Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stefan Rothenburg | + 1573 word(s) | 1573 | 2021-07-06 10:15:10 | | | |
| 2 | Vicky Zhou | Meta information modification | 1573 | 2021-07-24 02:03:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rothenburg, S. Poxviruses. Encyclopedia. Available online: https://encyclopedia.pub/entry/12371 (accessed on 07 February 2026).
Rothenburg S. Poxviruses. Encyclopedia. Available at: https://encyclopedia.pub/entry/12371. Accessed February 07, 2026.
Rothenburg, Stefan. "Poxviruses" Encyclopedia, https://encyclopedia.pub/entry/12371 (accessed February 07, 2026).
Rothenburg, S. (2021, July 23). Poxviruses. In Encyclopedia. https://encyclopedia.pub/entry/12371
Rothenburg, Stefan. "Poxviruses." Encyclopedia. Web. 23 July, 2021.
Copy Citation
Poxviruses possess a single, linear double-stranded DNA (dsDNA) genome, which ranges in size from 127 to 456 kb and encodes several hundred gene products. Unlike most other DNA viruses, poxviruses replicate exclusively within the cytoplasm of permissive cells.
poxviruses
vaccinia virus
pattern recognition receptors
PKR
RNase L
ZBP1
cGAS–STING
Toll-like receptors
inflammasome
1. Introduction
Members of the family Poxviridae can infect a diverse range of vertebrates and invertebrates, although some poxviruses have narrow host ranges and others have very broad host ranges [1]. Poxvirus infections have posed serious threats to both humans and animals worldwide [2]. Poxviridae is a large family of DNA viruses comprised of two subfamilies: Chordopoxvirinae and Entomopoxvirinae. There are currently 18 recognized genera of Chordopoxvirinae, which infect vertebrates, and 4 genera of Entomopoxvirinae, which infect invertebrates [1]. Poxviruses possess a single, linear double-stranded DNA (dsDNA) genome, which ranges in size from 127 to 456 kb and encodes several hundred gene products. Unlike most other DNA viruses, poxviruses replicate exclusively within the cytoplasm of permissive cells [3].
One of the best known poxviruses is variola virus (VARV), a member of the orthopoxvirus genus. VARV is the causative agent of human smallpox, which was one of the most devastating human diseases in history [4]. Despite the success of global smallpox eradication through a vaccination campaign led by the World Health Organization, other pathogenic poxviruses, such as monkeypox virus, cowpox viruses (CPXV), camelpox virus, tanapox virus, and capripoxviruses remain threats to human and animal health [1][2][5][6]. The most intensively studied poxviruses, such as vaccinia virus (VACV) and myxoma virus (MYXV), have proven to be excellent research models to study host innate recognition and virus–host protein interactions and have provided important insights into the fields of virology and immunology [7][8]. In turn, these fundamental insights have direct translational applications to improve the development of safer and more effective attenuated viral vectors for vaccines, cancer therapeutics, and other treatment modalities [9]. For example, in VACV inactivation of either IL-1β-binding protein or IL-18-binding protein, encoded by B15R and C12L, respectively, enhanced CD8+ T cell memory responses after immunization and improved the protection against virulent VACV WR challenge [10][11]. In addition, VACV has the genomic capacity to incorporate >25 kb of foreign DNA without noticeable impacts on viral replication. This capacity has been employed to genetically engineer VACV chimeras carrying multiple heterologous genes, as both polyvalent vaccines and treatment of various genetic diseases [12][13][14][15]. Multiple poxviruses are being investigated for use in oncolytic virotherapy. Myxoma virus (MYXV) is one such preclinical candidate oncolytic virus, and recombinant MYXV lacking various viral death modulator genes, such as M-T5 [16], M11 [17], M13 [18], and Serp2 [19], have enhanced anti-tumor activity that appears to be mediated through viral induction of programmed cell death rather than through viral replication (reviewed in [20]).
The recognition of viral pathogens and the host defense against them are provided by the innate and adaptive immune systems. The adaptive immune system is broadly comprised of antigen-specific CD8+ T cells, CD4+ helper T cells, and B cell antibody responses for specific, anamnestic protection against distinct pathogens [21][22][23]. Prior to the initiation of the adaptive immune response, pathogen-associated molecular patterns (PAMPs) derived from poxviruses, such as DNA and RNA, as well as envelope or core proteins, can be sensed by a diverse set of pattern recognition receptors (PRRs) to initiate the faster but less specific innate immune responses [24][25][26][27][28][29][30][31]. The innate immune response provides the first line of host defense and includes antiviral proteins that can lead to the direct elimination of viruses or induce the expression of type I interferons (IFNs), proinflammatory cytokines, chemokines, and other antiviral proteins [32][33]. These effector molecules mediate direct antiviral effects or orchestrate the adaptive immune response to contain poxvirus infections at various stages. In particular, type I IFNs, the hallmark effector of antiviral responses, are essential to initiate innate immunity and also to mediate the subsequent development of adaptive immunity against invading poxviruses [34]. In addition, type I IFNs upregulate the expression of hundreds of IFN-stimulated genes (ISGs) that directly influence protein synthesis, cell growth, and survival to establish an antiviral state [35][36]. Furthermore, multiple cytokines, such as interleukin-6 (IL-6), IL-12, and tumor necrosis factor-alpha (TNFα), can be induced during poxvirus infections which then act systemically to induce immune responses [37][38].
Over recent decades, substantial progress has been made in defining the roles of PRRs and the subsequent signaling pathways that are involved in the sensing of and response to poxviruses. IFN expression is transcriptionally regulated through activation of IFN regulatory factor (IRF) family members or coordinated activation of IRFs and nuclear factor kappa B (NF-κB) [39]. The stimulation of proinflammatory genes depends on activation of the transcription factors NF-κB and activator protein 1 (AP1) [40]. Despite the diversity of PRR ligands, many PRR-regulated signaling pathways share common downstream molecules, such as myeloid differentiation primary response gene 88 (MyD88) and Toll/interleukin-1 receptor domain-containing adapter-inducing interferon-β (TRIF). Thus, there is substantial crosstalk and overlap between the signaling cascades stimulated by different PRRs, which lead to IFN activation and also drive the production of other cytokines [41][42][43].
A broad spectrum of PRRs has been implicated in poxvirus recognition, including RNA sensors, cytosolic DNA sensors, multiple Toll-like receptors (TLRs), and components of the inflammasome. In order to establish successful infections in the face of this multi-pronged immune response, poxviruses evade host antiviral responses by expressing a variety of viral proteins. Those viral proteins interact with and antagonize the key components of these intracellular signal transduction pathways.
2. Inflammasome Recognition of Poxviruses and Poxvirus Antagonists
Inflammasomes are multiprotein signaling complexes responsible for the production of proinflammatory cytokines and the induction of pyroptosis, an inflammatory lytic programmed cell death, to halt viral replication and induce nearby cells to adopt antiviral states [44]. Inflammasome activation mediates the conversion of inactive precursor proteins pro-interleukin (IL)-1β and pro-IL-18 into the bioactive forms IL-1β and IL-18, which play important roles in host defense against a variety of bacterial, fungal, and viral infections [45][46]. Certain PRRs have been implicated in canonical inflammasome assembly, including NOD-like receptors and AIM2-like receptors [47][48]. After PAMP and cell damage-associated signal recognition, adaptors are recruited, such as apoptosis-associated speck-like protein (ASC) [49]. This process results in cytokine secretion and pyroptosis as proteolytically active caspases mediate the maturation and secretion of proinflammatory cytokines IL-1β and IL-18, while cleavage of gasdermin-D (GSDMD), a key pyroptotic substrate of inflammatory caspases, induces pyroptosis (Figure 1) [50]. Specifically, the inflammasome proteins NACHT, LRR, and PYD domains-containing protein (NLRP3) and AIM2 have been implicated in the recognition of poxvirus infections [29][30][51].
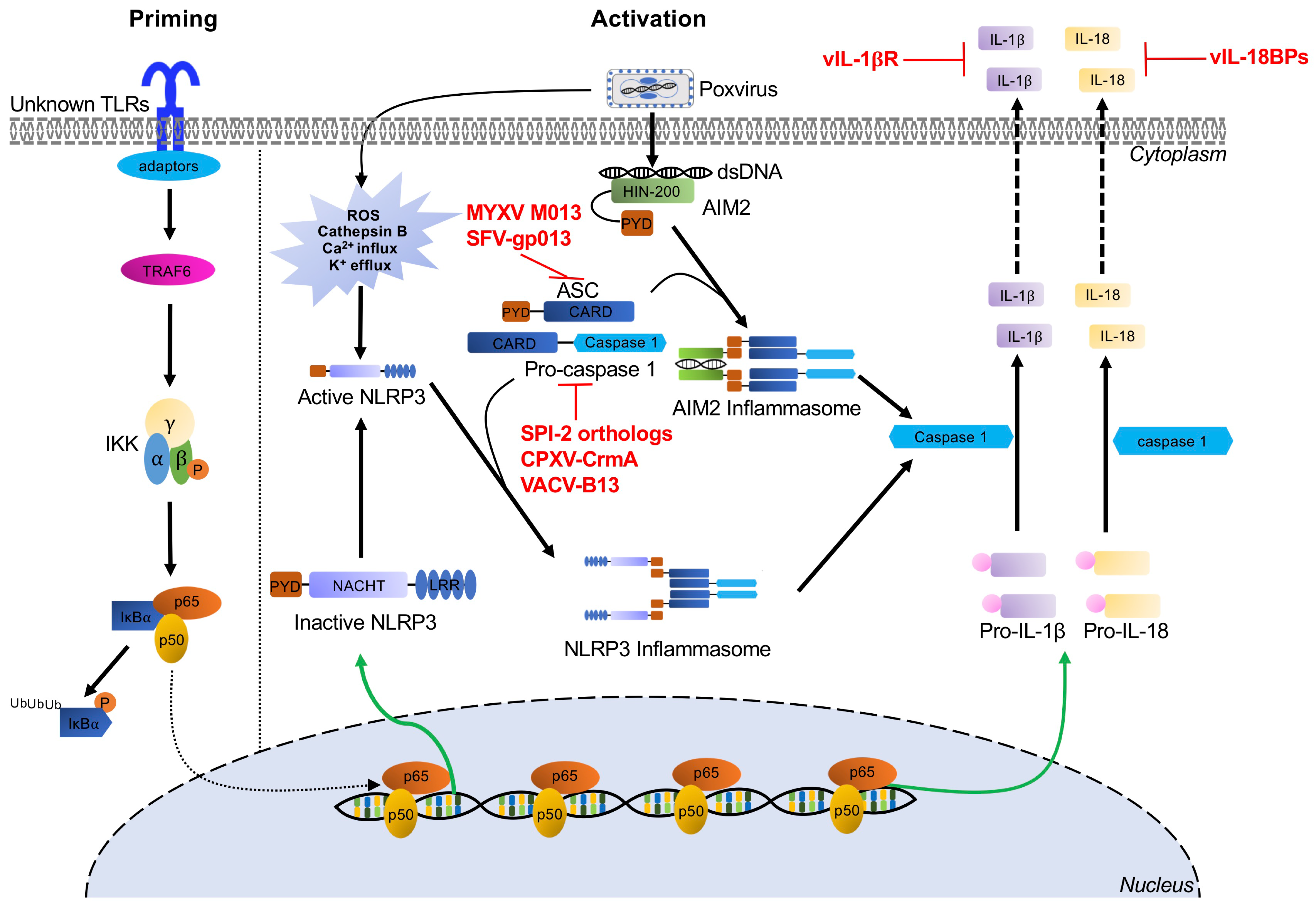
Figure 1. Inflammasome-mediated signaling pathways and poxvirus antagonists. NLRP3 and AIM2 inflammasome-mediated recognition of poxvirus infection and the priming and activation pathways for maturation and secretion of IL-1β and IL-18 effectors are indicated by black arrows. Poxviruses express several viral inhibitors or viral homologs of cellular proteins (shown in red) to interfere with these inflammasome pathways at different stages. Abbreviations used in this figure include AIM2: absent in melanoma 2; ASC: apoptosis-associated speck-like protein containing a CARD; CARD: caspase activating and recruiting domains; CPXV: cowpox virus; CrmA: cytokine response modifier A; dsDNA: double-stranded DNA; HIN-200: hematopoietic interferon-inducible nuclear proteins with a 200 amino acid repeat; IKKα: IκBα kinase α; IKKβ: IκBα kinase β; IKKγ: IκBα kinase γ; IL-18: interleukin-18; IL-1β: interleukin-1β; IκBα: inhibitor κBα; LRR: leucine-rich repeats; NF-κB: nuclear factor kappa B; NLRP3: NOD, LRR and pyrin domains-containing protein 3; NOD; nucleotide binding and oligomerization domain; p65/p50: NF-κB heterodimer p50/p65 subunit; PYD: pyrin domain; RFV: rabbit fibroma virus; ROS: reactive oxygen species; SPI-2: serine proteinase inhibitor 2; TLRs: Toll-like receptors; TRAF6: tumor necrosis factor receptor-associated factor 6; VACV: vaccinia virus.
3. Conclusions and Outlook
In this review, we have discussed multiple PRRs and their roles in sensing poxviruses infections and subsequently initiating innate immune responses. Individual PRRs have unique molecular mechanisms for sensing ligands and triggering antiviral responses via diverse adapters and effectors. Furthermore, redundancy, cooperation, and crosstalk among the various PRRs increase this complexity. This crosstalk and redundancy in immune pathways are also reflected in the viral antagonists, with multiple PRR pathways targeted by the same viral proteins. For example, E3 binds dsRNA and blocks the activation of PKR, OAS/RNase L, and TLRs [52][53][54][55]. However, it is currently an open question as to whether these multiple functions, for E3 and other antagonists, are truly redundant, or if there are situational differences in the activity of E3 against various pathways. Thus, the overall picture of host recognition of poxviruses is multifaceted and far from clear. Investigation of the cooperation and crosstalk between PRRs will help define the innate immune network(s) elicited by these various PRRs both individually and in combination.
On the other side of this battle, the specific poxviral ligands are not yet known for all of these PRRs. Identification of these ligands, characterization of their structures or motifs, and their interactions with the sensors themselves are necessary to reveal how the signal is initiated by receptors during infection of poxviruses. Furthermore, the molecular mechanisms underlying cell type-specific or virus-specific recognition and signaling by certain PAMPs during poxvirus infections are still largely unknown. Finally, it is becoming more apparent that the diversity in the poxvirus family is also reflected by the range of activities discovered for individual viral gene orthologs, and their implications for viral host range and virulence. This observation is most strongly supported for rapidly evolving genes such as viral immune regulators and their implications for virus host range and virulence [4][56][57][58]. Therefore, the data presented in this view representing the response to a handful of poxviruses may not capture the full spectrum of poxvirus responses, and viral orthologs from other poxviruses should also be examined.
References
- Haller, S.L.; Peng, C.; McFadden, G.; Rothenburg, S. Poxviruses and the evolution of host range and virulence. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2014, 21, 15–40.
- Silva, N.I.O.; de Oliveira, J.S.; Kroon, E.G.; Trindade, G.D.S.; Drumond, B.P. Here, There, and Everywhere: The Wide Host Range and Geographic Distribution of Zoonotic Orthopoxviruses. Viruses 2020, 13, 43.
- Moss, B. Poxvirus DNA replication. Cold Spring Harb. Perspect. Biol. 2013, 5.
- McFadden, G. Poxvirus tropism. Nat. Rev. Microbiol. 2005, 3, 201–213.
- Durski, K.N.; McCollum, A.M.; Nakazawa, Y.; Petersen, B.W.; Reynolds, M.G.; Briand, S.; Khalakdina, A. Emergence of monkeypox in West Africa and Central Africa, 1970–2017. Relev. Epidemiol. Hebd. 2018, 93, 125–132.
- Tuppurainen, E.S.M.; Venter, E.H.; Shisler, J.L.; Gari, G.; Mekonnen, G.A.; Juleff, N.; Lyons, N.A.; De Clercq, K.; Upton, C.; Bowden, T.R.; et al. Review: Capripoxvirus Diseases: Current Status and Opportunities for Control. Transbound. Emerg. Dis. 2017, 64, 729–745.
- Spiesschaert, B.; McFadden, G.; Hermans, K.; Nauwynck, H.; Van De Walle, G.R. The current status and future directions of myxoma virus, a master in immune evasion. Vet. Res. 2011, 42, 76.
- Smith, G.L.; Benfield, C.T.O.; Maluquer de Motes, C.; Mazzon, M.; Ember, S.W.J.; Ferguson, B.J.; Sumner, R.P. Vaccinia virus immune evasion: Mechanisms, virulence and immunogenicity. J. Gen. Virol. 2013, 94, 2367–2392.
- Albarnaz, J.D.; Torres, A.A.; Smith, G.L. Modulating Vaccinia Virus Immunomodulators to Improve Immunological Memory. Viruses 2018, 10, 101.
- Staib, C.; Kisling, S.; Erfle, V.; Sutter, G. Inactivation of the viral interleukin 1beta receptor improves CD8+ T-cell memory responses elicited upon immunization with modified vaccinia virus Ankara. J. Gen. Virol. 2005, 86, 1997–2006.
- Falivene, J.; Del Médico Zajac, M.P.; Pascutti, M.F.; Rodríguez, A.M.; Maeto, C.; Perdiguero, B.; Gómez, C.E.; Esteban, M.; Calamante, G.; Gherardi, M.M. Improving the MVA vaccine potential by deleting the viral gene coding for the IL-18 binding protein. PLoS ONE 2012, 7, e32220.
- Smith, G.L.; Moss, B. Infectious poxvirus vectors have capacity for at least 25 000 base pairs of foreign DNA. Gene 1983, 25, 21–28.
- Liu, R.; Americo, J.L.; Cotter, C.A.; Earl, P.L.; Erez, N.; Peng, C.; Moss, B. One or two injections of MVA-vectored vaccine shields hACE2 transgenic mice from SARS-CoV-2 upper and lower respiratory tract infection. Proc. Natl. Acad. Sci. USA 2021, 118.
- Wyatt, L.S.; Xiao, W.; Americo, J.L.; Earl, P.L.; Moss, B. Novel Nonreplicating Vaccinia Virus Vector Enhances Expression of Heterologous Genes and Suppresses Synthesis of Endogenous Viral Proteins. MBio 2017, 8.
- Sutter, G.; Moss, B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 1992, 89, 10847–10851.
- Werden, S.J.; McFadden, G. The role of cell signaling in poxvirus tropism: The case of the M-T5 host range protein of myxoma virus. Biochim. Biophys. Acta 2008, 1784, 228–237.
- Pisklakova, A.; McKenzie, B.; Zemp, F.; Lun, X.; Kenchappa, R.S.; Etame, A.B.; Rahman, M.M.; Reilly, K.; Pilon-Thomas, S.; McFadden, G.; et al. M011L-deficient oncolytic myxoma virus induces apoptosis in brain tumor-initiating cells and enhances survival in a novel immunocompetent mouse model of glioblastoma. Neuro. Oncol. 2016, 18, 1088–1098.
- Johnston, J.B.; Barrett, J.W.; Nazarian, S.H.; Goodwin, M.; Ricciuto, D.; Wang, G.; McFadden, G. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity 2005, 23, 587–598.
- Urbasic, A.S.; Hynes, S.; Somrak, A.; Contakos, S.; Rahman, M.M.; Liu, J.; MacNeill, A.L. Oncolysis of canine tumor cells by myxoma virus lacking the serp2 gene. Am. J. Vet. Res. 2012, 73, 1252–1261.
- Rahman, M.M.; McFadden, G. Oncolytic Virotherapy with Myxoma Virus. J. Clin. Med. 2020, 9, 171.
- Burshtyn, D.N. NK cells and poxvirus infection. Front. Immunol. 2013, 4, 7.
- Medeiros-Silva, D.C.; Dos Santos Moreira-Silva, E.A.; de Assis Silva Gomes, J.; da Fonseca, F.G.; Correa-Oliveira, R. CD4 and CD8 T cells participate in the immune memory response against Vaccinia virus after a previous natural infection. Results Immunol. 2013, 3, 104–113.
- Pütz, M.M.; Midgley, C.M.; Law, M.; Smith, G.L. Quantification of antibody responses against multiple antigens of the two infectious forms of Vaccinia virus provides a benchmark for smallpox vaccination. Nat. Med. 2006, 12, 1310–1315.
- Zhu, J.; Martinez, J.; Huang, X.; Yang, Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood 2007, 109, 619–625.
- Hutchens, M.; Luker, K.E.; Sottile, P.; Sonstein, J.; Lukacs, N.W.; Núñez, G.; Curtis, J.L.; Luker, G.D. TLR3 increases disease morbidity and mortality from vaccinia infection. J. Immunol. 2008, 180, 483–491.
- Samuelsson, C.; Hausmann, J.; Lauterbach, H.; Schmidt, M.; Akira, S.; Wagner, H.; Chaplin, P.; Suter, M.; O’Keeffe, M.; Hochrein, H. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. J. Clin. Investig. 2008, 118, 1776–1784.
- Wolferstätter, M.; Schweneker, M.; Späth, M.; Lukassen, S.; Klingenberg, M.; Brinkmann, K.; Wielert, U.; Lauterbach, H.; Hochrein, H.; Chaplin, P.; et al. Recombinant modified vaccinia virus Ankara generating excess early double-stranded RNA transiently activates protein kinase R and triggers enhanced innate immune responses. J. Virol. 2014, 88, 14396–14411.
- Rice, A.D.; Turner, P.C.; Embury, J.E.; Moldawer, L.L.; Baker, H.V.; Moyer, R.W. Roles of vaccinia virus genes E3L and K3L and host genes PKR and RNase L during intratracheal infection of C57BL/6 mice. J. Virol. 2011, 85, 550–567.
- Delaloye, J.; Roger, T.; Steiner-Tardivel, Q.-G.; Le Roy, D.; Knaup Reymond, M.; Akira, S.; Petrilli, V.; Gomez, C.E.; Perdiguero, B.; Tschopp, J.; et al. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009, 5, e1000480.
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009, 458, 514–518.
- El-Jesr, M.; Teir, M.; Maluquer de Motes, C. Vaccinia Virus Activation and Antagonism of Cytosolic DNA Sensing. Front. Immunol. 2020, 11, 568412.
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103.
- Gasteiger, G.; D’Osualdo, A.; Schubert, D.A.; Weber, A.; Bruscia, E.M.; Hartl, D. Cellular Innate Immunity: An Old Game with New Players. J. Innate Immun. 2017, 9, 111–125.
- Johnston, J.B.; McFadden, G. Poxvirus immunomodulatory strategies: Current perspectives. J. Virol. 2003, 77, 6093–6100.
- Roberts, W.K.; Hovanessian, A.; Brown, R.E.; Clemens, M.J.; Kerr, I.M. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature 1976, 264, 477–480.
- Hovanessian, A.G. On the discovery of interferon-inducible, double-stranded RNA activated enzymes: The 2′-5′oligoadenylate synthetases and the protein kinase PKR. Cytokine Growth Factor Rev. 2007, 18, 351–361.
- Tuazon Kels, M.J.; Ng, E.; Al Rumaih, Z.; Pandey, P.; Ruuls, S.R.; Korner, H.; Newsome, T.P.; Chaudhri, G.; Karupiah, G. TNF deficiency dysregulates inflammatory cytokine production, leading to lung pathology and death during respiratory poxvirus infection. Proc. Natl. Acad. Sci. USA 2020, 117, 15935–15946.
- Liu, L.; Xu, Z.; Fuhlbrigge, R.C.; Peña-Cruz, V.; Lieberman, J.; Kupper, T.S. Vaccinia virus induces strong immunoregulatory cytokine production in healthy human epidermal keratinocytes: A novel strategy for immune evasion. J. Virol. 2005, 79, 7363–7370.
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49.
- Platanitis, E.; Decker, T. Regulatory Networks Involving STATs, IRFs, and NFκB in Inflammation. Front. Immunol. 2018, 9, 2542.
- Ullah, M.O.; Sweet, M.J.; Mansell, A.; Kellie, S.; Kobe, B. TRIF-dependent TLR signaling, its functions in host defense and inflammation, and its potential as a therapeutic target. J. Leukoc. Biol. 2016, 100, 27–45.
- Piras, V.; Selvarajoo, K. Beyond MyD88 and TRIF Pathways in Toll-Like Receptor Signaling. Front. Immunol. 2014, 5, 70.
- Thaiss, C.A.; Levy, M.; Itav, S.; Elinav, E. Integration of Innate Immune Signaling. Trends Immunol. 2016, 37, 84–101.
- Shrivastava, G.; León-Juárez, M.; García-Cordero, J.; Meza-Sánchez, D.E.; Cedillo-Barrón, L. Inflammasomes and its importance in viral infections. Immunol. Res. 2016, 64, 1101–1117.
- Hayward, J.A.; Mathur, A.; Ngo, C.; Man, S.M. Cytosolic Recognition of Microbes and Pathogens: Inflammasomes in Action. Microbiol. Mol. Biol. Rev. 2018, 82.
- Man, S.M.; Karki, R.; Kanneganti, T.-D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75.
- Platnich, J.M.; Muruve, D.A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019, 670, 4–14.
- Sharma, M.; de Alba, E. Structure, Activation and Regulation of NLRP3 and AIM2 Inflammasomes. Int. J. Mol. Sci. 2021, 22, 872.
- Nambayan, R.J.T.; Sandin, S.I.; Quint, D.A.; Satyadi, D.M.; de Alba, E. The inflammasome adapter ASC assembles into filaments with integral participation of its two Death Domains, PYD and CARD. J. Biol. Chem. 2019, 294, 439–452.
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665.
- Rahman, M.M.; McFadden, G. Myxoma virus lacking the pyrin-like protein M013 is sensed in human myeloid cells by both NLRP3 and multiple Toll-like receptors, which independently activate the inflammasome and NF-κB innate response pathways. J. Virol. 2011, 85, 12505–12517.
- Myskiw, C.; Arsenio, J.; Hammett, C.; van Bruggen, R.; Deschambault, Y.; Beausoleil, N.; Babiuk, S.; Cao, J. Comparative analysis of poxvirus orthologues of the vaccinia virus E3 protein: Modulation of protein kinase R activity, cytokine responses, and virus pathogenicity. J. Virol. 2011, 85, 12280–12291.
- Rivas, C.; Gil, J.; Mĕlková, Z.; Esteban, M.; Díaz-Guerra, M. Vaccinia virus E3L protein is an inhibitor of the interferon (i.f.n.)-induced 2-5A synthetase enzyme. Virology 1998, 243, 406–414.
- Dempsey, A.; Keating, S.E.; Carty, M.; Bowie, A.G. Poxviral protein E3-altered cytokine production reveals that DExD/H-box helicase 9 controls Toll-like receptor-stimulated immune responses. J. Biol. Chem. 2018, 293, 14989–15001.
- Dai, P.; Cao, H.; Merghoub, T.; Avogadri, F.; Wang, W.; Parikh, T.; Fang, C.-M.; Pitha, P.M.; Fitzgerald, K.A.; Rahman, M.M.; et al. Myxoma virus induces type I interferon production in murine plasmacytoid dendritic cells via a TLR9/MyD88-, IRF5/IRF7-, and IFNAR-dependent pathway. J. Virol. 2011, 85, 10814–10825.
- Park, C.; Peng, C.; Brennan, G.; Rothenburg, S. Species-specific inhibition of antiviral protein kinase R by capripoxviruses and vaccinia virus. Ann. N. Y. Acad. Sci. 2019, 1438, 18–29.
- Park, C.; Peng, C.; Rahman, M.J.; Haller, S.L.; Tazi, L.; Brennan, G.; Rothenburg, S. Orthopoxvirus K3 orthologs show virus- and host-specific inhibition of the antiviral protein kinase PKR. PLoS Pathog. 2021, 17, e1009183.
- Rothenburg, S.; Brennan, G. Species-Specific Host–Virus Interactions: Implications for Viral Host Range and Virulence. Trends Microbiol. 2020, 28, 46–56.
More
Information
Subjects:
Virology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Revisions:
2 times
(View History)
Update Date:
24 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




