
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Leixuri Aguirre | + 2435 word(s) | 2435 | 2021-06-16 09:31:49 | | | |
| 2 | Lindsay Dong | Meta information modification | 2435 | 2021-07-23 09:52:22 | | | | |
| 3 | Lindsay Dong | Meta information modification | 2435 | 2021-07-23 09:53:09 | | | | |
| 4 | Lindsay Dong | Meta information modification | 2435 | 2021-07-26 05:14:20 | | |
Video Upload Options
Non-alcoholic fatty liver disease (NAFLD) covers a wide spectrum of histopathological abnormalities ranging from simple steatosis to steatohepatitis (NASH). Algae represent a good source of proteins, vitamins, minerals and fiber. In addition, they are rich in a great number of bioactive compounds, such as peptides, pigments, phenolic compounds and fatty acids with potential applications in health, due to their antioxidant, antimicrobial, anti-inflammatory, anticancer, antidiabetic, antihypertensive, antihiperlipidaemic and antiobesity effect.
1. Introduction
Hepatic steatosis is the most benign and common form of NAFLD that is defined as intrahepatic fat accumulation of at least 5% of liver weight. However, this condition can evolve to more advanced stages if hepatocytes are exposed to stress, causing cell death, apoptosis, inflammation and fibrosis and leading to NASH. This NASH can result in cirrhosis and hepatocellular carcinoma [1][2]. The prevalence of NAFLD shows a high variability, ranging from 6 to 35% in the general population. These rates are experiencing an upward trend due to the current epidemic of obesity [3] and type 2 diabetes [2]; in fact, about 50% of NAFLD patients and 80% of patients with NASH are obese [4].
Currently, there is no specific treatment for liver steatosis. The first step in its management consists of lifestyle intervention with caloric intake restriction and exercise. However, patients find it difficult to implement and achieve these lifestyle modifications. At present, no drugs have yet been approved, and pharmacological treatment devotes efforts to associated co-morbidities that contribute to the pathogenesis of NAFLD, such as, obesity, type 2 diabetes mellitus or dyslipidemia [4]. Due to the increasing prevalence and treatment limitations of NAFLD, there is an urgent need to seek new sources of bioactive compounds with potential preventive and/or therapeutic action.
2. Effect of Microalgae and Macroalgae Extracts on Non-Alcoholic Fatty Liver Disease
2.1. Animal Studies
The vast majority of algae extract effects have been studied in rodent models (Figure 1).
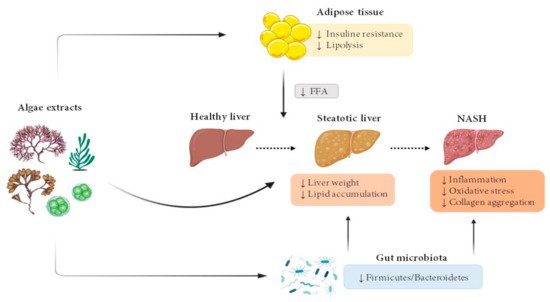
Figure 1. Effects of microalgae and macroalgae extracts on metabolic alterations leading to steatohepatitis. FFA, free fatty acids; NASH, non-alcoholic steatohepatitis.
2.1.1. Microalgae
As far as we know, only four studies analysing the effects of microalgae extracts on NAFLD in animal models have been published to date. (Table 1).
| Author | Algae Species | Animal Model and Experimental Period Length | Experimental Groups | Effects | Mechanisms |
|---|---|---|---|---|---|
| Kumar et al., 2015 [5] | Scenedesmus dimorphus + Schroederiella apiculate mixture (green microalgae) | Male Wistar rats 8 weeks |
Corn starch diet (C) High-carbohydrate high-fat diet (H) Corn starch diet + 5% microalgae mixture (CSC) High-carbohydrate high-fat diet + 5% microalgae mixture (HSC) |
↓ Liver weight ↓ Enlargement of fat vacuoles in hepatocytes (HC vs. H) ↓ Inflammation ↓ ALT and AST activities (HC vs. H) Improved glucose tolerance and insulin sensitivity (HC vs. H) |
↓ Infiltration of inflammatory cells |
| Nakashima et al., 2018 [6] | Euglena gracilis (green microalgae) | Male STAM mice 27 days |
High-fat diet High-fat diet + 3 g/kg BW/day E. gracilis High-fat diet + 3 g/kg BW/day Paramylon High-fat diet + 10 mg/kg BW/day Telmisartan |
Liver weight: NS Liver TG: NS ↓ Collagen aggregation (Euglena vs. vehicle) NAS Score: NS Serum ALT: NS |
↓ Immunostaining of F4/80, α-SMA (trend) Inflammation-related genes: NS Fibrosis-related genes: NS |
| Pham et al., 2019 [7] | Spirulina platensis (blue-green microalgae) | Male C57BL/6J mice 20 weeks |
Low-fat (LF) High-fat/high-sucrose/high-cholesterol (HF) HF + 2.5% S. platensis (HF/SP) |
Liver weight: NS Liver TG and cholesterol: NS ↓ Plasma ALT level Improvement of glucose tolerance Hepatic collagen accumulation: NS |
mRNA levels of Col1a1 in liver: NS ↓ Il-1β mRNA levels in splenocytes |
| Mayer et al., 2021 [8] | Tisochrysis lutea (brown-golden microalgae) | Male Wistar rats 8 weeks |
Standard diet (CTRL) High-fat high-fructose diet (HF) High-fat high-fructose + 12% T. lutea (HF-Tiso) |
↓ Liver TG and cholesterol ↓ Plasma AST, AST/ALT ↓ Plasma glucose, insulin, leptin ↓ Plasma TNF-α ↓ HOMAR-IR |
No information provided |
To sum up, after reviewing the literature concerning the effects of microalgae on NAFLD, it can be pointed out that although the results reported are encouraging, the number of studies is still very scarce. In addition, the reported studies address the effects of different microalgae, and thus only one source of information is available for each one. According to the experimental design used, all the reported studies analysed the preventive effects of microalgae because these were included in the diet that induced liver alterations. Although in all of them microalgae improved several alterations, there is a lack of consensus on the specific effects observed. Thus, whereas the three studies that analysed this parameter reported a reduction of liver inflammation, only two of these studies described a reduction of liver triglyceride content (the other two studies did not find significant changes). Moreover, two of the studies analysed the effects of fibrosis and only one observed a significant improvement. In this scenario, further research is needed in order to assess the effects of microalgae on NAFLD.
2.1.2. Macroalgae
Several studies have analysed the effects of green, red and brown macroalgae on NAFLD using different animal models.
Two green algae have been studied in the reported literature: Caulerpa lentillifera and Ulva prolifera (Table 2).
| Author | Algae Species | Animal Model and Experimental Period Length | Experimental Groups | Effects | Mechanisms |
|---|---|---|---|---|---|
| Sharma et al., 2017 [9] | Caulerpa lentillifera | Male C57BL/6J mice 10 weeks |
Standard diet High-fat diet High-fat diet + 250 mg/kg BW/day of C. lentillifera |
↓ Liver weight ↓ Liver TG, TC and FFA ↓ Plasma FFA, glucose and insulin |
No information provided |
| du Preez et al., 2020 [10] | Caulerpa lentillifera | Male Wistar rats 16 weeks |
Corn starch (C) Standard diet (C) High-carbohydrate high-fat (H) Standard diet + 5% C. lentillifera (CCL) High-carbohydrate high-fat + 5% C. lentillifera (HCL) |
↓ Liver TG content (H vs. HCL) Inflammatory cell infiltration: NS Plasma ALT, AST: NS ↓Firmicutes/Bacteroidetes ratio (H vs. HCL) |
No information provided |
| Song et al., 2018 [11] | Ulva prolifera | Male C57BL/6 mice 8 weeks |
Standard diet High-fat diet High-fat diet + 2% ethanol extract of U. prolifera in drinking water High-fat diet + 5% ethanol extract of U. prolifera in drinking water |
↓ Liver weight ↓ Liver TG content ↓ Serum insulin ↓ Oxidative stress |
↓ Dgat1 and Dgat2 liver mRNA levels ↑ Cpt-1a, Acadm and Acox1 liver mRNA levels ↓ Serum IL-1β, IL-6 and TNF-α ↓ Il-1β, Il-6 and Tnf-α liver mRNA levels ↓ Liver ROS ↑ GSH content and GSHPx activity |
In summary, as in the case of microalgae, the number of studies addressing the preventive effect of green algae on NAFLD is still scarce. The three reported works (two of them carried out with Caulerpa lentillifera) revealed a reduction in hepatic triglyceride accumulation when animals were fed with a high-fat diet, but only one of the studies addressed several aspects of the potential mechanisms of action (insulin resistance, fatty acid and triglyceride metabolism, oxidative stress). In one of these pieces of research, the authors analysed the effects of seaweed supplementation on gut microbiota composition, but they did not establish a clear relationship between these changes and the effects on liver steatosis, just a significant correlation.
Five red algae were studied in the reported literature: Plocamium telfairiae, Palmaria mollis, Sarconema filiforme Grateloupia elliptica and Gromphadorhina oblongata (Table 3).
| Author | Algae Species | Animal Model and Experimental Period Length | Experimental Groups | Effects | Mechanisms |
|---|---|---|---|---|---|
| Kang et al., 2016 [12] | Plocamium telfairiae | Male C57BL/6 mice 14 weeks |
Standard diet High-fat diet High-fat diet +100 mg/kg BW/day of P. telfairiae |
↓ Liver steatosis ↓ Serum glucose |
No information provided |
| Lu et al., 2020 [13] | Plocamium telfairiae | Male C57BL/6 mice 7 weeks |
Standard diet High-fat diet High-fat diet +100 mg/kg BW/day of P. telfairiae High-fat diet +165 mg/kg BW/day of P. telfairiae High-fat diet + 300 mg/kg BW/day of P. telfairiae |
↓ Hepatic steatosis (all doses) | No information provided |
| Nakayama et al., 2018 [14] | Palmaria mollis | Male NSY/HOS mice 4 weeks |
Standard diet High-fat diet High-fat diet + 2.5% of P. mollis |
↓ Liver TG content | ↑ Pparα, C/ebpα and Acox1 mRNA levels ↓ Pparɣ mRNA levels Acadm and Srebf1 mRNA levels: NS |
| du Preez et al., 2020 [15] | Sarconema filiforme | Male Wistar rats 16 weeks |
Corn starch diet Corn starch diet + 5% S. filiforme High-carbohydrate high-fat diet High-carbohydrate high-fat diet + 5% S. filiforme (drinking water of rats fed the steatotic diet was supplemented with 25% fructose) |
↓ Liver steatosis and infiltration of inflammatory cells (high-carbohydrate high-fat diet 5% S. filiforme vs. high-carbohydrate high-fat diet) Serum glucose: NS ↓ Serum ALT and AST (high-carbohydrate high-fat diet 5% S. filiforme vs. high-carbohydrate high-fat diet) Firmicutes/Bacteroidetes: NS |
No information provided |
| Lee et al., 2020 [16] | Grateloupia elliptica | Male C57BL/6 mice 7 weeks |
Standard diet High-fat high-sucrose diet High-fat high-sucrose diet +125 mg/kg BW/day of G. elliptica High-fat high-sucrose diet +250 mg/kg BW/day of G. elliptica |
↓ Hepatic steatosis | No information provided |
| Nabil-Adam et al., 2021 [17] | Gromphadorhina oblongata | BALB/C mice 1 week |
Negative control: saline solution Induction control: 5 mg/kg BW/day of LPS Protected group: 200 mg/kg BW/day of G. oblongata 2 h before LPS Positive control: 200 mg/kg BW/day of G. oblongata without LPS |
↓ Liver injury (inflammation and oxidative stress) ↑ Liver apoptosis ↓ Serum ALT, AST |
No information provided |
According to the reported studies, it can be concluded that the five red algae analysed were able to prevent liver triglyceride accumulation induced by diets rich in fat and those rich in fat and sugars. This positive effect is found in both rats and mice. The majority of the reported studies did not address the mechanisms of action involved in this effect. Interestingly, two studies that were focused on Plocamium telfairiae showed that the positive effects on liver fat accumulation were observed with quite different experimental period lengths (7 and 14 weeks).
Four brown algae have been researched in the studies reported in the literature: Undaria pinnatifida, Fucus vesiculosus, Ascophyllum nodosum Sargassum thunbergii and Sargassum horneri (Table 4).
| Author | Algae Species | Animal Model and Experimental Period Length | Experimental Groups | Effects | Mechanisms |
|---|---|---|---|---|---|
| Murata et al., 1999 [18] | Undaria pinnatifida | Male Sprague–Dawley rats 3 weeks |
Standard diet Standard diet + 0.5% U. pinnatifida Standard diet + 1% U. pinnatifida Standard diet + 2% U.pinnatifida Standard diet + 5% U. pinnatifida Standard diet + 10% U.pinnatifida |
↓ Liver TG content in 1, 2, 5 and 10% groups ↓ Liver TC content in 10% group |
↓G6PD activity in 5 and 10% groups ↑ CPT activity in 10% group ↑ ACADs activity in 5 and 10% groups ↑ ACO in 10% group ↑ DECR1 in 5 and 10% groups |
| Murata et al., 2002 [19] | Undaria pinnatifida | Male Sprague–Dawley rats 4 weeks |
Standard diet Standard diet + 19.1% U. pinnatifida |
↓ Liver weight ↓ Hepatic TG, TC and phospholipids levels |
↓ G6PD activity ↑ ACO and 3-hydroxiacil-CoA dehydrogenase activities CPT activity: NS |
| Li et al., 2020 [20] | Undaria pinnatifida | Male C57BL/6 mice 10 weeks |
Standard diet Standard diet + 10% U. pinnatifida High-fat diet High-fat diet + 10% U. pinnatifida |
↓ Liver steatosis ↓ Glucose levels |
No information provided |
| Gabbia et al., 2020 [21] | Fucus vesiculosus + Ascophyllum nodosum | Male Wistar rats 5 weeks |
High-fat diet (HFD) High-fat diet + 7.5 mg/kg BW/day of F. vesiculosus and A. nodosum |
↓ Liver weight ↓ Microvesicular steatosis ↓ Plasma ALT and AST levels Lower and delayed glucose peak |
No information provided |
| Kang et al., 2020 [22] | Sargassum thunbergii | Male C57BL/6 mice 7 weeks |
Standard diet High-fat diet High-fat diet + 100 mg/kg BW/day of S. thunbergii High-fat diet + 300 mg/kg BW/day of S. thunbergii |
↓ Lipid steatosis | No information provided |
| Murakami et al., 2021 [23] | Sargassum horneri | Male C57BL/6J mice 13 weeks |
Standard diet High-fat diet (HF) High-fat diet + 2% S. horneri (HF + ShL) High-fat diet + 6% S. horneri (HF + ShH) |
↓ Liver weight ↓ Liver TG content ↓ Serum glucose, insulin, ALT, AST, ALP and LAP levels ↑ Serum adiponectin ↓ Serum TNF-α |
↓ Pancreatic lipase activity |
Among the brown macroalgae, the most frequently analysed is Undaria pinnatifida, which has been demonstrated to be effective in both rats and mice after medium length and longer experimental periods. Nevertheless, in the three studies reported, where this type of alga has been used, the diets administered to animals were standard diets providing normal amounts of fat and sugars, the two nutrients that increase liver triglyceride accumulation. Consequently, further studies that make use of the diet that induces steatosis are needed to confirm the preventive effects of this seaweed. In the case of Sargassum thunbergii, this seaweed prevents the liver steatosis induced by a high-fat diet, in both mice and rats, in a dose-dependent manner.
2.2. Human Studies
To date, very little information aimed at analysing the effects of algae in humans has been reported. Ebrahimi-Mameghani et al. [24] carried out a double-blind, placebo-controlled, randomised clinical trial in 55 obese patients aged 20–50 years with confirmed NAFLD by ultrasonography. Individuals in the intervention group (29) received 1200 mg/day of Chlorella vulgaris dispensed in four tablets of 300 mg and 400 mg/day of vitamin E, whereas the placebo group (26) received 400 mg/day of vitamin E and four placebos for eight weeks. In this study, the only parameters related to the liver were serum transaminases, which did not yield differences between both experimental groups.
Li et al. [25] studied the association of algae consumption with newly diagnosed NAFLD by ultrasound in the adult population. To do so, they carried out a cross-sectional study involving 24,572 adult subjects from The Republic of China. The authors observed that algae consumption, assessed using a food frequency questionnaire, was negatively associated with the prevalence of NAFLD, especially in non-obese patients. Adjustments for several factors were implemented: age, sex, body mass index (BMI), smoking status, alcohol drinking status, socioeconomic status, physical activity, family history of disease (including cardiovascular disease, hypertension, hyperlipidaemia and diabetes), hypertension, hyperlipidaemia, diabetes and total energy intake. Additional adjustments were also applied for “fruits and sweet”, “healthy” and “animal foods” dietary pattern scores. The authors stated that to clarify the causality, more prospective studies and clinical trials were required.
3. Concluding Remarks
Taking into account that the susceptibility to develop NAFLD depends on genetic background, among other factors [26], it is important to address future studies devoted to analysing potential interactions of algae treatments with genetics and epigenetics, in order to established which subjects can get the most benefit, in the framework of personalised nutrition. Another factor with an important role in the development of NAFLD is gut microbiota [27]. Considering that several components of algae are able to modify microbiota composition [28][29][30], an interesting field of future research is to establish the relationship between these modifications and the improvement of NAFLD produced by algae.
References
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell. Mol. Life Sci. 2019, 76, 99–128.
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544.
- Than, N.N.; Newsome, P.N. A concise review of non-alcoholic fatty liver disease. Atherosclerosis 2015, 239, 192–202.
- Makri, E.; Goulas, A.; Polyzos, S.A. Epidemiology, Pathogenesis, Diagnosis and Emerging Treatment of Nonalcoholic Fatty Liver Disease. Arch. Med. Res. 2021, 52, 25–37.
- Kumar, S.A.; Magnusson, M.; Ward, L.C.; Paul, N.A.; Brown, L. A green algae mixture of Scenedesmus and Schroederiella attenuates obesity-linked metabolic syndrome in rats. Nutrients 2015, 7, 2771–2787.
- Nakashima, A.; Sugimoto, R.; Suzuki, K.; Shirakata, Y.; Hashiguchi, T.; Yoshida, C.; Nakano, Y. Anti-fibrotic activity of Euglena gracilis and paramylon in a mouse model of non-alcoholic steatohepatitis. Food Sci. Nutr. 2018, 7, 139–147.
- Pham, T.X.; Lee, Y.; Bae, M.; Hu, S.; Kang, H.; Kim, M.B.; Park, Y.K.; Lee, J.Y. Spirulina supplementation in a mouse model of diet-induced liver fibrosis reduced the pro-inflammatory response of splenocytes. Br. J. Nutr. 2019, 121, 748–755.
- Mayer, C.; Richard, L.; Côme, M.; Ulmann, L.; Nazih, H.; Chénais, B.; Ouguerram, K.; Mimouni, V. The Marine Microalga, Tisochrysis lutea, protects agains metabolic disorders associated with metabolic syndrome and obesity. Nutrients 2021, 13, 430.
- Sharma, B.R.; Kim, H.J.; Kim, M.S.; Park, C.M.; Rhyu, D.Y. Caulerpa okamurae extract inhibits adipogenesis in 3T3-L1 adipocytes and prevents high-fat diet-induced obesity in C57BL/6 mice. Nutr. Res. 2017, 47, 44–52.
- du Preez, R.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Caulerpa lentillifera (Sea Grapes) Improves Cardiovascular and Metabolic Health of Rats with Diet-Induced Metabolic Syndrome. Metabolites 2020, 10, 500.
- Song, W.; Wang, Z.; Zhang, X.; Li, Y. Ethanol Extract from Ulva prolifera prevents High-fat diet-induced insulin resistance, oxidative stress, and inflammation response in mice. Biomed. Res. Int. 2018, 2018, 1374565.
- Kang, M.C.; Kang, N.; Ko, S.C.; Kim, Y.B.; Jeon, Y.J. Anti-obesity effects of seaweeds of Jeju Island on the differentiation of 3T3-L1 preadipocytes and obese mice fed a high-fat diet. Food Chem. Toxicol. 2016, 90, 36–44.
- Lu, Y.A.; Lee, H.G.; Li, X.; Hyun, J.M.; Kim, H.S.; Kim, T.H.; Kim, H.M.; Lee, J.J.; Kang, M.C.; Jeon, Y.J. Anti-obesity effects of red seaweed, Plocamium telfairiae, in C57BL/6 mice fed a high-fat diet. Food Funct. 2020, 11, 2299–2308.
- Nakayama, H.; Shimada, Y.; Zang, L.; Terasawa, M.; Nishiura, K.; Matsuda, K.; Toombs, C.; Langdon, C.; Nishimura, N. Novel Anti-Obesity Properties of Palmaria mollis in Zebrafish and Mouse Models. Nutrients 2018, 10, 1401.
- du Preez, R.; Paul, N.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Carrageenans from the red seaweed Sarconema filiforme attenuate symptoms of diet-induced metabolic syndrome in rats. Mar. Drugs 2020, 18, 97.
- Lee, H.G.; Lu, Y.A.; Li, X.; Hyun, J.M.; Kim, H.S.; Lee, J.J.; Kim, T.H.; Kim, H.M.; Kang, M.C.; Jeon, A.Y. Anti-Obesity effects of Grateloupia elliptica, a red seaweed, in mice with high-fat diet-induced obesity via suppression of adipogenic factors in white adipose tissue and increased thermogenic factors in brown adipose tissue. Nutrients 2020, 12, 308.
- Nabil-Adam, A.; Shreadah, M.A. Red algae natural products for prevention of lipopolysaccharides (LPS)-induced liver and kidney inflammation and injuries. Biosci. Rep. 2021, 41.
- Murata, M.; Ishihara, K.; Saito, H. Hepatic fatty acid oxidation enzyme activities are stimulated in rats fed the brown seaweed, Undaria pinnatifida (wakame). J. Nutr. 1999, 129, 146–151.
- Murata, M.; Sano, Y.; Ishihara, K.; Uchida, M. Dietary fish oil and Undaria pinnatifida (wakame) synergistically decrease rat serum and liver triacylglycerol. J. Nutr. 2002, 132, 742–747.
- Li, L.; Wang, Y.; Yuan, J.; Liu, Z.; Ye, C.; Qin, S. Undaria pinnatifida improves obesity-related outcomes in association with gut microbiota and metabolomics modulation in high-fat diet-fed mice. Appl. Microbiol. Biotechnol. 2020, 104, 10217–10231.
- Gabbia, D.; Saponaro, M.; Sarcognato, S.; Guido, M.; Ferri, N.; Carrara, M.; De Martin, S. Fucus vesiculosus and Aescophyllum nodosum ameliorate liver function by reducing diet-induced steatosis in rats. Mar. Drugs 2020, 18, 62.
- Kang, M.C.; Lee, H.G.; Kim, H.S.; Song, K.M.; Chun, Y.G.; Lee, M.H.; Kim, B.K.; Jeon, Y.J. Anti-Obesity effects of Sargassum thunbergii via downregulation of adipogenesis gene and upregulation of thermogenic genes in high-fat diet-induced obese mice. Nutrients 2020, 12, 3325.
- Murakami, S.; Hirazawa, C.; Ohya, T.; Yoshikawa, R.; Mizutani, T.; Ma, N.; Moriyama, M.; Ito, T.; Matsuzaki, C. The edible brown seaweed Sargassum horneri (Turneri) C. Agardh ameliorated high-fat diet-induced obesity, diabetes, and hepatic steatosis in mice. Nutrients 2021, 13, 551.
- Ebrahimi-Mameghani, M.; Aliashrafi, S.; Javadzadeh, Y.; AsghariJafarabadi, M. The Effect of Chlorella vulgaris Supplementation on Liver En-zymes, Serum Glucose and Lipid Profile in Patients with Non-Alcoholic Fatty Liver Disease. Health Promot. Perspect. 2014, 4, 107–115.
- Li, H.; Gu, Y.; Wu, X.; Rayamajhi, S.; Bian, S.; Zhang, Q.; Meng, G.; Liu, L.; Wu, H.; Zhang, S.; et al. Association between consumption of edible seaweeds and newly diagnosed non-alcohol fatty liver disease: The TCLSIH Cohort Study. Liver Int. 2021, 41, 311–320.
- Meroni, M.; Longo, M.; Rustichelli, A.; Dongiovanni, P. Nutrition and Genetics in NAFLD: The Perfect Binomium. Int. J. Mol. Sci. 2020, 21, 2986.
- Meroni, M.; Longo, M.; Dongiovanni, P. The Role of Probiotics in Nonalcoholic Fatty Liver Disease: A New Insight into Therapeutic Strategies. Nutrients 2019, 11, 2642.
- Cian, R.E.; Drago, S.R.; de Medina, F.S.; Martínez-Augustin, O. Proteins and Carbohydrates from Red Seaweeds: Evidence for Beneficial Effects on Gut Function and Microbiota. Mar. Drugs 2015, 13, 5358–5383.
- de Jesus Raposo, M.F.; de Morais, A.M.; de Morais, R.M. Emergent Sources of Prebiotics: Seaweeds and Microalgae. Mar. Drugs 2016, 14, 27.
- Zheng, L.X.; Chen, X.Q.; Cheong, K.L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol. 2020, 151, 344–354.




