Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Domenico Azzolino | + 1861 word(s) | 1861 | 2021-07-09 05:27:21 | | | |
| 2 | Peter Tang | Meta information modification | 1861 | 2021-07-21 11:30:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Azzolino, D. Sarcopenia and Oral Status. Encyclopedia. Available online: https://encyclopedia.pub/entry/12230 (accessed on 08 February 2026).
Azzolino D. Sarcopenia and Oral Status. Encyclopedia. Available at: https://encyclopedia.pub/entry/12230. Accessed February 08, 2026.
Azzolino, Domenico. "Sarcopenia and Oral Status" Encyclopedia, https://encyclopedia.pub/entry/12230 (accessed February 08, 2026).
Azzolino, D. (2021, July 20). Sarcopenia and Oral Status. In Encyclopedia. https://encyclopedia.pub/entry/12230
Azzolino, Domenico. "Sarcopenia and Oral Status." Encyclopedia. Web. 20 July, 2021.
Copy Citation
Aging is accompanied by profound changes in many physiological functions, leading to a decreased ability to cope with stressors. Many changes are subtle, but can negatively affect nutrient intake, leading to overt malnutrition. Poor oral health may affect food selection and nutrient intake, leading to malnutrition and, consequently, to frailty and sarcopenia.
sarcopenia
nutrition
oral health
older people
malnutrition
swallowing
life course approach
1. Introduction
Advancing age is characterized by a progressive decline in multiple physiological functions, leading to an increased vulnerability to stressors and augmented risk of adverse outcomes [1][2][3]. During the aging process, several factors may affect body shape from both clinical and functional perspectives. Reduction in smell and taste senses, poor appetite (the so-called “anorexia of aging”), and decreased energy expenditure may all contribute to poor nutrition. Moreover, illnesses, medications, as well as poor oral health (for example, due to teeth loss and poorly fitting dentures) can exacerbate anorexia [4][5][6]. Nutritional status among older people may be also influenced by living or eating alone, poor financial status, dismobility, and decreased ability to shop or prepare meals [7][8]. Psychosocial factors including loneliness, sleep disorders, dementia, and depression are also recognized to have a negative impact on the dietary intake of older subjects [9].
Furthermore, with aging, there is a progressive loss in muscle mass and strength, whereas fat mass and fat infiltration of muscle increase [10][11]. Sarcopenia is the term, introduced for the first time in 1988 by Irwin Rosenberg, to indicate the pathologic reduction in muscle mass and strength leading to a poor function [12][13]. Interestingly, in recent years, it has been highlighted that sarcopenia is not limited to lower limbs, but is a whole-body process [14][15][16], also affecting the muscles devoted to chewing and swallowing [10][17], with a negative impact on food intake. In fact, atrophy of muscles critical for the respiratory and swallowing functions has been reported [14][18][19][20][21][22].
The variety of dental problems experienced by older people can result in chewing difficulties determining changes in food selection, thus leading to malnutrition and consequently to frailty [23] and sarcopenia [10][23]. Poor oral status may also predispose one to a chronic low-grade systemic inflammation through periodontal disease [24][25], which has an increased prevalence in those who are not able to perform the daily oral hygiene procedures [26], and it is a well-known risk factor in the pathogenesis of frailty [27] and sarcopenia [28]. Furthermore, periodontal disease has been associated with faster decline in handgrip strength [29], and recent studies showed an association between chewing difficulties and frailty [24].
Therefore, a hypothetical triangle oral status–nutrition–sarcopenia, exposing the older person to the frailty disabling cascade, may be suggested, as seen in Figure 1.
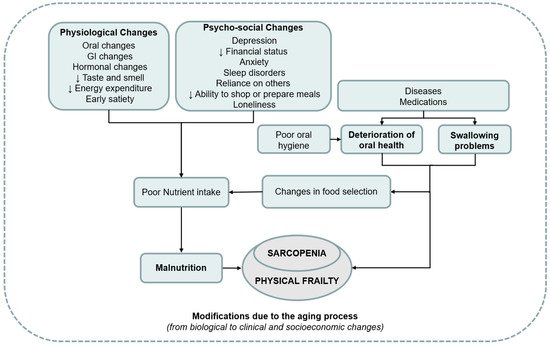
Figure 1. Overview of the interplay between poor oral status, malnutrition, and sarcopenia. GI—gastrointestinal.
2. Oral Changes with Aging
Poor oral health is not an inevitable part of aging since good care throughout the life course can result in the maintenance of functional teeth later in life [24]. Throughout a lifetime, the oral cavity experiences a variety of physiological modifications, such as enamel changes, fractures lines and stains, as well as dentin exposure and darkening of the tooth. At the same time, in the inner part of the tooth, several changes, such as the deposition of secondary dentin reducing the size of the pulp chamber and canals, may also occur [30]. Furthermore, in older people, tooth wear is frequently observed, affecting more than 85% of all the teeth groups in both the mandible and maxilla [31]. Additionally, a loss in terms of elastic fibers in the connective tissue has been documented and, subsequently, the oral mucosa becomes less resilient [32].
However, older people, especially those who are institutionalized or with limited financial resources, may experience problems to access oral care. Furthermore, it has been documented that older people frequently have difficulty expressing complaints and assign low priority to oral health until dental problems become intolerable [33]. Oral problems among older people have been implicated in a high prevalence of tooth loss, dental caries, periodontal disease, xerostomia, and oral precancer/cancer lesions [34]. Periodontitis and dental caries are very common diseases, especially in older people, and are considered the main cause of tooth loss [35].
Around the age of 70, there is also a peak of root/cementum caries, as a result of both tooth retention and major exposure of these surfaces following periodontal support loss. Moreover, older people are at higher risk of periodontitis since it is a cumulative disease, especially with regard to the multirooted teeth [36].
3. Sarcopenia and Oral Status
Sarcopenia, defined as the progressive and accelerated loss of muscle mass and function, is a major determinant of several adverse outcomes including frailty, disability, and mortality [13][37]. Although sarcopenia is a condition commonly observed with the aging process, it can also occur earlier in life [38]. Since 2016, sarcopenia has been recognized as an independent condition with an International Classification of Disease, 10th Revision, Clinical Modification (ICD-10-CM) Diagnosis Code [39]. Recently, the European Working Group on Sarcopenia in Older People (EWGSOP) [38] updated their consensus on definition and diagnosis (EWGSOP2). In this revised consensus, low muscle strength is considered a key characteristic of sarcopenia, and poor physical performance is identified as indicative of severe sarcopenia. Moreover, EWGSOP2 have recommended specific cut-off points to identify and characterize the sarcopenic condition, and provide an algorithm that can be used for case-finding.
Sarcopenia has a complex multifactorial pathogenesis, which involves lifestyle habits (i.e., malnutrition, physical inactivity), disease triggers, and age-dependent biological changes (i.e., chronic inflammation, mitochondrial abnormalities, loss of neuromuscular junctions, reduced satellite cell numbers, hormonal alterations) [40][41]. Sarcopenia is a whole-body process, affecting not only lower extremities, but also muscles dedicated to breathing, mastication, and swallowing [14][18][19][20][21][22]. In particular, swallowing is a complex mechanism involving several head and neck muscles simultaneously and in conjunction to coordinate the entire process [42]. Several age-related changes, such as as reduction of tissue elasticity, changes of the head and neck anatomy, reduced oral and pharyngeal sensitivity, and impaired dental status, may contribute to different degrees to a subtle swallowing impairment, the so called “presbyphagia”. It is usually an asymptomatic condition in which swallowing function is preserved, but tends to slowly worsen as the aging process advances [16][43]. Presbyphagia may increase the risk of dysphagia and aspiration in older people, especially during acute illnesses and other stressors [44]. Moreover, reductions in muscle mass of the geniohyoid, pterygoid, masseter, tongue, and pharyngeal muscles have been documented in older individuals [20][45][46][47]. Several authors also reported a decline in the strength of the swallowing muscles with aging or sarcopenia [48]. Maximal tongue strength decreases with aging [48][49][50][51], and there is some evidence that aging leads to a decreased jaw-opening force in older men. Several authors also reported an association between tongue strength and handgrip strength [52][53]. A decrease in tongue strength has been associated with a decline of activities of daily living [54], and a reduced tongue thickness has been noted in people with low body weight [20].
Lip function is also important for feeding. In fact, poor lip muscle closure may cause leakage through the corners of the mouth [55]. Additionally, decreased lip strength has been suggested to occur due to sarcopenia and to be related to difficulties in eating and drinking (i.e., dysphagia) [49]. Lip force has been associated with hand grip strength and lip pendency has been associated with aging [49][56].
Indeed, since it has been shown that skeletal muscle mass and strength decline may affect both swallowing and general muscle groups, a new condition, called “sarcopenic dysphagia” has been coined [22][56][57]. Swallowing muscles are characterized by a high percentage of type II fibers, which are more easily affected by malnutrition and sarcopenia than type I muscle fibers [22]. However, some cranial muscles, including the jaw-closers, are very different in fiber-type composition than other skeletal muscle groups (i.e., limbs or abdomen). For instance, the masseter muscle, which originates from the zygomatic arch, contains both type I and type II fibers, but shows a predominance of type I muscle fibers, which are more strongly affected by inactivity rather than aging [58][59]. Given that the meal texture of older people frequently becomes softer, less power of tongue movement and of masseter muscle is required, which may result in decreased activity of these muscles.
Interestingly, poor oral health may predispose one to a chronic low-grade inflammatory state through periodontal disease, which is a well-known risk factor for frailty and sarcopenia [25][60][61]. In fact, the detrimental effects of periodontitis are not confined solely to the oral cavity, but extend systemically, leading to metabolic alterations [62], including insulin resistance [63], diabetes [63][64], arthritis [65], and heart disease [66]. Furthermore, alterations in mitochondrial function leading to oxidative stress through the production of reactive oxygen species (ROS) have also been reported to mediate both oral and systemic pathologies (i.e., sarcopenia) [40][67][68][69]. Given their regulatory role as signaling molecules in autophagy, it has been speculated that elevated ROS production in periodontal disease could lead to autophagic alterations [70]. Bullon et al. [71] found high levels of mitochondrial-derived ROS, accompanied by mitochondrial dysfunction in peripheral blood mononuclear cells from patients with periodontitis. Moreover, oral gingiva seems to be highly responsive to the lipopolysaccharides (LPS), which are bacterial endotoxins prevalent in periodontal disease. In fact, gingival fibroblasts, which play an important role in remodeling periodontal soft tissues, may directly interact with LPS. In particular, LPS from Porphyromonas gingivalis enhances the production of inflammatory cytokines [72]. Porphyromonas gingivalis has been found to be responsible for high mitochondrial ROS and coenzyme Q10 levels, and for mitochondrial dysfunction, given its influence on the amount of respiratory chain complex I and III [70][71]. Indeed, LPS-mediated mitochondrial dysfunction could explain the oxidative stress onset in patients with periodontitis. Furthermore, Hamalainen et al. [29] reported an association between periodontitis and quicker declines in handgrip strength.
On the other hand, as discussed in the previous section, the variety of dental problems experienced by older people can lead to a decline in general health through poor nutrient intake, pain, and low quality of life [25]. Poor oral status has been reported to affect 71% of patients in rehabilitation settings [73] and 91% of people in acute-care hospitals [74], and has been associated with malnutrition, dysphagia, and reduced activities of daily living [17]. Hence, poor oral status may lead to sarcopenia through poor nutrient intake. Moreover, inflammation further contributes to malnutrition through various mechanisms, such as anorexia, decreased nutrient intake, altered metabolism (i.e., elevation of resting energy expenditure), and increased muscle catabolism [75]. Chronic inflammation is a common underlying factor, not only in the etiology of sarcopenia, but also for frailty. In fact, sarcopenia and frailty are closely related and show a remarkable overlap especially in the physical function domain [76][77][78]. The presence of oral problems, alone or in combination with sarcopenia, may thus represent the biological substratum of the disabling cascade experienced by many frail individuals.
4. Interventions
The management of older people should be multimodal and multidisciplinary, especially for those with or at risk of malnutrition [79], in order to improve different conditions (i.e., oral problems and sarcopenia). From a practical point of view, comprehensive geriatric assessment (CGA) is the multidimensional, interdisciplinary diagnostic and therapeutic process aimed at determining the medical, psychological, and functional problems of older people. The CGA’s objective is the development of a coordinated and integrated plan for treatment and follow-up in order to maximize overall health with aging [80]. To date, increasing evidence suggests that prosthodontic treatment in combination with personalized dietary counselling may improve the nutritional status of patients [81].
References
- Bales, C.W.; Ritchie, C.S. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu. Rev. Nutr. 2002, 22, 309–323.
- Palmer, K.; Onder, G.; Cesari, M. The geriatric condition of frailty. Eur. J. Intern. Med. 2018, 56, 1–2.
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217.
- Leslie, W.; Hankey, C. Aging, Nutritional Status and Health. Healthcare 2015, 3, 648–658.
- Roberts, H.C.; Lim, S.E.R.; Cox, N.J.; Ibrahim, K. The Challenge of Managing Undernutrition in Older People with Frailty. Nutrients 2019, 11, 808.
- Hickson, M. Malnutrition and ageing. Postgrad. Med. J. 2006, 82, 2–8.
- Schilp, J.; Wijnhoven, H.A.H.; Deeg, D.J.H.; Visser, M. Early determinants for the development of undernutrition in an older general population: Longitudinal Aging Study Amsterdam. Br. J. Nutr. 2011, 106, 708–717.
- Locher, J.L.; Ritchie, C.S.; Roth, D.L.; Sen, B.; Vickers, K.S.; Vailas, L.I. Food choice among homebound older adults: Motivations and perceived barriers. J. Nutr. Health Aging 2009, 13, 659–664.
- Bloom, I.; Lawrence, W.; Barker, M.; Baird, J.; Dennison, E.; Sayer, A.A.; Cooper, C.; Robinson, S. What influences diet quality in older people? A qualitative study among community-dwelling older adults from the Hertfordshire Cohort Study, UK. Public Health Nutr. 2017, 20, 2685–2693.
- Cichero, J.A.Y. Age-Related Changes to Eating and Swallowing Impact Frailty: Aspiration, Choking Risk, Modified Food Texture and Autonomy of Choice. Geriatrics 2018, 3, 69.
- Calvani, R.; Miccheli, A.; Landi, F.; Bossola, M.; Cesari, M.; Leeuwenburgh, C.; Sieber, C.C.; Bernabei, R.; Marzetti, E. Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J. Frailty Aging 2013, 2, 38–53.
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127 (Suppl. S5), 990S–991S.
- Cruz-Jentoft, A.J.; Landi, F. Sarcopenia. Clin. Med. 2014, 14, 183–186.
- Komatsu, R.; Okazaki, T.; Ebihara, S.; Kobayashi, M.; Tsukita, Y.; Nihei, M.; Sugiura, H.; Niu, K.; Ebihara, T.; Ichinose, M. Aspiration pneumonia induces muscle atrophy in the respiratory, skeletal, and swallowing systems. J. Cachexia Sarcopenia Muscle 2018, 9, 643–653.
- Beckwée, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J. Nutr. Health Aging 2019, 23, 494–502.
- Azzolino, D.; Damanti, S.; Bertagnoli, L.; Lucchi, T.; Cesari, M. Sarcopenia and swallowing disorders in older people. Aging. Clin. Exp. Res. 2019, 22, 1–7.
- Shiraishi, A.; Yoshimura, Y.; Wakabayashi, H.; Tsuji, Y. Prevalence of stroke-related sarcopenia and its association with poor oral status in post-acute stroke patients: Implications for oral sarcopenia. Clin. Nutr. 2018, 37, 204–207.
- Iee Shin, H.; Kim, D.-K.; Seo, K.M.; Kang, S.H.; Lee, S.Y.; Son, S. Relation Between Respiratory Muscle Strength and Skeletal Muscle Mass and Hand Grip Strength in the Healthy Elderly. Ann. Rehabil. Med. 2017, 41, 686–692.
- Fujishima, I.; Fujiu-Kurachi, M.; Arai, H.; Hyodo, M.; Kagaya, H.; Maeda, K.; Mori, T.; Nishioka, S.; Oshima, F.; Ogawa, S.; et al. Sarcopenia and dysphagia: Position paper by four professional organizations. Geriatr. Gerontol. Int. 2019, 19, 91–97.
- Tamura, F.; Kikutani, T.; Tohara, T.; Yoshida, M.; Yaegaki, K. Tongue thickness relates to nutritional status in the elderly. Dysphagia 2012, 27, 556–561.
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646.
- Wakabayashi, H.; Sakuma, K. Rehabilitation nutrition for sarcopenia with disability: A combination of both rehabilitation and nutrition care management. J. Cachexia Sarcopenia Muscle 2014, 5, 269–277.
- Castrejón-Pérez, R.C.; Jiménez-Corona, A.; Bernabé, E.; Villa-Romero, A.R.; Arrivé, E.; Dartigues, J.-F.; Gutiérrez-Robledo, L.M.; Borges-Yáñez, S.A. Oral Disease and 3-Year Incidence of Frailty in Mexican Older Adults. J. Gerontol. Ser. A 2017, 72, 951–957.
- Woo, J.; Tong, C.; Yu, R. Chewing Difficulty Should be Included as a Geriatric Syndrome. Nutrients 2018, 10, 2019. Available online: (accessed on 11 October 2019).
- Castrejón-Pérez, R.C.; Borges-Yáñez, S.A.; Gutiérrez-Robledo, L.M.; Avila-Funes, J.A. Oral health conditions and frailty in Mexican community-dwelling elderly: A cross sectional analysis. BMC Public Health 2012, 12, 773.
- Lertpimonchai, A.; Rattanasiri, S.; Arj-Ong Vallibhakara, S.; Attia, J.; Thakkinstian, A. The association between oral hygiene and periodontitis: A systematic review and meta-analysis. Int. Dent. J. 2017, 67, 332–343.
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty measurement in research and clinical practice: A review. Eur. J. Intern. Med. 2016, 31, 3–10.
- Rolland, Y.; Czerwinski, S.; Abellan Van Kan, G.; Morley, J.E.; Cesari, M.; Onder, G.; Woo, J.; Baumgartner, R.; Pillard, F.; Boirie, Y.; et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging 2008, 12, 433–450.
- Hämäläinen, P.; Rantanen, T.; Keskinen, M.; Meurman, J.H. Oral health status and change in handgrip strength over a 5-year period in 80-year-old people. Gerodontology 2004, 21, 155–160.
- Lamster, I.B.; Asadourian, L.; Del Carmen, T.; Friedman, P.K. The aging mouth: Differentiating normal aging from disease. Periodontol 2000 2016, 72, 96–107.
- Liu, B.; Zhang, M.; Chen, Y.; Yao, Y. Tooth wear in aging people: An investigation of the prevalence and the influential factors of incisal/occlusal tooth wear in northwest China. BMC Oral Health 2014, 14, 65.
- Klein, D.R. Oral soft tissue changes in geriatric patients. Bull. N. Y. Acad. Med. 1980, 56, 721–727.
- MacEntee, M.I.; Donnelly, L.R. Oral health and the frailty syndrome. Periodontology 2000 2016, 72, 135–141.
- Razak, P.A.; Richard, K.M.J.; Thankachan, R.P.; Hafiz, K.A.A.; Kumar, K.N.; Sameer, K.M. Geriatric Oral Health: A Review Article. J. Int. Oral Health 2014, 6, 110–116.
- Chapple, I.L.C.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.-C.; Cocco, F.; Nibali, L.; Hujoel, P.; Laine, M.L.; Lingstrom, P.; et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44 (Suppl S18), S39–S51.
- Hirotomi, T.; Yoshihara, A.; Yano, M.; Ando, Y.; Miyazaki, H. Longitudinal study on periodontal conditions in healthy elderly people in Japan. Community Dent. Oral Epidemiol. 2002, 30, 409–417.
- Cruz-Jentoft, A.J.; Landi, F.; Topinková, E.; Michel, J.-P. Understanding sarcopenia as a geriatric syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 1–7.
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31.
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514.
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Salini, S.; Sisto, A.; Picca, A.; et al. Sarcopenia: An Overview on Current Definitions, Diagnosis and Treatment. Curr. Protein Pept. Sci. 2018, 19, 633–638.
- Liguori, I.; Russo, G.; Aran, L.; Bulli, G.; Curcio, F.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Sarcopenia: Assessment of disease burden and strategies to improve outcomes. Clin. Interv. Aging 2018, 13, 913–927.
- McCulloch, T.M.; Jaffe, D. Head and neck disorders affecting swallowing. GI Motil. Online 2006. Available online: (accessed on 11 October 2019).
- Wirth, R.; Dziewas, R.; Beck, A.M.; Clavé, P.; Hamdy, S.; Heppner, H.J.; Langmore, S.; Leischker, A.H.; Martino, R.; Pluschinski, P.; et al. Oropharyngeal dysphagia in older persons—From pathophysiology to adequate intervention: A review and summary of an international expert meeting. Clin. Interv. Aging 2016, 11, 189–208.
- Robbins, J.; Bridges, A.D.; Taylor, A. Oral, pharyngeal and esophageal motor function in aging. GI Motil. Online 2006. Available online: (accessed on 11 October 2019).
- Feng, X.; Todd, T.; Lintzenich, C.R.; Ding, J.; Carr, J.J.; Ge, Y.; Browne, J.D.; Kritchevsky, S.B.; Butler, S.G. Aging-related geniohyoid muscle atrophy is related to aspiration status in healthy older adults. J. Gerontol. Ser. A 2013, 68, 853–860.
- Newton, J.P.; Yemm, R.; Abel, R.W.; Menhinick, S. Changes in human jaw muscles with age and dental state. Gerodontology 1993, 10, 16–22.
- Wakabayashi, H.; Takahashi, R.; Watanabe, N.; Oritsu, H.; Shimizu, Y. Prevalence of skeletal muscle mass loss and its association with swallowing function after cardiovascular surgery. Nutrition 2017, 38, 70–73.
- Machida, N.; Tohara, H.; Hara, K.; Kumakura, A.; Wakasugi, Y.; Nakane, A.; Minakuchi, S. Effects of aging and sarcopenia on tongue pressure and jaw-opening force. Geriatr. Gerontol. Int. 2017, 17, 295–301.
- Sakai, K.; Nakayama, E.; Tohara, H.; Kodama, K.; Takehisa, T.; Takehisa, Y.; Ueda, K. Relationship between tongue strength, lip strength, and nutrition-related sarcopenia in older rehabilitation inpatients: A cross-sectional study. Clin. Interv. Aging 2017, 12, 1207–1214.
- Sporns, P.B.; Muhle, P.; Hanning, U.; Suntrup-Krueger, S.; Schwindt, W.; Eversmann, J.; Warnecke, T.; Wirth, R.; Zimmer, S.; Dziewas, R. Atrophy of Swallowing Muscles Is Associated with Severity of Dysphagia and Age in Patients with Acute Stroke. J. Am. Med. Dir. Assoc. 2017, 18, 635.e1–635.e7.
- Maeda, K.; Akagi, J. Decreased tongue pressure is associated with sarcopenia and sarcopenic dysphagia in the elderly. Dysphagia 2015, 30, 80–87.
- Butler, S.G.; Stuart, A.; Leng, X.; Wilhelm, E.; Rees, C.; Williamson, J.; Kritchevsky, S.B. The relationship of aspiration status with tongue and handgrip strength in healthy older adults. J. Gerontol. Ser. A 2011, 66, 452–458.
- Buehring, B.; Hind, J.; Fidler, E.; Krueger, D.; Binkley, N.; Robbins, J. Tongue strength is associated with jumping mechanography performance and handgrip strength but not with classic functional tests in older adults. J. Am. Geriatr. Soc. 2013, 61, 418–422.
- Tsuga, K.; Yoshikawa, M.; Oue, H.; Okazaki, Y.; Tsuchioka, H.; Maruyama, M.; Yoshida, M.; Akagawa, Y. Maximal voluntary tongue pressure is decreased in Japanese frail elderly persons. Gerodontology 2012, 29, e1078–e1085.
- Ertekin, C.; Aydogdu, I. Neurophysiology of swallowing. Clin. Neurophysiol. 2003, 114, 2226–2244.
- Sakai, K.; Sakuma, K. Sarcopenic Dysphagia as a New Concept. Frailty Sarcopenia Onset Dev. Clin. Chall. 2017. Available online: (accessed on 11 October 2019).
- Wakabayashi, H. Presbyphagia and Sarcopenic Dysphagia: Association between Aging, Sarcopenia, and Deglutition Disorders. J. Frailty Aging 2014, 3, 97–103.
- Rowlerson, A.; Raoul, G.; Daniel, Y.; Close, J.; Maurage, C.-A.; Ferri, J.; Sciote, J.J. Fiber-type differences in masseter muscle associated with different facial morphologies. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 37–46.
- Yamaguchi, K.; Tohara, H.; Hara, K.; Nakane, A.; Kajisa, E.; Yoshimi, K.; Minakuchi, S. Relationship of aging, skeletal muscle mass, and tooth loss with masseter muscle thickness. BMC Geriatr. 2018, 18, 67.
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522.
- Yao, X.; Li, H.; Leng, S.X. Inflammation and immune system alterations in frailty. Clin. Geriatr. Med. 2011, 27, 79–87.
- Napa, K.; Baeder, A.C.; Witt, J.E.; Rayburn, S.T.; Miller, M.G.; Dallon, B.W.; Gibbs, J.L.; Wilcox, S.H.; Winden, D.R.; Smith, J.H.; et al. LPS from, P. gingivalis Negatively Alters Gingival Cell Mitochondrial Bioenergetics. Int. J. Dent. 2017, 2017. Available online: (accessed on 11 October 2019).
- Taylor, G.W.; Burt, B.A.; Becker, M.P.; Genco, R.J.; Shlossman, M.; Knowler, W.C.; Pettitt, D.J. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J. Periodontol. 1996, 67 (Suppl. S10), 1085–1093.
- Chee, B.; Park, B.; Bartold, P.M. Periodontitis and type II diabetes: A two-way relationship. Int. J. Evid. Based Healthc. 2013, 11, 317–329.
- Fuggle, N.R.; Smith, T.O.; Kaul, A.; Sofat, N. Hand to Mouth: A Systematic Review and Meta-Analysis of the Association between Rheumatoid Arthritis and Periodontitis. Front. Immunol. 2016, 7, 80.
- Beck, J.D.; Offenbacher, S.; Williams, R.; Gibbs, P.; Garcia, R. Periodontitis: A risk factor for coronary heart disease? Ann. Periodontol. 1998, 3, 127–141.
- D′Aiuto, F.; Nibali, L.; Parkar, M.; Patel, K.; Suvan, J.; Donos, N. Oxidative stress, systemic inflammation, and severe periodontitis. J. Dent. Res. 2010, 89, 1241–1246.
- Borges, I.; Moreira, E.A.M.; Filho, D.W.; de Oliveira, T.B.; da Silva, M.B.S.; Fröde, T.S. Proinflammatory and oxidative stress markers in patients with periodontal disease. Mediat. Inflamm. 2007, 2007, 45794.
- Horton, A.L.; Boggess, K.A.; Moss, K.L.; Beck, J.; Offenbacher, S. Periodontal disease, oxidative stress, and risk for preeclampsia. J. Periodontol. 2010, 81, 199–204.
- Bullon, P.; Cordero, M.D.; Quiles, J.L.; del Carmen Ramirez-Tortosa, M.; Gonzalez-Alonso, A.; Alfonsi, S.; García-Marín, R.; de Miguel, M.; Battino, M. Autophagy in periodontitis patients and gingival fibroblasts: Unraveling the link between chronic diseases and inflammation. BMC Med. 2012, 10, 122.
- Bullon, P.; Cordero, M.D.; Quiles, J.L.; Morillo, J.M.; del Carmen Ramirez-Tortosa, M.; Battino, M. Mitochondrial dysfunction promoted by Porphyromonas gingivalis lipopolysaccharide as a possible link between cardiovascular disease and periodontitis. Free Radic. Biol. Med. 2011, 50, 1336–1343.
- Wang, P.-L.; Ohura, K. Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors. Crit. Rev. Oral Biol. Med. 2002, 13, 132–142.
- Andersson, P.; Hallberg, I.R.; Lorefält, B.; Unosson, M.; Renvert, S. Oral health problems in elderly rehabilitation patients. Int. J. Dent. Hyg. 2004, 2, 70–77.
- Hanne, K.; Ingelise, T.; Linda, C.; Ulrich, P.P. Oral status and the need for oral health care among patients hospitalised with acute medical conditions. J. Clin. Nurs. 2012, 21, 2851–2859.
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9.
- Cesari, M.; Landi, F.; Vellas, B.; Bernabei, R.; Marzetti, E. Sarcopenia and Physical Frailty: Two Sides of the Same Coin. Front. Aging Neurosci 2014, 6, 192. Available online: (accessed on 5 November 2019).
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48.
- Landi, F.; Cherubini, A.; Cesari, M.; Calvani, R.; Tosato, M.; Sisto, A.; Martone, A.M.; Bernabei, R.; Marzetti, E. Sarcopenia and frailty: From theoretical approach into clinical practice. Eur. Geriatr. Med. 2016, 7, 197–200. Available online: (accessed on 11 October 2019).
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559.
- Ellis, G.; Whitehead, M.A.; O′Neill, D.; Langhorne, P.; Robinson, D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane. Database. Syst. Rev. 2011, 7, CD006211.
- Kossioni, A.E. The Association of Poor Oral Health Parameters with Malnutrition in Older Adults: A Review Considering the Potential Implications for Cognitive Impairment. Nutrients 2018, 10, 1709.
More
Information
Subjects:
Dentistry, Oral Surgery & Medicine
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
876
Revisions:
2 times
(View History)
Update Date:
21 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




