
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gianniantonio Petruzzelli | + 1179 word(s) | 1179 | 2021-07-21 08:06:30 | | | |
| 2 | Lily Guo | Meta information modification | 1179 | 2021-07-26 02:58:33 | | |
Video Upload Options
Tungsten (W) occurs naturally in soils and the Earth’s crust is the most important source of this element. Tungsten reserves have been estimated to be approximately 3.1 Mt in ore deposits, where the metal exists mainly as a component of several minerals, such as wolframite (Fe, MnWO4) and scheelite (CaWO4).
1. Introduction
2. Production and Uses of Tungsten
In many of these phases, tungsten can enter the soil ecosystem. In the mining phases, where the tungsten is extracted from ores, the W concentration in surrounding soils can reach values higher than 1000 mg kg-1 [31]. In the manufacturing and fabrication phases, in which products containing tungsten that are requested by the market are prepared, the production of dust and atmospheric transport must be considered as an important environmental issue, also in terms of deposition of airborne particles on soils [15].
The behavior of tungsten depends on the specific characteristics of the soils that regulate its distribution between the liquid and the solid phases. In soil, its oxidation state ranges from −2 to +6, the most common of which is +6. In fact, in soils, tungsten occurs mainly as tungstate anion (WO42−), which is thermodynamically very stable [13]. However, the dynamics of tungsten in soil are complex due to its tendency to form polymers, even with other ions present in the soil, such as phosphates and silicates (Figure 2).
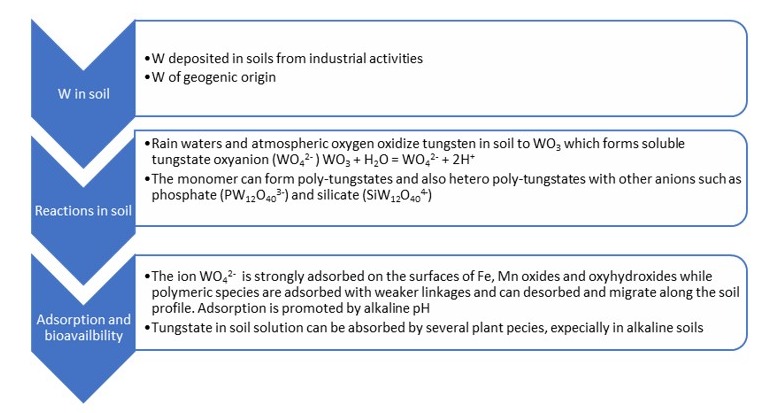
Figure 2. Schematic reactions of tungsten in soil.
The immediate source of tungsten that is available for biological processes in soil, including plant uptake, is the amount present in the soil solution. This quantity is determined by the solubility of tungsten compounds and is controlled by the actual concentration in the soil solution and the quantity weakly adsorbed on soil surfaces and easily releasable in the desorption processes. The sorption processes determine the environmental behavior of tungsten in soils and its bioavailability to plants.
References
- Shedd, K.B. U.S. Geological Survey Minerals Yearbook 2017: Tungsten; U.S. Department of the Interior: Reston, VA, USA, 2017.
- Che, X.D.; Linen, R.L.; Wang, R.C.; Aseri, A.; Thibault, Y. Tungsten solubility in evolved granitic melts: An evaluation of magmatic wolframite. Geochim. Cosmochim. Acta 2016, 106, 84–98.
- Hartung, M. Tungsten. In Metals and Their Compounds in the Environment; Merian, E., Ed.; VCH: Weinheim, Germany, 1991; pp. 1269–1272.
- Langard, S. Tungsten. In Patty’s Toxicology; Bingham, E., Cohrssen, B., Powell, C.H., Eds.; John Wiley: New York, NY, USA, 2001; pp. 106–128.
- Steenstra, P.; Strigul, N.; Harrison, J. Tungsten in Washington State surface waters. Chemosphere 2020, 242, 125151.
- Mohajerin, T.J.; Helz, G.R.; Johannesson, K.H. Tungsten—Molybdenum fractionation in estuarine environments. Geochem. Cosmochim. Acta 2016, 177, 105.
- Johannesson, K.H.; Dave, H.B.; Mohajerin, T.J.; Datta, S. Controls on tungsten concentrations in groundwater flow systems: The role of adsorption, aquifer sediment Fe (III) oxide/oxyhydroxide content, and thiotungstate formation. Chem. Geol. 2013, 351, 76e94.
- Strigul, N. Does speciation matter for tungsten ecotoxicology? Ecotoxicol. Environ. Saf. 2010, 73, 1099–1113.
- Hobson, C.; Kulkarni, H.V.; Johannesson, K.H.; Bednar, A.; Tappero, R.; Mohajerin, T.J.; Sheppard, P.R.; Witten, M.L.; Hettiarachchi, G.M.; Datta, S. Origin of tungsten and geochemical controls on its occurrence and mobilization in shallow sediments from Fallon, Nevada, USA. Chemosphere 2020, 260, 127577.
- Osseo-Asare, K. Solution chemistry of tungsten leaching systems. Metall. Mater. Trans. B 1982, 13, 555–563.
- Seiler, R.L.; Stollenwerk, K.G.; Garbarino, J.R. Factors controlling tungsten concentrations in ground water, Carson Desert, Nevada. Appl. Geochem. 2005, 20, 423–441.
- Steinberg, K.K.; Relling, M.V.; Gallagher, M.L.; Greene, C.N.; Rubin, C.S.; French, D.; Holmes, A.K.; Carroll, W.L.; Koontz, D.A.; Sampson, E.J.; et al. Genetic studies of a cluster of acute lymphoblastic leukemia cases in Churchill County, Nevada. Environ. Health Perspect. 2007, 115, 158–164.
- Koutsospyros, A.; Braida, W.J.; Christodoulatos, C.; Dermatas, D.; Strigul, N.S. A review of tungsten: From environmental obscurity to scrutiny. J. Hazard. Mater. 2006, 136, 1–19.
- Sheppard, P.R.; Speakman, R.J.; Farris, C.; Witten, M.L. Multiple environmental monitoring techniques for assessing spatial patterns of airborne tungsten. Environ. Sci. Technol. 2007, 41, 406–410.
- Sheppard, P.R.; Toepfer, P.; Schumacher, E.; Rhodes, K.; Ridenour, G.; Witten, M.L. Morphological and chemical characteristics of airborne tungsten particles of Fallon, Nevada. Microsc. Microanal. 2007, 13, 296–303.
- Bednar, A.J.; Mirecki, J.E.; Inouye, L.S.; Winfield, L.E.; Larson, S.L.; Ringelberg, D.B. The determination of tungsten, molybdenum, and phosphorus oxyanions by high performance liquid chromatography inductively coupled plasma mass spectrometery. Talanta 2007, 72, 1828–1832.
- Pardus, M.J.; Sueker, J.K. Occurrence and geochemistry of tungsten in the Carson River basin, Nevada, USA. Land Contam. Reclamat. 2009, 17, 9–29.
- Guilbert, C.; Kelly, A.D.R.; Petruccelli, L.A.; Lemaire, M.; Mann, K.K. Exposure to tungsten induces DNA damage and apoptosis in developing B lymphocytes. Leukemia 2011, 25, 1900.
- USEPA. Technical fact sheet–Tungsten. Office of Land and Emergency Management (5106P); EPA 505-F-17-004; Environmental Protection Agency: Washington, DC, USA, 2017.
- Sun, C.B.; Zhong, Y.W.; Fu, W.J.; Zhao, Z.Q.; Liu, J.; Ding, J.; Han, X.P.; Deng, Y.D.; Hu, W.B.; Zhong, C. Tungsten disulfide-based nanomaterials for energy conversion and storage. Tungsten 2020, 2, 109–133.
- Wataria, T.; Nansaia, K.; Nakajima, K. Review of critical metal dynamics to 2050 for 48 elements. Resour. Conserv. Recycl. 2020, 155, 104669.
- Tang, L.; Wang, P.; Graedel, T.E.; Pauliuk, S.; Xiang, K.; Ren, Y.; Chen, W. Refining the understanding of China’s tungsten dominance with dynamic material cycle analysis. Resour. Conserv. Recycl. 2020, 158, 104829.
- Graedel, T.E.; Harper, E.M.; Nassar, N.T.; Nuss, P.; Reck, B.K. Criticality of metals and metalloids. Proc. Natl. Acad. Sci. USA 2015, 112, 4257–4262.
- European Commision. Report on Critical Raw Materials for the European Critical Raw Materials Profiles. European Commision. 2014. Available online: (accessed on 4 May 2021).
- European Commision. Report on Critical Raw Materials and the Circular Economy. 2018. Available online: (accessed on 4 May 2021).
- Mudd, G.M.; Werner, T.T.; Weng, Z.H.; Yellishetty, M.; Yuan, Y.; McAlpine, S.R.B.; Skirrow, R.; Czarnota, K. Critical Minerals in Australia: A Review of Opportunities and Research Needs. 2019. Available online: (accessed on 4 May 2021).
- National Science and Technology Council (NSTC), Assessment of Cretical Minerals: Updated Application of Screening Methodology. 2018. Available online: (accessed on 4 May 2021).
- Schmidt, S. ITIA Newletters. Tungsten 2012, 4, 1–20. Available online: (accessed on 4 May 2021).
- Xu, H.; He, L.L.; Pei, Y.F.; Jiang, C.Z.; Li, W.Q.; Xiao, X.H. Recent progress of radiation response in nanostructured tungsten for nuclear application. Tungsten 2021, 3, 20–37.
- Tkaczyk, A.H.; Bartl, A.; Amato, A.; Lapkovskis, V.; Petranikova, M. Sustainability evaluation of essential critical raw materials: Cobalt, niobium, tungsten and rare earth elements. J. Phys. D Appl. Phys. 2018, 51, 203001.
- Clausen, J.L.; Korte, N. Environmental fate of tungsten from military use. Sci. Tot. Environ. 2009, 407, 2887–2893.
- USEPA. Technical Fact Sheet–Tungsten; USEPA: Washington, DC, USA, 2014.
- Koutsospyros, A.D.; Strigul, N.; Braida, W.; Christodoulatos, C. Tungsten: Environmental pollution and health effects. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Burlington, UK, 2011; pp. 418–426.
- Yin, S. Preface to the special issue: Novel functionalities of tungsten-related materials. Tungsten 2019, 1, 245–246.
- Day, G.; Virji, M.A.; Stefaniak, A.B. Characterization of Exposures among Cemented Tungsten Carbide Workers. Part II: Assessment of Surface Contamination and Skin Exposures to Cobalt, Chromium and Nickel. J. Exposure Sci. Environ. Epidemiol. 2009, 19, 423–434.
- Stefaniak, A.B.; Virji, M.A.; Day, G.A. Characterization of Exposures among Cemented Tungsten Carbide Workers. Part I: Size-Fractionated Exposures to Airborne Cobalt and Tungsten Particles. J. Exposure Sci. Environ. Epidemiol. 2009, 19, 475.
- Yao, Z.; Stiglich, J.J.; Sudarshan, T. Nanosized WC-Co Holds Promise for the Future. Met. Powder Rep. 1998, 53, 26–33.
- Ciacci, L.; Reck, B.K.; Nassar, N.T.; Graedel, T.E. Lost by design. Environ. Sci. Technol. 2015, 49, 9443–9451.




