
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Amy Arnold | + 1397 word(s) | 1397 | 2021-07-06 11:28:08 | | | |
| 2 | Amy Arnold | + 1 word(s) | 1398 | 2021-07-21 13:30:47 | | | | |
| 3 | Rita Xu | Meta information modification | 1398 | 2021-07-22 03:14:45 | | |
Video Upload Options
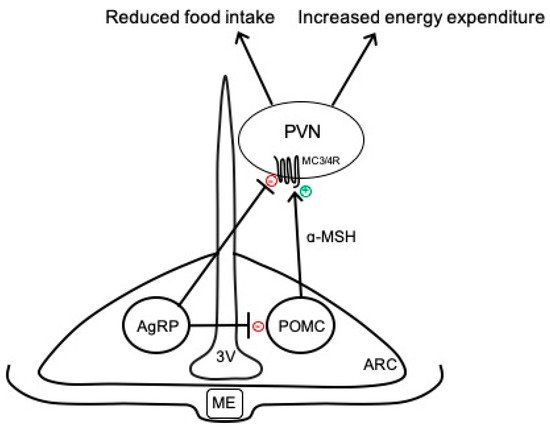
Obesity is a chronic state of energy imbalance that represents a major public health problem and greatly increases the risk for developing hypertension, hyperglycemia, and a multitude of related pathologies that encompass the metabolic syndrome. The underlying mechanisms and optimal treatment strategies for obesity, however, are still not fully understood. A growing area of research is to better understand how peripheral hormones interact with a widely distributed network of brain circuits involved in the control of energy balance. In this regard, the arcuate nucleus of the hypothalamus has emerged as an important brain region due to its ability to sense circulating hormones and to modulate neural pathways controlling food intake and energy expenditure, and blood pressure.
1. Introduction
2. Overview of Arcuate Neurocircuits Controlling Energy Balance
References
- Hales, C.M. Prevalence of Obesity among Adults and Youth: United States, 2015–2016; NCHS Data Brief no. 346; National Center for Health Statistics: Hyattsville, MD, USA, 2017; pp. 1–8.
- Sullivan, P.W.; Morrato, E.H.; Ghushchyan, V.; Wyatt, H.R.; Hill, J.O. Obesity, Inactivity, and the Prevalence of Diabetes and Diabetes-Related Cardiovascular Comorbidities in the U.S., 2000–2002. Diabetes Care 2005, 28, 1599–1603.
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction: The American Heart Association’s Strategic Impact Goal through 2020 and Beyond. Circulation 2010, 121, 586–613.
- Montesi, L.; El Ghoch, M.; Brodosi, L.; Calugi, S.; Marchesini, G.; Dalle Grave, R. Long-Term Weight Loss Maintenance for Obesity: A Multidisciplinary Approach. Diabetes Metab. Syndr. Obes. 2016, 9, 37–46.
- Rodgers, R.J.; Tschöp, M.H.; Wilding, J.P.H. Anti-Obesity Drugs: Past, Present and Future. Dis. Model. Mech. 2012, 5, 621–626.
- Mark, A.L. Cardiovascular Side Effects of Anti-Obesity Drugs: A Yellow Flag in the Race to Effective, Safe Pharmacotherapy for Obesity. Circulation 2009, 120, 719–721.
- Grill, H.J. Distributed Neural Control of Energy Balance: Contributions from Hindbrain and Hypothalamus. Obesity 2006, 14, 216S–221S.
- Münzberg, H.; Qualls-Creekmore, E.; Berthoud, H.-R.; Morrison, C.D.; Yu, S. Neural Control of Energy Expenditure. Handb. Exp. Pharmacol. 2016, 233, 173–194.
- Contreras, C.; Nogueiras, R.; Diéguez, C.; Rahmouni, K.; López, M. Traveling from the Hypothalamus to the Adipose Tissue: The Thermogenic Pathway. Redox Biol. 2017, 12, 854–863.
- Rahmouni, K. Cardiovascular Regulation by the Arcuate Nucleus of the Hypothalamus: Neurocircuitry and Signaling Systems. Hypertension 2016, 67, 1064–1071.
- Mercer, A.J.; Hentges, S.T.; Meshul, C.K.; Low, M.J. Unraveling the Central Proopiomelanocortin Neural Circuits. Front. Neurosci. 2013, 7, 19.
- Jégou, S.; Cone, R.D.; Eberlé, A.N.; Vaudry, H. Melanocortins. In Handbook of Biologically Active Peptides; Elsevier: Amsterdam, The Netherlands, 2013; pp. 838–844. ISBN 978-0-12-385095-9.
- Cai, M.; Hruby, V.J. The Melanocortin Receptor System: A Target for Multiple Degenerative Diseases. Curr. Protein Pept. Sci. 2016, 17, 488–496.
- Vargas-Castillo, A.; Fuentes-Romero, R.; Rodriguez-Lopez, L.A.; Torres, N.; Tovar, A.R. Understanding the Biology of Thermogenic Fat: Is Browning A New Approach to the Treatment of Obesity? Arch. Med. Res. 2017, 48, 401–413.
- Kim, J.D.; Leyva, S.; Diano, S. Hormonal Regulation of the Hypothalamic Melanocortin System. Front. Physiol. 2014, 5, 480.
- Toda, C.; Santoro, A.; Kim, J.D.; Diano, S. POMC Neurons: From Birth to Death. Annu. Rev. Physiol. 2017, 79, 209–236.
- Quarta, C.; Claret, M.; Zeltser, L.M.; Williams, K.W.; Yeo, G.S.H.; Tschöp, M.H.; Diano, S.; Brüning, J.C.; Cota, D. POMC Neuronal Heterogeneity in Energy Balance and beyond: An Integrated View. Nat. Metab. 2021, 3, 299–308.
- Williams, K.W.; Margatho, L.O.; Lee, C.E.; Choi, M.; Lee, S.; Scott, M.M.; Elias, C.F.; Elmquist, J.K. Segregation of Acute Leptin and Insulin Effects in Distinct Populations of Arcuate Proopiomelanocortin Neurons. J. Neurosci. 2010, 30, 2472–2479.
- Hentges, S.T.; Nishiyama, M.; Overstreet, L.S.; Stenzel-Poore, M.; Williams, J.T.; Low, M.J. GABA Release from Proopiomelanocortin Neurons. J. Neurosci. 2004, 24, 1578–1583.
- Wittmann, G.; Hrabovszky, E.; Lechan, R.M. Distinct Glutamatergic and GABAergic Subsets of Hypothalamic Pro-Opiomelanocortin Neurons Revealed by in Situ Hybridization in Male Rats and Mice. J. Comp. Neurol. 2013, 521, 3287–3302.
- Suyama, S.; Yada, T. New Insight into GABAergic Neurons in the Hypothalamic Feeding Regulation. J. Physiol. Sci. 2018, 68, 717–722.
- Tupone, D.; Madden, C.J.; Morrison, S.F. Autonomic Regulation of Brown Adipose Tissue Thermogenesis in Health and Disease: Potential Clinical Applications for Altering BAT Thermogenesis. Front. Neurosci. 2014, 8, 14.
- Song, N.-J.; Chang, S.-H.; Li, D.Y.; Villanueva, C.J.; Park, K.W. Induction of Thermogenic Adipocytes: Molecular Targets and Thermogenic Small Molecules. Exp. Mol. Med. 2017, 49, e353.
- Bartness, T.J.; Ryu, V. Neural Control of White, Beige and Brown Adipocytes. Int. J. Obes. Suppl. 2015, 5, S35–S39.
- Jeremic, N.; Chaturvedi, P.; Tyagi, S.C. Browning of White Fat: Novel Insight Into Factors, Mechanisms, and Therapeutics. J. Cell Physiol. 2017, 232, 61–68.
- Finlin, B.S.; Memetimin, H.; Confides, A.L.; Kasza, I.; Zhu, B.; Vekaria, H.J.; Harfmann, B.; Jones, K.A.; Johnson, Z.R.; Westgate, P.M.; et al. Human Adipose Beiging in Response to Cold and Mirabegron. JCI Insight 2018, 3, e121510.
- Lin, S.; Huang, X.F. Altered Hypothalamic C-Fos-like Immunoreactivity in Diet-Induced Obese Mice. Brain Res. Bull. 1999, 49, 215–219.
- Xin, X.; Storlien, L.H.; Huang, X.F. Hypothalamic C-Fos-like Immunoreactivity in High-Fat Diet-Induced Obese and Resistant Mice. Brain Res. Bull. 2000, 52, 235–242.
- Newton, A.J.; Hess, S.; Paeger, L.; Vogt, M.C.; Fleming Lascano, J.; Nillni, E.A.; Brüning, J.C.; Kloppenburg, P.; Xu, A.W. AgRP Innervation onto POMC Neurons Increases with Age and Is Accelerated with Chronic High-Fat Feeding in Male Mice. Endocrinology 2013, 154, 172–183.
- Deng, G.; Morselli, L.L.; Wagner, V.A.; Balapattabi, K.; Sapouckey, S.A.; Knudtson, K.L.; Rahmouni, K.; Cui, H.; Sigmund, C.D.; Kwitek, A.E.; et al. Single-Nucleus RNA Sequencing of the Hypothalamic Arcuate Nucleus of C57BL/6J Mice After Prolonged Diet-Induced Obesity. Hypertension 2020, 76, 589–597.
- Diano, S.; Liu, Z.-W.; Jeong, J.K.; Dietrich, M.O.; Ruan, H.-B.; Kim, E.; Suyama, S.; Kelly, K.; Gyengesi, E.; Arbiser, J.L.; et al. Peroxisome Proliferation-Associated Control of Reactive Oxygen Species Sets Melanocortin Tone and Feeding in Diet-Induced Obesity. Nat. Med. 2011, 17, 1121–1127.
- Betz, M.J.; Enerbäck, S. Targeting Thermogenesis in Brown Fat and Muscle to Treat Obesity and Metabolic Disease. Nat. Rev. Endocrinol. 2018, 14, 77–87.
- Rahmouni, K.; Morgan, D.A.; Morgan, G.M.; Mark, A.L.; Haynes, W.G. Role of Selective Leptin Resistance in Diet-Induced Obesity Hypertension. Diabetes 2005, 54, 2012–2018.
- Mark, A.L. Selective Leptin Resistance Revisited. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R566–R581.




