
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Takashi Eguchi | + 1330 word(s) | 1330 | 2021-07-02 11:54:45 |
Video Upload Options
A lung segmentectomy, a type of sublobar resection, preserves more pulmonary function than is lobectomy. The use of minimally invasive lung segmentectomy for early-stage lung cancer has been increasing. This procedure is associated with technical challenges because (1) it requires a thorough understanding of the complex segmental anatomy that frequently accompanies anomalies, and (2) it is difficult to confirm the location of small tumors during minimally invasive surgery, which makes it difficult to obtain adequate surgical margins.
1. Introduction
The basis of cancer treatment has been shifting from a “one-drug (treatment)-fits-all approach” to a “precision medicine” approach, which is a treatment tailored to individual patients. However, for thoracic surgeons, lobectomy has been the evidence-based standard surgical treatment for early-stage lung cancer since 1995 when a randomized control trial (LCSG 821) compared lobectomy and sublobar resection (including segmentectomy and wedge resection), demonstrating the former’s prognostic superiority [1]. Recently, the results of a large, multi-institutional, prospective randomized trial assessing the outcomes of segmentectomy and lobectomy (JCOG0802) were demonstrated (the 101st Annual Meeting of the American Association of Thoracic Surgery, 30 April–2 May 2021); the overall survival of patients who underwent segmentectomy was significantly superior to that of patients who underwent lobectomy, suggesting that segmentectomy is a potentially definitive, standard surgical approach in patients with small, early-stage lung cancers.
With the increased detection of early-stage lung cancer and the technical advancement of minimally invasive surgery (MIS) in the field of thoracic surgery, lung segmentectomy using MIS, including video- and robot-assisted thoracic surgery, has been widely adopted. However, lung segmentectomy can be technically challenging for thoracic surgeons due to (1) complex segmental and subsegmental anatomy with frequent anomalies and (2) difficulty in localizing deep, small, and impalpable tumors, leading to difficulty in obtaining adequate margins. To overcome these challenges, several studies have investigated lung segmental anatomy and preoperative simulation based on individual anatomy by three-dimensional (3D) CT [2][3][4][5][6][7], efforts to localize tumors intraoperatively [8][9][10] and identify the intersegmental plane [11][12].
2. Minimally Invasive Segmentectomy
Despite wide variations in lung segmentectomy, they can be classified into simple (typical) and complex (atypical) [13][14]. In general, the MIS approach for segmentectomies, particularly complex segmentectomies, requires greater skill than the open approach. Therefore, simple MIS segmentectomies have been relatively widely performed compared to complex MIS segmentectomies [13][15].
In 2005, Okada et al. introduced their techniques for anatomical segmentectomy using “hybrid VATS,” which is defined as a minimally invasive approach under direct visualization with muscle-sparing mini-thoracotomy and video assistance [16]. The technical and oncological feasibility of this MIS approach for complex segmentectomy has been reported [14][17]. Several reports have demonstrated that complete VATS, which refers to a procedure that is performed primarily with a monitored view without direct visualization or rib spreading, and a recently emerged uniport VATS are technically feasible for complex segmentectomy [18][19][20]. However, their learning curves and generalizability remain unclear. Recently, the RATS approach has gained popularity in the field of general thoracic surgery, with several reported advantages over VATS, including ergonomic design, 3D-binocular vision, elimination of tremors, increased degree of motion, and enhanced manipulation. Such advantages are considered to enhance precise movement, surgeon view, and dexterity; and make MIS segmentectomy easier to adopt and perform [21][22][23].
3.Planning and Navigation for Segmentectomy
In decision-making during lung segmentectomy, thoracic surgeons should be confident of the following: (i) which branches should be divided or preserved; (ii) the appropriate sequential order for dividing segmental branches of the pulmonary artery, pulmonary vein, and bronchi; (iii) the margin distance can be obtained by a planned procedure; and (iv) the location of the intersegmental plane and tumor. These may be challenging for thoracic surgeons because of the wide variety of segmental/subsegmental branching patterns of the pulmonary vessels and bronchi with frequent anomalies. Therefore, preoperative planning and intraoperative navigation are useful in segmentectomy.
Three-dimensional computed tomography (3DCT)-based anatomical evaluation in individual patients is useful for preoperative planning and intraoperative navigation for segmentectomy. However, there remain some potential drawbacks, as follows: (a) this is a time-consuming process that requires technical skill in preparing detailed 3D images for surgeons; (b) difficulty in developing 3DCT from CT scans without contrast; and (c) difficulty in obtaining more realistic simulation images, such as an image showing stumps of segmental branches with divided intersegmental planes and preserved intersegmental veins. Recently, volume-rendering reconstruction software dedicated to lung segmentectomy was approved in Japan (REVORAS, Ziosoft, Inc., Tokyo, Japan) (Figure 1). The advantages and novelty of this segmentectomy planning by REVORAS are as follows: (i) an easy-to-use tool for surgeons; (ii) accepted use of non-contrast CT for “one-click 3D reconstruction”; (iii) separate segmentectomy planning based on the dividing bronchus or pulmonary artery, providing more reliable planning with an accurate resection margin according to the intraoperative identification technique of the intersegmental plane (ventilation-based identification fits for planning based on the bronchial stump, whereas perfusion-based identification fits for planning based on the arterial stump); and (iv) surgeon-oriented, intraoperatively useful 3D images with “key parts for segmentectomy,” including the bronchial and vascular stumps and intersegmental plane.
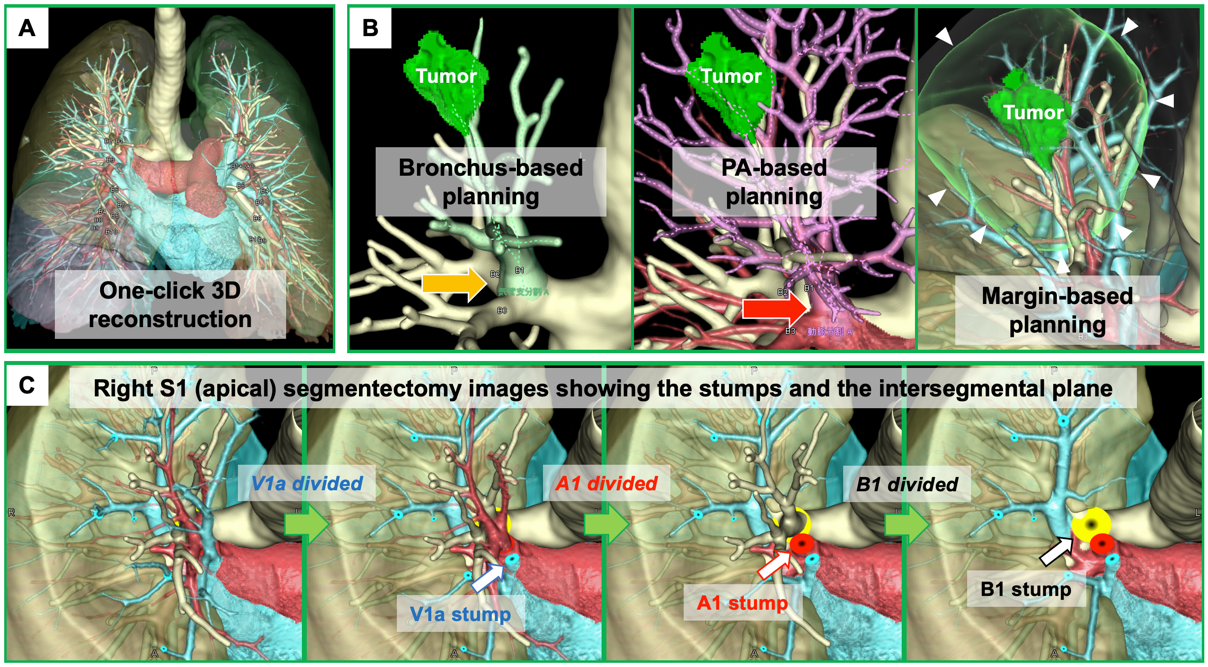 Figure 1. Three-dimensional computed tomography (3DCT) images in segmentectomy planning using a novel 3DCT processing software
Figure 1. Three-dimensional computed tomography (3DCT) images in segmentectomy planning using a novel 3DCT processing software
4. Localization of Small Tumors
Tiny nodules or subsolid nodules detected by CT scans are often difficult to palpate, particularly when they exist deep in the lung parenchyma. One of the drawbacks of the MIS approach is the difficulty in palpating small nodules. Therefore, intraoperative tumor localization without palpation is important, especially for MIS, and various localization strategies have been proposed. There are three categories in localization procedures based on their use of markers and the approach for placement: (1) CT-guided percutaneous marker placement, (2) bronchoscopic marker placement, and (3) intraoperative ultrasonography without marker placement. Important qualities in tumor localization are safety, technical feasibility, accurate intraoperative localization, and real-time monitoring of the tumor location.
Radiofrequency identification (RFID) is a newly developed technique for tumor localization [10][24][25][26] has been approved for clinical use in Japan (SuReFInD, Hogy Medical Co., Ltd., Tokyo, Japan). In clinical practice, the RFID localization technique involves the following four steps: (1) bronchoscopic placement of the marker into the peripheral bronchus within or adjacent to the tumor using a virtual bronchoscopic navigation technique under fluoroscopic or CT guidance within two days before surgery; (2) CT scan to confirm the exact location of the marker and the 3D relationship between the marker and the tumor; (3) intraoperative identification of the marker using a handheld, sterile detection probe; and (4) real-time monitoring of the tumor location when dividing the lung (Figure 2). The advantages of this procedure include: (i) the marker can stay in place for at least 48 h, (ii) we can detect multiple markers separately with a unique identification in each marker, and (iii) the location of the marker can be easily confirmed even when dividing the lungs (real-time monitoring) [27][28].
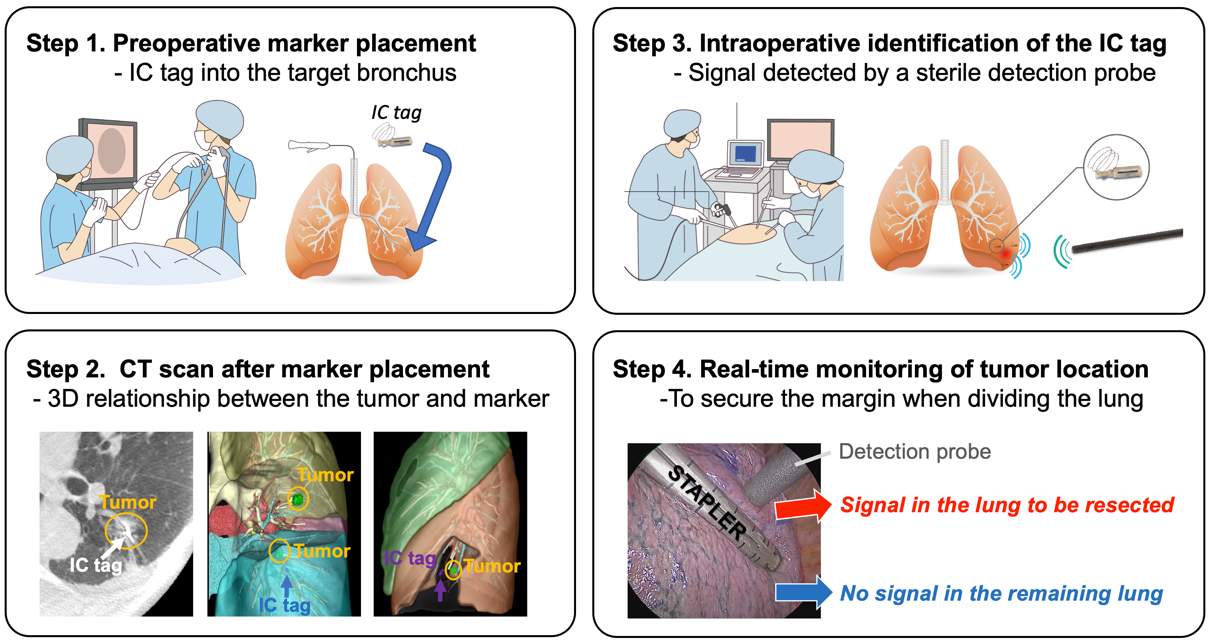 Figure 2. Schema of four steps in radiofrequency identification (RFID) marking.
Figure 2. Schema of four steps in radiofrequency identification (RFID) marking.
5. Precision Lung Segmentectomy in Shinshu University Hospital
To perform safe, secure, and precise lung segmentectomy, even for deep, small, and impalpable tumors, we adopted two novel technologies in Shinshu University Hospital: 1) surgeon-oriented 3DCT-based resection planning using a novel 3DCT processing software called “REVORAS,” and 2) tumor localization using a novel RFID technology called “SuReFInD,” details of which were described earlier. This section demonstrates the utilization of these novel techniques in clinical practice (Figure 3).
In Shinshu University, the criteria for lung segmentectomy for malignant diseases are shown in Figure 3. For all the patients who undergo segmentectomy, we create 3DCT images to perform segmentectomy planning. Based on the planning with expected volume loss and margin distance, we determine which segment(s) and/or subsegment(s) should be removed and which segmental/subsegmental branches should be divided or preserved. Then, we determine whether tumor localization is required for the planned segmentectomy with adequate margins. We visualize 3DCT images for segmentectomy planning for intraoperative navigation using two additional monitors next to the surgeons during surgery.
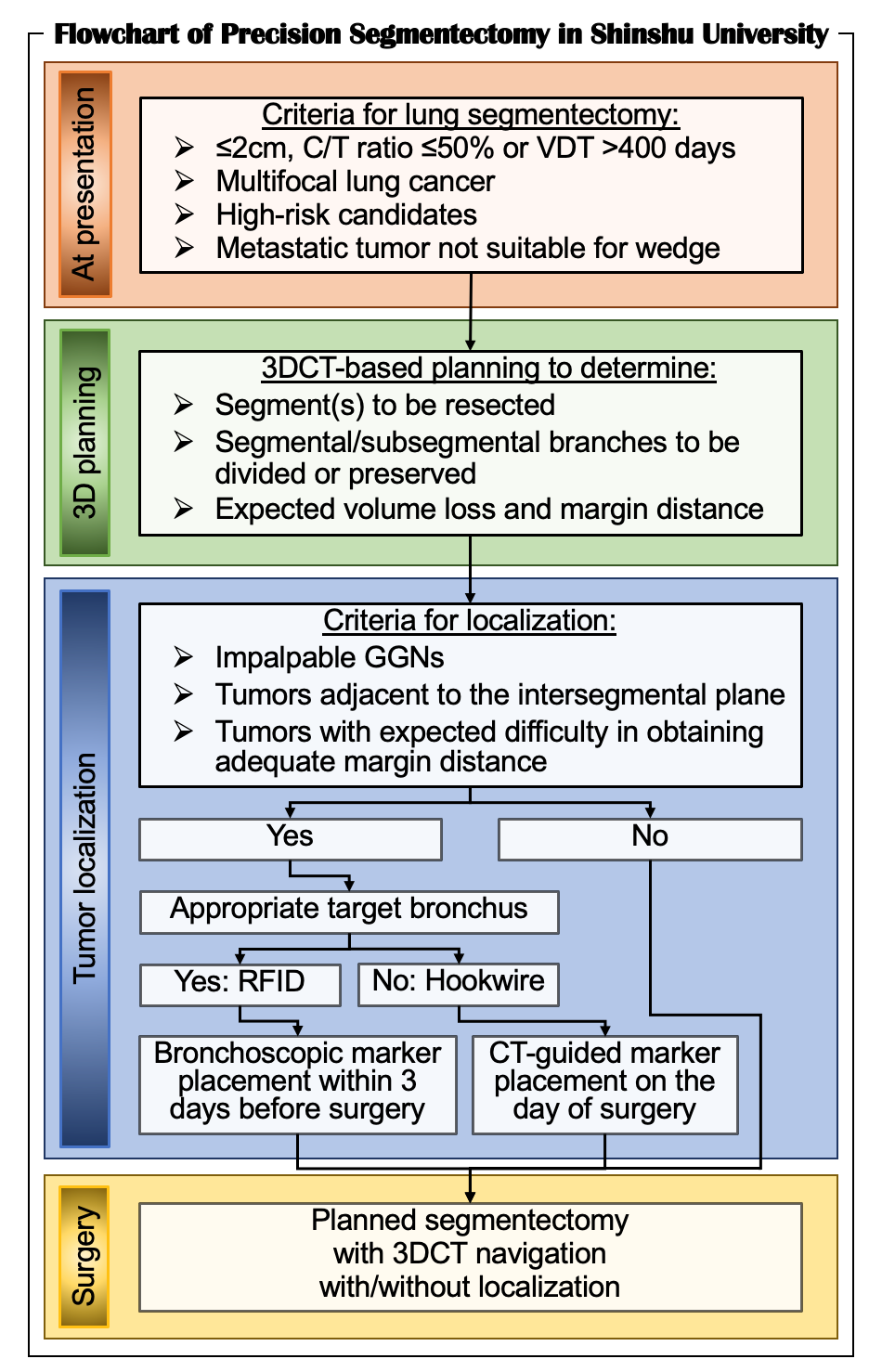 Figure 3. Flowchart of precision segmentectomy in Shinshu University.
Figure 3. Flowchart of precision segmentectomy in Shinshu University.
6. Conclusions
In this era of personalized cancer treatment, thoracic surgeons should be familiar with technical advances in MIS segmentectomy to provide safe and secure “precision segmentectomy” based on individual tumor location, lung function, and anatomy.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995; 60: 615-622; discussion 622-613.
- Eguchi T, Takasuna K, Kitazawa A et al. Three-dimensional imaging navigation during a lung segmentectomy using an iPad. Eur J Cardiothorac Surg 2012; 41: 893-897.
- Shimizu K, Nakazawa S, Nagashima T et al. 3D-CT anatomy for VATS segmentectomy. J Vis Surg 2017; 3: 88.
- Nakazawa S, Hanawa R, Nagashima T et al. Segmentectomy Guided by 3D Images Reconstructed from Non-enhanced Computed Tomography Data. Ann Thorac Surg 2020.
- Akiba T, Marushima H, Odaka M et al. Pulmonary vein analysis using three-dimensional computed tomography angiography for thoracic surgery. Gen Thorac Cardiovasc Surg 2010; 58: 331-335.
- Fukuhara K, Akashi A, Nakane S, Tomita E. Preoperative assessment of the pulmonary artery by three-dimensional computed tomography before video-assisted thoracic surgery lobectomy. Eur J Cardiothorac Surg 2008; 34: 875-877.
- Matsumoto T, Kanzaki M, Amiki M et al. Comparison of three software programs for three-dimensional graphic imaging as contrasted with operative findings. Eur J Cardiothorac Surg 2012; 41: 1098-1103.
- Sato M. Precise sublobar lung resection for small pulmonary nodules: localization and beyond. Gen Thorac Cardiovasc Surg 2020; 68: 684-691.
- Powell TI, Jangra D, Clifton JC et al. Peripheral lung nodules: fluoroscopically guided video-assisted thoracoscopic resection after computed tomography-guided localization using platinum microcoils. Ann Surg 2004; 240: 481-488; discussion 488-489.
- Yutaka Y, Sato T, Matsushita K et al. Three-dimensional Navigation for Thoracoscopic Sublobar Resection Using a Novel Wireless Marking System. Semin Thorac Cardiovasc Surg 2018; 30: 230-237.
- Andolfi M, Potenza R, Seguin-Givelet A, Gossot D. Identification of the intersegmental plane during thoracoscopic segmentectomy: state of the art. Interact Cardiovasc Thorac Surg 2020; 30: 329-336.
- Yajima T, Shimizu K, Mogi A et al. Pulmonary Artery Compression Facilitates Intersegmental Border Visualization. Ann Thorac Surg 2019; 108: e141-e143.
- Nakazawa S, Shimizu K, Mogi A, Kuwano H. VATS segmentectomy: past, present, and future. Gen Thorac Cardiovasc Surg 2018; 66: 81-90.
- Handa Y, Tsutani Y, Mimae T et al. Complex segmentectomy in the treatment of stage IA non-small-cell lung cancer. Eur J Cardiothorac Surg 2020; 57: 114-121.
- Ceppa DP, Balderson S, D'Amico TA. Technique of thoracoscopic basilar segmentectomy. Semin Thorac Cardiovasc Surg 2011; 23: 64-66.
- Okada M, Sakamoto T, Yuki T et al. Hybrid surgical approach of video-assisted minithoracotomy for lung cancer: significance of direct visualization on quality of surgery. Chest 2005; 128: 2696-2701.
- Okada M, Mimura T, Ikegaki J et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007; 133: 753-758.
- Yajima, T.; Shimizu, K.; Mogi, A.; Kosaka, T.; Nakazawa, S.; Shirabe, K. Thoracoscopic right middle lobe segmentectomy. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 344–347.
- Endoh, M.; Oizumi, H.; Kato, H.; Suzuki, J.; Watarai, H.; Masaoka, T.; Sadahiro, M. Posterior approach to thoracoscopic pulmonary segmentectomy of the dorsal basal segment: A single-institute retrospective review. J. Thorac. Cardiovasc. Surg. 2017, 154, 1432–1439.
- Yajima, T.; Shimizu, K.; Mogi, A.; Kosaka, T.; Nakazawa, S.; Shirabe, K. Medial-basal segment (S(7))-sparing right basal segmentectomy. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 306–309.
- Pardolesi A, Park B, Petrella F et al. Robotic anatomic segmentectomy of the lung: technical aspects and initial results. Ann Thorac Surg 2012; 94: 929-934.
- Veronesi G, Novellis P, Voulaz E, Alloisio M. Robot-assisted surgery for lung cancer: State of the art and perspectives. Lung Cancer 2016; 101: 28-34.
- Perroni G, Veronesi G. Robotic segmentectomy: indication and technique. J Thorac Dis 2020; 12: 3404-3410.
- Yutaka Y, Sato T, Zhang J et al. Localizing small lung lesions in video-assisted thoracoscopic surgery via radiofrequency identification marking. Surg Endosc 2017; 31: 3353-3362.
- Kojima F, Sato T, Takahata H et al. A novel surgical marking system for small peripheral lung nodules based on radio frequency identification technology: Feasibility study in a canine model. J Thorac Cardiovasc Surg 2014; 147: 1384-1389.
- Kojima F, Sato T, Tsunoda S et al. Development of a novel marking system for laparoscopic gastrectomy using endoclips with radio frequency identification tags: feasibility study in a canine model. Surg Endosc 2014; 28: 2752-2759.
- Kato A, Yasuo M, Tokoro Y et al. Virtual bronchoscopic navigation as an aid to CT-guided transbronchial biopsy improves the diagnostic yield for small peripheral pulmonary lesions. Respirology 2018; 23: 1049-1054.
- Sato T, Yutaka Y, Ueda Y et al. Diagnostic yield of electromagnetic navigational bronchoscopy: results of initial 35 cases in a Japanese institute. J Thorac Dis 2018; 10: S1615-s1619.




