neuronal stem/progenitor cells in the vertebrate eye. Dev. Growth Differ. 2008, 50, 253–259.
- Birke, M.T.; Lipo, E.; Adhi, M.; Birke, K.; Kumar-Singh, R. AAV-Mediated expression of human PRELP inhibits complement activation, choroidal neovascularization and deposition of membrane attack complex in mice. Gene Ther. 2014, 21, 507–513.
- Kraljević Pavelić, S.; Klobučar, M.; Sedić, M.; Micek, V.; Gehrig, P.; Grossman, J.; Pavelić, K.; Vojniković, B. UV-induced retinal proteome changes in the rat model of age-related macular degeneration. Biochim. Biophys. Acta Mol. Basis Dis. 2015.
- Pellegrini, B.; Acland, G.M.; Ray, J. Cloning and characterization of opticin cDNA: Evaluation as a candidate for canine oculo-skeletal dysplasia. Gene 2002, 282, 121–131.
- Sallo, F.B.; Bereczki, E.; Csont, T.; Luthert, P.J.; Munro, P.; Ferdinandy, P.; Sántha, M.; Lengyel, I. Bruch’s membrane changes in transgenic mice overexpressing the human biglycan and apolipoprotein b-100 genes. Exp. Eye Res. 2009, 89, 178–186.
- Pinto, F.; Santos-Ferreira, L.; Pinto, M.T.; Gomes, C.; Reis, C.A. The extracellular small leucine-rich proteoglycan biglycan is a key player in gastric cancer aggressiveness. Cancers 2021, 13, 1330.
- Neill, T.; Schaefer, L.; Iozzo, R.V. Decorin: A guardian from the matrix. Am. J. Pathol. 2012, 181, 380–387.
- Csordas, G.; Santra, M.; Reed, C.C.; Eichstetter, I.; McQuillan, D.J.; Gross, D.; Nugent, M.A.; Hajnoczky, G.; Iozzo, R.V. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J. Biol. Chem. 2000, 275, 32879–32887.
- Iozzo, R.V.; Buraschi, S.; Genua, M.; Xu, S.Q.; Solomides, C.C.; Peiper, S.C.; Gomella, L.G.; Owens, R.C.; Morrione, A. Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling. J. Biol. Chem. 2011, 286, 34712–34721.
- Goldoni, S.; Humphries, A.; Nyström, A.; Sattar, S.; Owens, R.T.; McQuillan, D.J.; Ireton, K.; Iozzo, R.V. Decorin is a novel antagonistic ligand of the Met receptor. J. Cell Biol. 2009, 185, 743–754.
- Davies, J.E.; Tang, X.; Bournat, J.C.; Davies, S.J.A. Decorin promotes plasminogen/plasmin expression within acute spinal cord injuries and by adult microglia in vitro. J. Neurotrauma 2006, 23, 397–408.
- Begum, G.; O’neill, J.; Chaudhary, R.; Blachford, K.; Snead, D.R.J.; Berry, M.; Scott, R.A.H.; Logan, A.; Blanch, R.J. Altered decorin biology in proliferative vitreoretinopathy: A mechanistic and cohort study. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4929–4936.
- Du, S.; Wang, S.; Wu, Q.; Hu, J.; Li, T. Decorin inhibits angiogenic potential of choroid-retinal endothelial cells by downregulating hypoxia-induced Met, Rac1, HIF-1α and VEGF expression in cocultured retinal pigment epithelial cells. Exp. Eye Res. 2013, 116, 151–160.
- Wang, S.; Du, S.; Wu, Q.; Hu, J.; Li, T. Decorin prevents retinal pigment epithelial barrier breakdown under diabetic conditions by suppressing P38MAPK activation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2971–2979.
- Güler, S.D.; Balbaba, M.; Çolakoǧlu, N.; Bulmuş, Ö.; Ulaş, F.; Eröksüz, Y. Effect of Decorin and Bevacizumab on oxygen-induced retinopathy in rat models: A comparative study. Indian J. Ophthalmol. 2021, 69, 369–373.
- Sjöberg, A.; Önnerfjord, P.; Mörgelin, M.; Heinegård, D.; Blom, A.M. The extracellular matrix and inflammation: Fibromodulin activates the classical pathway of complement by directly binding C1q. J. Biol. Chem. 2005, 280, 32301–32308.
- Chakravarti, S.; Paul, J.; Roberts, L.; Chervoneva, I.; Oldberg, A.; Birk, D.E. Ocular and scleral alterations in gene-targeted lumican-fibromodulin double-null mice. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2422–2432.
- Saika, S.; Miyamoto, T.; Tanaka, S.I.; Tanaka, T.; Ishida, I.; Ohnishi, Y.; Ooshima, A.; Ishiwata, T.; Asano, G.; Chikama, T.I.; et al. Response of lens epithelial cells to injury: Role of lumican in epithelial-mesenchymal transition. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2094–2102.
- Happonen, K.E.; Fürst, C.M.; Saxne, T.; Heinegård, D.; Blom, A.M. PRELP protein inhibits the formation of the complement membrane attack complex. J. Biol. Chem. 2012, 287, 8092–8100.
- Ramesh, S.; Bonshek, R.E.; Bishop, P.N. Immunolocalisation of opticin in the human eye. Br. J. Ophthalmol. 2004, 88, 697–702.
- Le Goff, M.M.; Sutton, M.J.; Slevins, M.; Latif, A.; Humphries, M.J.; Bishop, P.N. Opticin exerts its anti-angiogenic activity by regulating extracellular matrix adhesiveness. J. Biol. Chem. 2012, 287, 28027–28036.
- Le Goff, M.M.; Lu, H.; Ugarte, M.; Henry, S.; Takanosu, M.; Mayne, R.; Bishop, P.N. The vitreous glycoprotein opticin inhibits preretinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2012, 53, 228–234.
- Nobl, M.; Reich, M.; Dacheva, I.; Siwy, J.; Mullen, W.; Schanstra, J.P.; Choi, C.Y.; Kopitz, J.; Kretz, F.T.A.; Auffarth, G.U.; et al. Proteomics of vitreous in neovascular age-related macular degeneration. Exp. Eye Res. 2016, 146, 107–117.
- Sanders, E.J.; Walter, M.A.; Parker, E.; Arámburo, C.; Harvey, S. Opticin Binds Retinal Growth Hormone in the Embryonic Vitreous. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5404–5409.
- Tasheva, E.S.; An, K.; Boyle, D.L.; Conrad, G.W. Expression and localization of leucine-rich B7 protein in human ocular tissues. Mol. Vis. 2005, 11, 452–460.
- Pearring, J.N.; Bojang, P.; Shen, Y.; Koike, C.; Furukawa, T.; Nawy, S.; Gregg, R.G. A role for nyctalopin, a small leucine-rich repeat protein, in localizing the TRP melastatin 1 channel to retinal depolarizing bipolar cell dendrites. J. Neurosci. 2011, 31, 10060–10066.
- Gregg, R.G.; Kamermans, M.; Klooster, J.; Lukasiewicz, P.D.; Peachey, N.S.; Vessey, K.A.; McCall, M.A. Nyctalopin expression in retinal bipolar cells restores visual function in a mouse model of complete X-linked congenital stationary night blindness. J. Neurophysiol. 2007, 98, 3023–3033.
- Hasan, N.; Pangeni, G.; Ray, T.A.; Fransen, K.M.; Noel, J.; Borghuis, B.G.; McCall, M.A.; Gregg, R.G. LRIT3 is required for nyctalopin expression and normal ON and OFF pathway signaling in the retina. eNeuro 2020, 7.
- Demas, J.; Sagdullaev, B.T.; Green, E.; Jaubert-Miazza, L.; McCall, M.A.; Gregg, R.G.; Wong, R.O.L.; Guido, W. Failure to Maintain Eye-Specific Segregation in nob, a Mutant with Abnormally Patterned Retinal Activity. Neuron 2006, 50, 247–259.
- Pusch, C.M.; Zeitz, C.; Brandau, O.; Pesch, K.; Achatz, H.; Feil, S.; Scharfe, C.; Maurer, J.; Jacobi, F.K.; Pinckers, A.; et al. The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nat. Genet. 2000, 26, 324–327.
- Pardue, M.T.; Faulkner, A.E.; Fernandes, A.; Yin, H.; Schaeffel, F.; Williams, R.W.; Pozdeyev, N.; Iuvone, P.M. High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Investig. Ophthalmol. Vis. Sci. 2008, 49, 706–712.




