Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marcin Samiec | + 1783 word(s) | 1783 | 2021-07-21 10:05:27 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Samiec, M. Cloned Goats. Encyclopedia. Available online: https://encyclopedia.pub/entry/12272 (accessed on 08 February 2026).
Samiec M. Cloned Goats. Encyclopedia. Available at: https://encyclopedia.pub/entry/12272. Accessed February 08, 2026.
Samiec, Marcin. "Cloned Goats" Encyclopedia, https://encyclopedia.pub/entry/12272 (accessed February 08, 2026).
Samiec, M. (2021, July 21). Cloned Goats. In Encyclopedia. https://encyclopedia.pub/entry/12272
Samiec, Marcin. "Cloned Goats." Encyclopedia. Web. 21 July, 2021.
Copy Citation
The Cloned Goats or Transgenic Cloned Goats are generated and/or multiplied by one the most advanced and widely applied assisted reproductive technologies (ARTs) designated as somatic cell cloning or somatic cell nuclear transfer (SCNT). The SCNT-derived goats can provide a valuable tool for a variety of transgenic, biomedical, biopharmaceutical and nutri-biotechnological research.
domestic goat
somatic cell cloning
SCNT-derived embryo
genetically engineered specimen
gene targeting
genome editing
1. Introduction
One of the most rapidly developing strategies for reproductive biotechnology in mammals, including farm livestock species, is cloning by somatic cell nuclear transfer (SCNT) (Figure 1).
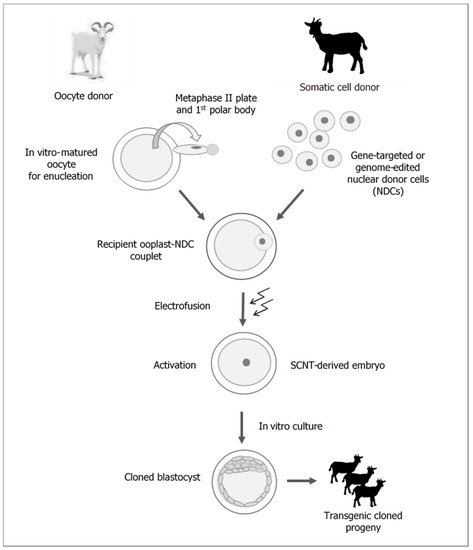
Figure 1. Generation of transgenic cloned goats by somatic cell nuclear transfer (SCNT).
It is beyond any doubt that the attractiveness of cloning techniques results from their potential to generate and multiply transgenic animals, which are valuable due to the expression of modified genes (Figure 1). Furthermore, this attractiveness also depends, to a lesser degree, on the possibility to replicate individuals with excellent, highly heritable breeding (genetic) and performance traits, which may shorten the generation interval and increase the rate of breeding progress. However, the aforementioned areas of research are being explored on a limited scale due to the high costs associated with the cloning procedure resulting from the low efficiency of the method. It is beyond any doubt that the widespread use of cloning methods will be possible once efficacy and repeatable results are guaranteed [1][2][3][4].
The main reason for low pre- and postimplantation developmental potential and poor quality of SCNT-derived embryos is the abnormal adaptation of the transferred somatic cell nuclei to the biochemical conditions of the oocyte cytoplasmic microenvironment, i.e., their incomplete or improper remodeling and reprogramming in the cytoplasm of nuclear-transferred oocytes. The latter also gives rise to the relatively high incidence of congenital malformations (anatomo-, histo-, and physiopathological changes) in cloned fetuses and offspring. This calls for studies aimed at the precise determination of the conditions that facilitate epigenetic reprogramming in the nuclear donor cell genome during the pre- and postimplantation development of SCNT-generated embryos and fetuses of different mammalian species, including the domestic goat [5][6][7][8][9][10]. Promising results were achieved by investigations that focused on the use of extrinsic nonselective agents for stimulating the epigenetically regulated transcriptional activity of genomic DNA in both nuclear donor somatic cells and SCNT-cloned embryos [11][12][13][14][15].
2. Key Issues Related to Biological, Molecular, and Epigenetic Determinants Affecting the Efficacy of Somatic Cell Cloning in Goats
The provenance of somatic cells is a factor that can have a significant impact on cloning efficiency in goats. Relatively few types of nuclear donor cells have been tested for their suitability for the production of cloned embryos, fetuses, and/or offspring in this livestock species. Those that have been tested include cells stemming from several types of tissues collected from both caprine fetuses and adult animals of both sexes and of different ages. Among the nuclear donor cells (NDCs) used for SCNT procedures, mention should be made of: (1) in vitro cultured (transgenic or nontransgenic) fetal dermal fibroblasts [12][16][17][18][19][20][21][22]; (2) juvenile and adult dermal fibroblasts [14][23][24][25][26]; (3) mural granulosa cells isolated from antral ovarian follicles [18][27]; (4) cumulus oophorus cells [18][27][28].
Special consideration should be given to the use of pituicytes, i.e., endocrine cells originating from the anterior pituitary (also known as adenohypophysis) of postpubertal bucks, as a source of nuclear donors for SCNT in goats [29]. To date, this cerebral tissue-specific type of endocrine cell, which synthesizes and secretes tropic hormones, has not been used for SCNT in other species of farm and laboratory animals. It has been postulated, however, that artificial (ectopic) control of metabolic and secretory activities of the endocrine compartment in all lobes of the pituitary gland of livestock species might be feasible through genetic modification (transfection) of pituicytes at the in vitro culture level. In turn, utilizing genetically transformed pituitary-derived glandular cells, which are characterized by inducible expression of recombinant human hormonal proteins or polypeptides, for the generation of transgenic specimens of different mammalian species by somatic cell cloning opens up a variety of new application opportunities. The latter encompass the production of transgenic animal bioreactors, which provide xenogeneic (human) tropic hormones in cytosol extracts (homogenates) of pituicytes or in blood plasma. These hormones are indispensable for the clinical application of therapies for many human monogenic diseases, which induce endocrine-mediated congenital malformations. The transfer of caprine SCNT embryos that had been reconstructed with pituicytes into the reproductive tract of hormonally synchronized recipient females resulted in the birth of a cloned male kid. The results of these experiments confirmed that even the nuclear genome of terminally differentiated somatic cells such as pituicytes can successfully undergo the complete processes of epigenetic remodeling and reprogramming in pre- and postimplantation cloned goat embryos [29].
Incomplete or incorrect epigenetic reprogramming of epigenetic memory, which is encoded in extragenic covalent modifications of the somatic cell nuclear genome, was found to be one of the main factors decreasing the efficiency of somatic cell cloning in mammals, including the domestic goat. Reductions in this efficiency are reflected in the weakened in vitro and/or in vivo developmental potential of SCNT-derived embryos [7][8][30]. Methylation of cytosine residues in CpG islands/dinucleotides is a widely explored/recognized modification of the somatic cell nuclear genome in cloned embryos [6][9][10][31]. Han et al. [32] demonstrated that enzymatic activity of ten-eleven translocation methylcytosine dioxygenase 3 (TET3) is a key molecular mechanism underlying active DNA demethylation in preimplantation goat embryos created by somatic cell cloning. Knocking out the TET3 gene led to the inhibition of active (i.e., DNA replication-independent) demethylation of 5-methylcytosine (5-mC) residues in 2-blastomere-stage cloned goat embryos. As a consequence, this brought about the downregulation of the expression of the pluripotency-related Nanog gene in the inner cell mass (ICM) compartment of the generated blastocysts. In turn, overexpression of the TET3 gene that had been induced by transgenization of in vitro cultured somatic cells resulted in: (1) abundant demethylation of DNA 5-mC residues; (2) declined quantitative profile of 5-mC moieties; (3) increased incidence of 5-hydroxymethylcytosine residues; (4) intensified transcriptional activity of crucial pluripotency-related genes. Furthermore, the use of genetically transformed somatic cells displaying overexpression of the TET3 gene—as nuclear donors for the reconstruction of caprine enucleated oocytes—contributed to an enhancement in the extent of active demethylation of 5-mC residues within somatic cell-inherited nuclear DNA. The latter perpetuated hypomethylation of the somatic cell-derived genome in cleaved SCNT embryos, subsequently triggering remarkable improvements in their in vitro and in vivo developmental capabilities. It follows that overexpression of the TET3 gene in NDCs significantly ameliorates the efficacy of somatic cell cloning in goats.
The developmental potential of the mammalian SCNT embryos, including their caprine representatives, which inherit the somatic cell nuclear genome as a result of the reconstruction of enucleated oocytes, is highly dependent on the level of epigenetic modifications within DNA and chromatin-derived histones of the NDCs undergoing long-term in vitro culture [33][34][35]. One of the strategies to reverse advanced alterations in the pattern of epigenetic covalent modifications within somatic cell nuclei, which encompass rapid DNA methylation and a decrease in the quantitative profile of histone protein acetylation, appears to be the exposure of NDCs, SCNT-derived oocytes, and corresponding embryos to reversible agents inhibiting biocatalytic activity of DNA methyltransferases (DNMTs) and/or histone deacetylases (HDACs). The use of nonselective or selective promoters of epigenetically determined transcriptional activity of genomic DNA in both in vitro cultured NDCs and cloned embryos is supposed to be an approach that allows for proper reprogramming of somatic cell nuclei [7][12][14]. Exogenously modulating the epigenetic memory profile of genomic DNA appears to contribute to successfully reversing the “transcriptional clock” of a differentiated somatic cell nucleus to the status of a cell nucleus characteristic of a totipotent or pluripotent embryonic cell. As a consequence, such efforts induce the restoration of the expression pattern that is seen in genes that are inevitable in the initiation and progress of the developmental program of SCNT-derived embryos [36][37]. This results in a reduction in the methylation degree of DNA cytosine residues and an increase in the acetylation of nuclear chromatin histone proteins [38][39]. In turn, the previously specified processes were shown to bring about recapitulation and perpetuation of the correct and faithful profiles of transcriptional activities observed both for the genes indispensable to induce and maintain the totipotency/pluripotency states and for the genes encoding enzymes responsible for endogenous epigenetic modifications during pre- and postimplantation embryogenesis. The totipotency/pluripotency-related genes encompass those that encode such proteins as: e.g., octamer-binding transcription factor 3/4 (Oct3/4), the homeobox-containing transcription factor Nanog, whose name stems from the Celtic/Irish mythical word Tír na nÓg (i.e., The Land of the Ever-Young), DNA-binding proto-oncogenic/oncogenic transcription factor c-Myc, sex-determining region Y (SRY)-box 2 transcription factor (Sox2), Krüppel-like factor 4 (Klf4), reduced expression protein 1 (Rex1), and caudal-type homeobox protein 2 (Cdx2). The epigenetic modifier genes involve those that encode such enzymatic proteins as: e.g., DNA methyltransferase type 1 (DNMT1), DNA methyltransferase type 3a (DNMT3a), DNA methyltransferase type 3b (DNMT3b), histone deacetylase type 1 (HDAC1), histone deacetylase type 2 (HDAC2), histone methyltransferase (HMT), and histone acetyltransferase (HAT) [3][40][41]. In the wake of recent research, innovative and highly efficient methods were developed to modulate the epigenetic memory profile of mammalian SCNT embryos, including their caprine counterparts. These methods are focused on applying exogenous nonselective HDAC inhibitors (such as trichostatin A, valproic acid, and scriptaid) and/or nonselective DNMT inhibitors (such as 5-aza-2′-deoxycytidine) or selective inhibitors lysine K4 demethylases specific for histones H3 within the nucleosomal core of nuclear chromatin (such as trans-2-phenylcyclopropylamine (tranylcypromine; 2-PCPA)). The aforementioned strategies may considerably modify the epigenetically determined reprogramming of the somatic cell nuclear genome in SCNT-derived embryos. The final results of these innovative solutions turn out to be significant enhancements of the pre- and/or postimplantation developmental competence and an improvement in the molecular quality of cloned embryos in mammals, including the domestic goat [12][13][14][41][42][43].
3. Conclusions and Future Goals
Although the efficiency of somatic cell cloning in goats remains relatively low, further studies are necessary because modern ART has important implications in the fields of goat breeding, the transgenics of this mammalian species, agri-food biotechnology, biomedicine, and biopharmacy.
An increase in the efficiency of somatic cell cloning techniques in the domestic goat can be brought about by further intensive research into improving both developmental competence and the parameters related to the molecular and epigenetic quality of SCNT-derived embryos. The latter can be achieved by efforts aimed at using nonselective or selective inhibitors of DNMTs and HDACs, which would in turn lead to enhancements in the reprogrammability of the epigenetic memory profile within genomic DNA of NDCs, nuclear-transferred oocytes, and the corresponding caprine cloned embryos. This is a sine qua non condition for the practical use of SCNT-based cloning, and thus for the production of genetically transformed goats for the purposes of human nutrition technology based on a meat diet.
References
- Samiec, M.; Skrzyszowska, M. Transgenic mammalian species, generated by somatic cell cloning, in biomedicine, biopharmaceutical industry and human nutrition/dietetics—Recent achievements. Pol. J. Vet. Sci. 2011, 14, 317–328.
- Samiec, M.; Skrzyszowska, M. The possibilities of practical application of transgenic mammalian species generated by somatic cell cloning in pharmacology, veterinary medicine and xenotransplantology. Pol. J. Vet. Sci. 2011, 14, 329–340.
- Hall, V.; Hinrichs, K.; Lazzari, G.; Betts, D.H.; Hyttel, P. Early embryonic development, assisted reproductive technologies, and pluripotent stem cell biology in domestic mammals. Vet. J. 2013, 197, 128–142.
- Skrzyszowska, M.; Samiec, M. Generation of monogenetic cattle by different techniques of embryonic cell and somatic cell cloning—Their application to biotechnological, agricultural, nutritional, biomedical and transgenic research—A review. Ann. Anim. Sci. 2021, 21, 1–15.
- Martins, L.T.; Neto, S.G.; Tavares, K.C.; Calderón, C.E.; Aguiar, L.H.; Lazzarotto, C.R.; Ongaratto, F.L.; Rodrigues, V.H.; Carneiro Ide, S.; Rossetto, R.; et al. Developmental outcome and related abnormalities in goats: Comparison between somatic cell nuclear transfer- and in vivo-derived concepti during pregnancy through term. Cell. Reprogram. 2016, 18, 264–279.
- Samiec, M.; Skrzyszowska, M. Can reprogramming of overall epigenetic memory and specific parental genomic imprinting memory within donor cell-inherited nuclear genome be a major hindrance for the somatic cell cloning of mammals?—A review. Ann. Anim. Sci. 2018, 18, 623–638.
- Samiec, M.; Skrzyszowska, M. Intrinsic and extrinsic molecular determinants or modulators for epigenetic remodeling and reprogramming of somatic cell-derived genome in mammalian nuclear-transferred oocytes and resultant embryos. Pol. J. Vet. Sci. 2018, 21, 217–227.
- Yang, M.; Perisse, I.; Fan, Z.; Regouski, M.; Meyer-Ficca, M.; Polejaeva, I.A. Increased pregnancy losses following serial somatic cell nuclear transfer in goats. Reprod. Fertil. Dev. 2018, 30, 1443–1453.
- Deng, M.; Liu, Z.; Chen, B.; Wan, Y.; Yang, H.; Zhang, Y.; Cai, Y.; Zhou, J.; Wang, F. Aberrant DNA and histone methylation during zygotic genome activation in goat cloned embryos. Theriogenology 2020, 148, 27–36.
- Deng, M.; Zhang, G.; Cai, Y.; Liu, Z.; Zhang, Y.; Meng, F.; Wang, F.; Wan, Y. DNA methylation dynamics during zygotic genome activation in goat. Theriogenology 2020, 156, 144–154.
- Iager, A.E.; Ragina, N.P.; Ross, P.J.; Beyhan, Z.; Cunniff, K.; Rodriguez, R.M.; Cibelli, J.B. Trichostatin A improves histone acetylation in bovine somatic cell nuclear transfer early embryos. Cloning Stem Cells 2008, 10, 371–379.
- Mao, T.; Han, C.; Deng, R.; Wei, B.; Meng, P.; Luo, Y.; Zhang, Y. Treating donor cells with 2-PCPA corrects aberrant histone H3K4 dimethylation and improves cloned goat embryo development. Syst. Biol. Reprod. Med. 2018, 64, 174–182.
- Samiec, M.; Romanek, J.; Lipiński, D.; Opiela, J. Expression of pluripotency-related genes is highly dependent on trichostatin A-assisted epigenomic modulation of porcine mesenchymal stem cells analysed for apoptosis and subsequently used for generating cloned embryos. Anim. Sci. J. 2019, 90, 1127–1141.
- Skrzyszowska, M.; Samiec, M. Enhancement of in vitro developmental outcome of cloned goat embryos after epigenetic modulation of somatic cell-inherited nuclear genome with trichostatin A. Ann. Anim. Sci. 2020, 20, 97–108.
- Wiater, J.; Samiec, M.; Skrzyszowska, M.; Lipiński, D. Trichostatin A-assisted epigenomic modulation affects the expression profiles of not only recombinant human α1,2-fucosyltransferase and α-galactosidase A enzymes but also Galα1→3Gal epitopes in porcine bi-transgenic adult cutaneous fibroblast cells. Int. J. Mol. Sci. 2021, 22, 1386.
- Reggio, B.C.; James, A.N.; Green, H.L.; Gavin, W.G.; Behboodi, E.; Echelard, Y.; Godke, R.A. Cloned transgenic offspring resulting from somatic cell nuclear transfer in the goat: Oocytes derived from both follicle-stimulating hormone-stimulated and nonstimulated abattoir-derived ovaries. Biol. Reprod. 2001, 65, 1528–1533.
- Keefer, C.L.; Baldassarre, H.; Keyston, R.; Wang, B.; Bhatia, B.; Bilodeau, A.S.; Zhou, J.F.; Leduc, M.; Downey, B.R.; Lazaris, A.; et al. Generation of dwarf goat (Capra hircus) clones following nuclear transfer with transfected and nontransfected fetal fibroblasts and in vitro-matured oocytes. Biol. Reprod. 2001, 64, 849–856.
- Keefer, C.L.; Keyston, R.; Lazaris, A.; Bhatia, B.; Begin, I.; Bilodeau, A.S.; Zhou, F.J.; Kafidi, N.; Wang, B.; Baldassarre, H.; et al. Production of cloned goats after nuclear transfer using adult somatic cells. Biol. Reprod. 2002, 66, 199–203.
- Zou, X.; Wang, Y.; Cheng, Y.; Yang, Y.; Ju, H.; Tang, H.; Shen, Y.; Mu, Z.; Xu, S.; Du, M. Generation of cloned goats (Capra hircus) from transfected foetal fibroblast cells, the effect of donor cell cycle. Mol. Reprod. Dev. 2002, 61, 164–172.
- Baldassarre, H.; Wang, B.; Pierson, J.; Neveu, N.; Lapointe, J.; Cote, F.; Kafidi, N.; Keefer, C.L.; Lazaris, A.; Karatzas, C.N. Prepubertal propagation of transgenic cloned goats by laparoscopic ovum pick-up and in vitro embryo production. Cloning Stem Cells 2004, 6, 25–29.
- Zhu, H.; Hu, L.; Liu, J.; Chen, H.; Cui, C.; Song, Y.; Jin, Y.; Zhang, Y. Generation of β-lactoglobulin-modified transgenic goats by homologous recombination. FEBS J. 2016, 283, 4600–4613.
- Yuan, Y.G.; Song, S.Z.; Zhu, M.M.; He, Z.Y.; Lu, R.; Zhang, T.; Mi, F.; Wang, J.Y.; Cheng, Y. Human lactoferrin efficiently targeted into caprine beta-lactoglobulin locus with transcription activator-like effector nucleases. Asian Australas. J. Anim. Sci. 2017, 30, 1175–1182.
- Behboodi, E.; Memili, E.; Melican, D.T.; Destrempes, M.M.; Overton, S.A.; Williams, J.L.; Flanagan, P.A.; Butler, R.E.; Liem, H.; Chen, L.H.; et al. Viable transgenic goats derived from skin cells. Transgenic Res. 2004, 13, 215–224.
- Wan, Y.J.; Zhang, Y.L.; Zhou, Z.R.; Jia, R.X.; Li, M.; Song, H.; Wang, Z.Y.; Wang, L.Z.; Zhang, G.M.; You, J.H.; et al. Efficiency of donor cell preparation and recipient oocyte source for production of transgenic cloned dairy goats harboring human lactoferrin. Theriogenology 2012, 78, 583–592.
- Zhou, Z.R.; Zhong, B.S.; Jia, R.X.; Wan, Y.J.; Zhang, Y.L.; Fan, Y.X.; Wang, L.Z.; You, J.H.; Wang, Z.Y.; Wang, F. Production of myostatin-targeted goat by nuclear transfer from cultured adult somatic cells. Theriogenology 2013, 79, 225–233.
- Kumar, D.; Sarkhel, B.C. Differential expression pattern of key regulatory developmental genes in pre-implant zona free cloned vs in vitro fertilized goat embryos. Gene Expr. Patterns 2017, 25, 118–123.
- Baldassarre, H.; Wang, B.; Kafidi, N.; Keefer, C.L.; Lazaris, A.; Karatzas, C.N. Advances in the production and propagation of transgenic goats using laparoscopic ovum pick-up and in vitro embryo production technologies. Theriogenology 2002, 57, 275–284.
- Cheng, Y.; Wang, Y.G.; Luo, J.P.; Shen, Y.; Yang, Y.F.; Ju, H.M.; Zou, X.G.; Xu, S.F.; Lao, W.D.; Du, M. Cloned goats produced from the somatic cells of an adult transgenic goat. Sheng Wu Gong Cheng Xue Bao (Chin. J. Biotechnol.) 2002, 18, 79–83. (In Chinese)
- Ohkoshi, K.; Takahashi, S.; Koyama, S.; Akagi, S.; Adachi, N.; Furusawa, T.; Fujimoto, J.; Takeda, K.; Kubo, M.; Izaike, Y.; et al. In vitro oocyte culture and somatic cell nuclear transfer used to produce a live-born cloned goat. Cloning Stem Cells 2003, 5, 109–115.
- Wang, F.; Kou, Z.; Zhang, Y.; Gao, S. Dynamic reprogramming of histone acetylation and methylation in the first cell cycle of cloned mouse embryos. Biol. Reprod. 2007, 77, 1007–1016.
- Deng, M.; Ren, C.; Liu, Z.; Zhang, G.; Wang, F.; Wan, Y. Epigenetic status of H19-Igf2 imprinted genes and loss of 5-hydroxymethylcytosine in the brain of cloned goats. Cell. Reprogram. 2017, 19, 199–207.
- Han, C.; Deng, R.; Mao, T.; Luo, Y.; Wei, B.; Meng, P.; Zhao, L.; Zhang, Q.; Quan, F.; Liu, J.; et al. Overexpression of Tet3 in donor cells enhances goat somatic cell nuclear transfer efficiency. FEBS J. 2018, 285, 2708–2723.
- Yang, F.; Hao, R.; Kessler, B.; Brem, G.; Wolf, E.; Zakhartchenko, V. Rabbit somatic cell cloning: Effects of donor cell type, histone acetylation status and chimeric embryo complementation. Reproduction 2007, 133, 219–230.
- Yamanaka, K.; Sugimura, S.; Wakai, T.; Kawahara, M.; Sato, E. Acetylation level of histone H3 in early embryonic stages affects subsequent development of miniature pig somatic cell nuclear transfer embryos. J. Reprod. Dev. 2009, 55, 638–644.
- Wan, Y.; Deng, M.; Zhang, G.; Ren, C.; Zhang, H.; Zhang, Y.; Wang, L.; Wang, F. Abnormal expression of DNA methyltransferases and genomic imprinting in cloned goat fibroblasts. Cell Biol. Int. 2016, 40, 74–82.
- Xiong, X.; Lan, D.; Li, J.; Zhong, J.; Zi, X.; Ma, L.; Wang, Y. Zebularine and scriptaid significantly improve epigenetic reprogramming of yak fibroblasts and cloning efficiency. Cell. Reprogram. 2013, 15, 293–300.
- Xiong, X.R.; Li, J.; Fu, M.; Gao, C.; Wang, Y.; Zhong, J.C. Oocyte extract improves epigenetic reprogramming of yak fibroblast cells and cloned embryo development. Theriogenology 2013, 79, 462–469.
- Zhang, Y.; Li, J.; Villemoes, K.; Pedersen, A.M.; Purup, S.; Vajta, G. An epigenetic modifier results in improved in vitro blastocyst production after somatic cell nuclear transfer. Cloning Stem Cells 2007, 9, 357–363.
- Wang, Y.S.; Xiong, X.R.; An, Z.X.; Wang, L.J.; Liu, J.; Quan, F.S.; Hua, S.; Zhang, Y. Production of cloned calves by combination treatment of both donor cells and early cloned embryos with 5-aza-2’-deoxycytidine and trichostatin A. Theriogenology 2011, 75, 819–825.
- Dutta, R.; Malakar, D.; Khate, K.; Sahu, S.; Akshey, Y.; Mukesh, M. A comparative study on efficiency of adult fibroblast, putative embryonic stem cell and lymphocyte as donor cells for production of handmade cloned embryos in goat and characterization of putative ntES cells obtained from these embryos. Theriogenology 2011, 76, 851–863.
- Gómez, M.C.; Biancardi, M.N.; Jenkins, J.A.; Dumas, C.; Galiguis, J.; Wang, G.; Earle Pope, C. Scriptaid and 5-aza-2’deoxycytidine enhanced expression of pluripotent genes and in vitro developmental competence in interspecies black-footed cat cloned embryos. Reprod. Domest. Anim. 2012, 47, 130–135.
- Zhao, J.; Hao, Y.; Ross, J.W.; Spate, L.D.; Walters, E.M.; Samuel, M.S.; Rieke, A.; Murphy, C.N.; Prather, R.S. Histone deacetylase inhibitors improve in vitro and in vivo developmental competence of somatic cell nuclear transfer porcine embryos. Cell. Reprogram. 2010, 12, 75–83.
- Kim, Y.J.; Ahn, K.S.; Kim, M.; Shim, H. Comparison of potency between histone deacetylase inhibitors trichostatin A and valproic acid on enhancing in vitro development of porcine somatic cell nuclear transfer embryos. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 283–289.
More
Information
Subjects:
Genetics & Heredity
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.1K
Revision:
1 time
(View History)
Update Date:
21 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




