Malaria is the most common vector-borne parasitic infection causing significant human morbidity and mortality in nearly 90 tropical/sub-tropical countries worldwide. Significant differences exist in the incidence of malaria cases, dominant Plasmodium species, drug-resistant strains and mortality rates in different countries. Six Gulf Cooperation Council (GCC) countries (Bahrain, Kuwait, Qatar, Oman, Saudi Arabia and United Arab Emirates, UAE) in the Middle East region with similar climates, population demographics and economic prosperity are aiming to achieve malaria elimination. In this narrative review, all studies indexed in PubMed describing epidemiological characteristics of indigenous and imported malaria cases, vector control status and how malaria infections can be controlled to achieve malaria elimination in GCC countries were reviewed and discussed.
1. Introduction
Significant progress has been made in human healthcare during the last several decades. However, infectious diseases caused by human parasites continue to inflict considerable suffering and death worldwide, particularly in poor and developing countries. Estimates of global burden of diseases have suggested that more than 2 billion cases of parasitic infections afflicted mankind in the year 2013
[1]. Among human parasitic diseases, vector-borne parasitic infections are particularly important as they are responsible for more than 15% of all infectious diseases worldwide, causing more than 600,000 deaths every year, mostly in poor tropical/sub-tropical countries
[2][3]. Malaria is an important vector-borne parasitic infection which alone is responsible for more than 400,000 fatalities every year around the world with most of the deaths occurring in younger (less than 5-year old) children
[3].
Five
Plasmodium species,
P. falciparum, P. vivax, P. ovale, P. malariae and
P. knowlesi, cause malaria in humans and the disease spreads from person to person through the mosquito vector. Zoonotic malaria cases have also been reported due to
P. knowlesi in some countries in Southeast Asia. Malaria cases are reported from 87 different countries and significant differences exist in the incidence of malaria cases, dominant
Plasmodium species causing the infections, resistance to antimalarial drugs and mortality rates among infected patients in different countries. Infections by
P. falciparum and
P. vivax are most common and account for most of the global malaria cases. Most malaria infections in Africa are caused by
P. falciparum while the highest burden of
P. vivax infections is seen in South/Southeast Asian countries (including India) and South America
[4]. In most African countries,
P. vivax infection is absent or very rare due to lack of Duffy antigen receptor for chemokines on the surface of red blood cells that is involved in the parasite invasion of erythrocytes among the population. However, since 2010, there has been a growing evidence of presence of
P. vivax infections in all regions of Africa, including
P. vivax-infected Duffy-negative individuals
[5]. Although
P. falciparum is the more virulent and causes the majority of malarial mortality, particularly in Africa, increasing prevalence of
P. vivax malaria in some countries, particularly in the Indian subcontinent, poses unique diagnostic and therapeutic challenges. Several reports have described acute respiratory distress syndrome, cerebral malaria, multi-organ failure, dyserythropoiesis and anemia following
P. vivax infection
[4][5]. The
P. vivax malaria also triggers higher inflammatory responses and exacerbated clinical symptoms (fever and chills) than
P. falciparum malaria
[5]. The
P. vivax infection also results in persistence of the parasite, as dormant liver-stage hypnozoites can cause recurrent episodes of malaria
[4].
The United Nations Millennium Summit included three major infectious diseases, viz. malaria, human immunodeficiency virus (HIV) infection and tuberculosis for special attention and created a global fund to support control and eradication of these infections
[6]. For malaria, the term ‘elimination’ is used when disease transmission is no longer occurring in a specific geographic area while the term ‘eradication’ is used to describe malaria elimination from the whole world. Concerted worldwide efforts during the first decade of the new millennium resulted in significant progress as nearly 1.5 billion malaria infections and 7.6 million malaria-related deaths were averted
[6], according to the Malaria Atlas Project 2019,
https://malariaatlas.org/malaria-burden-data-download/ accessed on 18 March 2021.
An important factor that has halted the progress against malaria in recent years is the emergence of drug-resistant strains of
Plasmodium species which are currently posing serious threats to global malaria control efforts. Currently, the World Health Organization (WHO) recommends artemisinin-based combination therapies (ACT) as a first-line therapy in most malaria-endemic countries, as the older antimalarial drugs are now ineffective in treating malaria cases worldwide
[7][8]. In addition, judicious use of appropriate chemical insecticides through long-lasting insecticidal nets (LLINs), insecticide-treated mosquito nets (ITNs) and indoor residual spraying (IRS) have also been useful
[6]. Vector control through LLINs and IRS has reduced global malaria burden by nearly 40% in some countries during the last decade
[7]. However, recent reports of the rapid spread of pyrethroid resistance in malaria vectors across Africa have documented the reduced effectiveness of LLINs, thus leading to increased malaria transmission in sub-Saharan Africa
[7][9][10]. New types of LLINs (e.g., PBO-Py LLINs, Olyst Plus) are being manufactured which have shown improved effectiveness against pyrethroid-resistant mosquitoes
[11].
A new paradigm shift that has occurred in many malaria non-endemic countries/regions, particularly among those located in the temperate climate of Europe and North America and a few oil-rich Arabian Gulf countries, is the emergence of imported malaria cases
[12][13][14][15]. Many countries which were malaria free until a few years ago have seen a sharp increase in the number of imported malaria cases in recent years
[12][13][14][15]. This increase has occurred as a result of increased international travel for business or tourism particularly from or to malaria-endemic countries, migratory movements for employment and migration of large number of refugees from war-torn countries into non-endemic/malaria-free countries
[12][16][17][18].
Some other areas of concern have also been noted in recent years. Deletions in the
P. falciparum histidine-rich protein (pfhrp)2 and pfhrp3 genes have been confirmed in 11 African countries
[6][19][20]. Deletions of pfhrp2 and pfhrp3 genes reduce the diagnostic utility of rapid tests based on detection of HRP2. Mutations in PfKelch13 have also been detected in malaria patients in many malaria-endemic countries. Some of these mutations confer partial resistance to artemisinin, the first-line treatment for
P. falciparum infections
[4][21]. Of the 81 malaria-endemic countries that provided data for vector resistance to insecticides during 2010 to 2019, 28 countries detected resistance to all four of the most commonly used insecticide classes in at least one malaria vector and one collection site, and 73 detected resistances to at least one insecticide class. Only eight countries did not detect resistance to any insecticide class
[6].
The effects of the COVID-19 pandemic on the substantial progress made in reducing the burden of malaria is still being evaluated. However, it is most likely that this pandemic will severely disrupt important malaria control interventions, i.e., distribution of LLINs, ITNs and antimalarial. It has been suggested that these disruptions will potentially lead to greater increases in malaria incidence in subsequent years and the malaria mortality will likely double from 386,400 to 768,600 individuals. In Nigeria alone, reducing case management for 6 months and delaying LLIN campaigns could result in 81,000 (44,000–119,000) additional deaths
[6][22][23]. On the other hand, strict travel restrictions due to COVID-19 may have decreased the number of imported malaria cases in non-endemic countries. For instance, the number of imported malaria cases in Kuwait during the COVID-19 pandemic year 2020 decreased by more than 80%, as the State of Kuwait put in place strict travel restrictions on people coming to Kuwait from Africa and South Asia during this period (personal communication, Infectious Diseases Hospital, Kuwait).
2. Malaria Status among Countries Contributing Most of the Imported Malaria Cases in GCC Countries
Only two GCC countries (Saudi Arabia and Oman) have recently reported indigenous transmission of malaria while the remaining countries have been malaria-free for the last several years. However, GCC countries continue to have imported malaria cases as they all have large expatriate populations
[24][25][26][27][28][29][30]. Most of the expatriates in GCC countries originate from India, Pakistan, Afghanistan, Bangladesh and Philippines, located in South/Southeast Asia, and Sudan, Ethiopia, and Nigeria, located in sub-Saharan Africa.
Table 1 presents number of local citizens and expatriate population (originating from malaria-endemic countries) among the six GCC countries. Expatriates from India, Pakistan, Afghanistan, Bangladesh, Philippines, Nigeria, Sudan and Ethiopia total more than 2 million individuals, which is more than the local Kuwaiti population of 1.43 million people (
Table 1). Most of the countries from which expatriates originate are endemic or highly endemic for malaria
[6][14]. Details regarding the incidence of malaria cases and malaria deaths reported by some countries during 2000–2019, and from where most of the expatriates working in GCC countries originate, are provided in
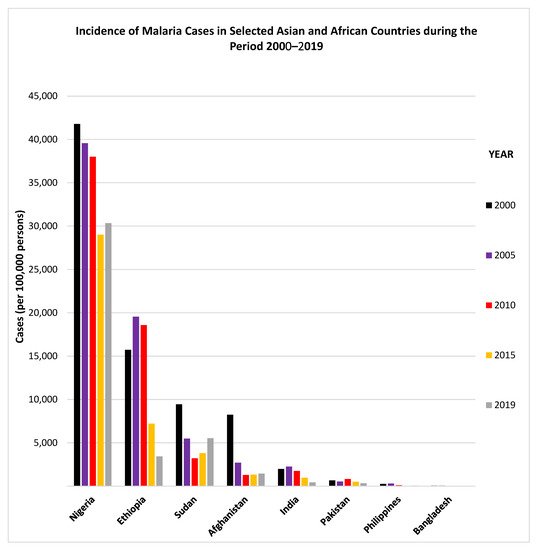
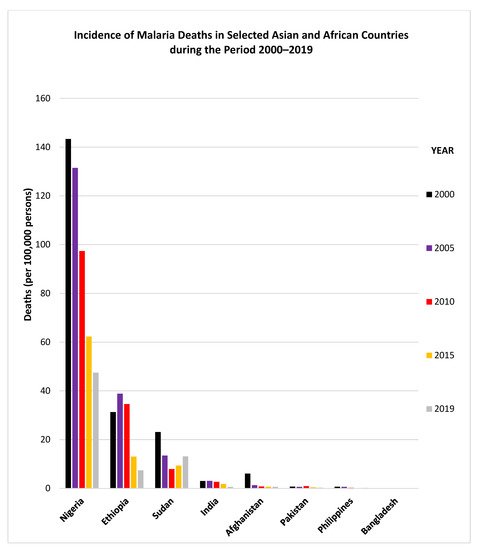
Figure 1 and
Figure 2. The data show that since 2010, the incidence of malaria cases has generally declined considerably in Ethiopia, India and Pakistan while it has shown an upward trend in Sudan (
Figure 1). Similarly, the incidence of malaria deaths has also declined considerably since 2010 in Nigeria, Ethiopia and India while it has shown an upward trend in Sudan (
Figure 2).
Figure 1. Incidence of malaria cases in selected Asian and African countries during the period 2000–2019. The graphs were made using data adopted from WHO, World Malaria Reports, 2019 and 2020 and the Malaria Atlas Project 2019.
Figure 2. Incidence of malaria deaths in selected Asian and African countries during the period 2000–2019. The graphs were made using data adopted from WHO, World Malaria Reports, 2019 and 2020 and the Malaria Atlas Project 2019.
Table 1. Number of local citizens and expatriate population (originating from malaria-endemic countries) among the GCC countries.
| GCC |
Total Population in |
Population of Citizens |
Population of Expatriates from Different Countries in GCC Countries in 2019 |
| Country |
2019 (in Millions) |
in 2019 (in Millions) |
India |
Pakistan |
Afghanistan |
Bangladesh |
Philippines |
Nigeria |
Sudan |
Ethiopia |
| Bahrain |
1.59 |
1.21 |
318,547 |
78,638 |
690 |
82,518 |
50,585 |
2054 |
7917 |
713 |
| Kuwait |
4.77 |
1.43 |
1,124,256 |
330,824 |
2826 |
370,844 |
192,143 |
4702 |
48,204 |
3806 |
| Qatar |
2.68 |
0.33 |
698,088 |
235,876 |
1602 |
263,086 |
168,461 |
4152 |
23,954 |
1700 |
| Oman |
4.7 |
2.6 |
1,325,444 |
240,965 |
N. A. |
304,917 |
44,546 |
N. A. |
19,155 |
N. A. |
| Saudi Arabia |
34 |
23.2 |
2,440,489 |
1,447,071 |
469,324 |
1,246,052 |
628,894 |
N. A. |
469,324 |
160,192 |
| UAE |
9.7 |
1.2 |
3,419,875 |
981,536 |
8071 |
1,079,013 |
556,407 |
15,465 |
131,254 |
10,886 |
It has recently been suggested that continuous increases in long-distance travel and recent large migratory movements have changed the epidemiological characteristics of imported malaria in countries where malaria is not endemic, such as GCC countries
[17]. Malaria infection is primarily imported to non-endemic countries by returning travelers from malaria-endemic countries. Many expatriates originating from malaria-endemic regions frequently (usually annually) travel to visit friends and relatives in their native countries. These individuals now make up the majority of malaria patients in non-endemic countries as they have partial immunity to malaria, resulting from repeated exposure to infection (
Table 1)
[24][25][26][27][28][29][30]. They also exhibit differences in the parasitological features, clinical manifestation and relatively lower odds for severe malaria compared to returning nationals of GCC countries, who have significantly higher odds for severe malaria because of their nonimmune status. Since the expatriate workers and their dependent family members contribute to a significantly high number of imported malaria cases in the GCC countries, a brief account of the current situation of malaria, including drug-resistant malaria, in these countries is also described here.
2.1. Characterization of Malaria Cases from India
India, with 1385 million people, has the second largest population (after China) in the world and Indians constitute the largest ethnic group among expatriate populations in nearly all GCC countries. India carries 4% of the global malaria burden and contributes nearly 87% of the total malaria cases in the WHO Southeast Asia Region. The latest WHO world malaria report has shown that nearly 5.6 million malaria cases occurred in India in 2019
[6]. However, the report also showed that India also contributed to the largest absolute reductions in malaria cases and deaths in the WHO Southeast Asia region from 2000 to 2019 (
Figure 1 and
Figure 2)
[6]. The malaria outbreaks usually peak during summer and fall monsoon seasons which raise the overall number of malaria cases and deaths in the country
[31][32].
Several studies have described the current situation of malaria in India. One study which employed a PCR-based assay reported an overall malaria prevalence of 19%, which varied from 6% in Oddanchatram, South India to 35% in Ratnagiri, West India
[33]. Among malaria-positive patients,
P. falciparum monoinfection was detected in 46% of the positive subjects. Furthermore, 38% subjects had
P. vivax infection, 5% had
P. malariae infection and 11% subjects had mixed infections with
P. falciparum and
P. vivax [33]. The study also showed that microscopy and rapid diagnostic tests had lower sensitivity than PCR-based assays, resulting in under diagnosis of malaria cases in the rural regions of India. One study carried out in the southwestern regions of India showed that
P. vivax infections account for approximately 80% of malaria cases, which have been reported to cause severe malaria, leading to more deaths than
P. falciparum infections
[34]. Another recent study carried out in Mangaluru city in southwestern India examined a total of 579 malaria patients and reported that 364 (62.9%) had
P. vivax infection, 150 (25.9%) had
P. falciparum infection while 65 (11.2%) patients had mixed infection by two
Plasmodium species
[35]. The study also reported that the majority (506 or 87%) of malaria patients had mild malaria, which may be attributed to prompt treatment with antimalarial drugs or previous exposure to the malarial parasite. Although
P. vivax was thought to dominate India in comparison to
P. falciparum, a large recent study which examined 2333 blood samples collected from nine malaria-endemic Indian states by using multiple diagnostic tests (microscopy, rapid diagnostic tests and PCR assay) reported that the ratio of
P. vivax to
P. falciparum infection was almost the same (1.04:1)
[36]. The study also reported that 13% of cases had
Plasmodium spp. mixed infection.
Several studies have shown that mixed
Plasmodium spp. infections are reported frequently from India
[32][35][36][37]. Data from more recent studies have shown that
P. falciparum, which had dominated India’s malaria cases previously, is now showing a decreasing trend over the past few years, from 65.4% in 2017 to 46.4% in 2019. India is a vast country with region-specific epidemiology of malaria. It has now become apparent that the majority of malaria cases in some regions in India are caused by
P. vivax. On the contrary, the rising number of malaria infections in some other regions are caused mostly by
P. falciparum, with only a minor contribution from
P. vivax. Recent estimates show that India alone harbors nearly 47% of all
P. vivax malaria cases worldwide, where 7 (Uttar Pradesh, Jharkhand, Chhattisgarh, West Bengal, Gujarat, Madhya Pradesh and Odisha) out of 36 Indian states account for nearly 90% of malaria cases
[3].
In addition to the problems of
P. vivax infection and mixed infections in some regions in India, increasing reports of drug resistance is another worrisome development
[4]. There is also a higher risk of
P. vivax parasitemia following treatment of
P. falciparum malaria
[38]. The epidemiology of drug-resistant malaria also varies considerably in different regions of India. One recent study has shown increasing prevalence of single nucleotide polymorphisms in the dihydrofolate reductase (
dhfr) gene of
P. falciparum from different regions in India
[39]. Although chloroquine had been discontinued for nearly a decade, a higher abundance of mutated
P. falciparum chloroquine-resistant transporter (
pfcrt) and a lower prevalence of mutated
P. falciparum multidrug resistance 1 (
pfmdr1), which confer resistance to chloroquine, were observed
[40][41]. Mutant alleles of
pvmdr1 gene were also observed among
P. vivax from New Delhi Capital Region
[42], Chandigarh in North India
[43] and the southwestern coastal region in South India
[44]. The presence of Kelch 13 mutations, which confer resistance to artemisinin-based combination therapy (such as artesunate/sulfadoxine/pyrimethamine), have also been reported from India
[45]. Detection of artemisinin-resistant malaria parasites, some of which carry novel mutations, and increasing incidence of combination therapy failures are worrisome developments for malaria control in India and beyond.
Previous studies carried out in all GCC countries have shown that travelers or returning travelers from India have contributed significantly to imported malaria cases in Bahrain
[24], Kuwait
[46][28], Oman
[25][47], Qatar
[26][29][48], Saudi Arabia
[30][49][50] and UAE
[27] (
Table 2).
Table 2. Indigenous and imported malaria cases reported in different recent studies from the GCC countries.
| Country |
Duration of Study |
No. of Indigenous and Imported Malaria Cases |
No. of Imported Malaria Cases Detected among Nationals (Citizens) or Expatriates |
Malaria Cases by Plasmodium spp. |
Reference |
| Citizens |
India |
Pakistan |
AFGN |
BGD |
Nigeria |
Sudan |
Other African Countries |
Others |
P. falciparum |
P. vivax |
Pf/Pv Mixed |
Others |
| Bahrain |
1992–2001 |
0 and 1572 |
N. A. |
629 |
566 |
N. A. |
31 |
NA |
63 |
N. A. |
283 |
220 |
1346 |
5 |
1 |
Ismaeel et al., 2004 [24] |
| Bahrain |
2017 |
0 and 133 |
N.A. |
N.A. |
N.A. |
N.A. |
N.A. |
N.A. |
N.A. |
N.A. |
N.A. |
|
|
|
|
WHO/EMRO Annual Report 2017 [51] |
| Kuwait |
1985–2000 |
0 and 6776 * |
39 |
3569 |
1057 |
1871 |
0 |
89 |
48 |
N. A. |
133 |
1137 |
5207 |
395 |
0 |
Iqbal et al., 2003 [46] |
| Kuwait |
2013–2018 |
0 and 1913 |
18 |
1012 |
390 |
94 |
5 |
16 |
48 |
275 |
55 |
361 |
124 |
1383 |
45 |
Iqbal et al., 2020 [28] |
| Oman |
2014 |
53 and 1 |
1 |
14 |
6 |
0 |
32 |
0 |
0 |
0 |
1 |
0 |
54 |
0 |
0 |
Simon et al., 2017 [25] |
| Oman |
2019 |
15 and 1323 |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
1080 |
206 |
0 |
52 |
MoH, Oman [47] |
| Qatar |
2004–2006 |
0 and 438 |
N. A. |
210 |
128 |
N. A. |
N. A. |
N. A. |
37 |
N. A. |
63 |
60 |
175 |
0 |
203 |
Al-Kuwari, 2009 [26] |
| Qatar a |
2008–2015 |
0 and 4092 |
14 |
812 |
772 |
0 |
0 |
200 |
0 |
0 |
14 |
404 |
2336 b |
0 |
229 b |
Farag et al. 2018 [29] |
| Qatar |
2013–2016 |
0 and 448 |
1 |
168 |
108 |
0 |
0 |
16 |
74 |
8 |
73 |
118 |
318 |
12 |
0 |
Al-Rumhi et al., 2020 [48] |
| Saudi Arabia |
2000–2014 |
5522 and 9930 |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
El Hassan et al., 2015 [52] |
| Saudi Arabia |
2008–2011 |
318 c |
16 |
37 |
108 |
5 |
3 |
53 |
12 |
7 |
77 |
204 |
103 |
0 |
11 |
Memish et al. 2014 [30] |
| Saudi Arabia |
2012–2015 |
121 and 224 |
113 |
28 |
73 |
3 |
0 |
0 |
48 |
37 |
43 |
212 |
128 |
2 |
3 |
Alshahrani et al., 2016 [49] |
| Saudi Arabia |
2016 |
4 and 22 |
4 |
4 |
5 |
0 |
0 |
2 |
10 |
1 |
0 |
13 |
13 |
0 |
0 |
Soliman et al. 2018 [50] |
| Saudi Arabia |
2016–2018 |
5 and 25 |
11 |
2 |
2 |
0 |
0 |
0 |
4 |
0 |
11 |
23 |
5 |
2 |
0 |
Hawash et al., 2019 [53] |
| Saudi Arabia |
2018 |
61 and 2650 |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
N. A. |
57 |
4 |
0 |
0 |
WHO Malaria Report 2019 [3] |
| UAE |
2008–2010 |
0 and 629 |
0 |
338 |
228 |
0 |
0 |
14 ** |
14 ** |
14 ** |
21 |
122 |
493 |
14 |
0 |
Nilles et al., 2014 [27] |
2.2. Characterization of Malaria Cases from Pakistan
Pakistan is another highly malaria-endemic country in South Asia, reporting more than 670,000 cases of malaria and 3159 deaths in the year 2013
[14]. The latest WHO world malaria report has shown that 700,000 malaria cases occurred in Pakistan in 2019
[6]. A recent study in a highly malaria-endemic district in Pakistan examined blood smears of 2033 individuals who were suspected of malaria infection. The study utilized microscopy, a rapid diagnostic test (RDT) and a PCR-based assay and found 429 (21.1%) malaria-positive cases by at least one diagnostic test
[54]. The data showed that the positivity by PCR-based assay, microscopy, and RDT was 30.5%, 17.7% and 16.4%, respectively. The study further showed that ~80% of malaria cases had
P. vivax infection. Of the remaining cases,
P. falciparum alone accounted for 11% of cases while
Plasmodium spp. mixed infections were seen in 9% of malaria cases
[54]. Another study had examined blood specimens from 216 febrile patients for the diagnosis of malaria in a tribe-governed region in Pakistan which also receives refugees from neighboring Afghanistan. The study found that most (86.5%) of the malaria cases were attributed to mono
P.
vivax infection. The authors showed that 11.8% patients had malaria due to
P.
falciparum and found that the ratio of
P. vivax to
P. falciparum infection has increased in provinces which border Afghanistan
[55]. Another recent study which used RDTs to screen 31,041 individuals for malaria infection in three (Bannu, Dera Ismail Khan and Lakki Marwat) highly malaria-endemic districts of Khyber Pakhtunkhwa province in northern Pakistan reported an overall malaria prevalence of 13.8% in the population. The study further showed that the species prevalence of
P. vivax,
P. falciparum and
Plasmodium spp. mixed infection were 92.4%, 4.7% and 2.9%, respectively
[56]. These reports clearly highlight an increasing dominance of
P. vivax in highly malaria-endemic regions of Pakistan.
Chloroquine-resistant
P. vivax is now considered as an emerging threat in Pakistan. Mutations in the
pvmdr1 gene responsible for chloroquine resistance and
pvdhfr,
pvdhps genes associated with sulphadoxine/pyrimethamine resistance in
P. vivax have been detected in recent studies from Pakistan
[57][58]. Previous studies performed in GCC countries have shown that travelers or returning travelers from Pakistan have contributed to imported malaria cases in the GCC countries, as described in
Table 1.
2.3. Characterization of Malaria Cases from Afghanistan
Afghanistan is another highly malaria-endemic country in Asia, where approximately half of the population is at risk of infection
[58]. Previous studies have shown that the incidence of malaria in Afghanistan exceeded 200,000 cases in the year 2013, with 1783 deaths
[14]. The latest WHO world malaria report has reported nearly 400,000 malaria cases in Afghanistan in 2019 and about 25% of these cases were due to
P. vivax [6]. A recent study performed to determine malaria prevalence in Jalalabad city, employing real-time PCR with high resolution melting analysis on 296 blood samples, reported asymptomatic malaria in 26 (7.8%) individuals
[59]. However, in this study 21 of 26 cases were due to
P. falciparum while only 1 case of
P. vivax was detected. The remaining 4 cases had mixed infection of
P. falciparum and
P. vivax. The study carried out in the southern region of Fars province in Iran also detected nearly all malaria cases among Afghan nationals, mostly including migrant workers, during 2006–2018
[60].
2.4. Characterization of Malaria Cases from Bangladesh
Bangladesh, another country with a population of more than 160 million people in the Indian subcontinent, is also a malaria-endemic country and nearly 17 million people are at risk of malaria infection
[6]. In recent years, Bangladesh has been successfully accelerating its efforts to eliminate malaria from the country. As a result of concerted malaria control efforts, Bangladesh has seen a steady decline in the number of malaria cases during the period 2000–2019. According to the latest world malaria report, Bangladesh reported nearly 50,000 malaria cases in 2019
[6]. Although most malaria infections are caused by
P. falciparum, the contribution of
P. vivax malaria and antimalarial drug resistance have increased over the last 20 years
[6][61]. Haque et al.
[61] reviewed antimalarial drug resistance data from Bangladesh until June 2013 and reported that
P. falciparum shows varying levels of resistance to chloroquine, mefloquine and sulfadoxine/pyrimethamine. A meta-analysis of data from malaria patients from three sites in Bangladesh has recently shown that 12–26% of
P. falciparum malaria patients are at risk of
P. vivax parasitemia after several weeks following treatment with antimalarial drugs
[38]. Imported malaria cases have also been detected among returning Bangladeshi workers in GCC countries.
2.5. Characterization of Malaria Cases from the Philippines
The Philippines, located in the WHO Western Pacific Region, is also a malaria-endemic country. Philippines reported more than 550,000 malaria cases and 230 deaths in the year 2013
[14]. However, the number of malaria cases and deaths have declined considerably from the year 2010 to 2018. The two regions with the highest prevalence of malaria in the Philippines are Palawan province and Mindanao Islands
[3][6]. According to the latest world malaria report, the Philippines reported nearly 40,000 malaria cases in 2019
[6]. Zoonotic malaria infections have also been reported from the Philippines in recent years. Some studies have shown mixed
P. falciparum/P. vivax malaria infections as well as zoonotic malaria due to
P. knowlesi in younger individuals who were working in agricultural fields within Palawan province in the Philippines
[62][63]. Drug-resistant malaria cases have also emerged as mutations in dihydropteroate synthase (pvdhps) and dihydrofolate reductase (pvdhfr), genes associated with sulfadoxine/pyrimethamine drug resistance, have been reported among
P. vivax isolates in Palawan province in the Philippines
[64]. Imported malaria cases have been detected among returning Filipino workers in GCC countries
[28][29].
2.6. Characterization of Malaria Cases from Nigeria
Nigeria, with a population of 208 million individuals and located in the sub-Saharan region, is the most populous country in Africa. Nigeria is the leading contributor of malaria cases and deaths not only in the WHO African Region but also globally. According to the latest WHO world malaria report, Nigeria reported nearly 60 million malaria cases (corresponding to 27% of total malaria cases) and 23% of total malaria deaths worldwide in 2019
[6]. Contrary to the malaria trends seen in most of the malaria-endemic countries of the world, the number of malaria cases as well as malaria-related deaths have increased in 2019 compared to 2018 in Nigeria
[6]. Nigeria is also one of eleven countries which have reported Pfhrp2/3 deletions in the
P. falciparum parasite, which compromise the utility of HPR2-based rapid diagnostic tests for the detection of malaria cases
[6]. One study showed that the levels of
P. falciparum resistance to sulfadoxine/pyrimethamine have remained low from 2000 to 2020 in western Africa, including in Nigeria
[65]. However, the rising incidence of polymorphisms in
P. falciparum genes (Pfk13, Pfmdr1, PfATPase6 and Pfcrt) associated with drug resistance to first-line drugs (artemisinin combination therapy) is a major challenge in malaria control efforts
[66]. Previous studies from GCC countries have shown that travelers or returning travelers from Nigeria have presented with imported malaria cases in Kuwait, Qatar, Saudi Arabia and UAE
[27][46][28][29][48][30][50].
2.7. Characterization of Malaria Cases from Ehiopia
Ethiopia, a land-locked country located in the Horn of Africa, is also a significant contributor for total malaria cases in the WHO African Region. According to the latest WHO world malaria report, Ethiopia reported nearly 2.5 million malaria cases in 2019
[6]. Although the number of total malaria infections in Ethiopia is still high, the country has shown considerable progress in reducing the total burden of malaria over the past two decades by employing widespread use of LLINs and IRS to protect individuals from mosquito bites
[67]. In recent years, Ethiopia has shifted efforts from the control of malaria to elimination of malaria in some regions of the country. The data reported in a recent study carried out in the Harari Region have shown that malaria incidence has declined from 42.9 cases per 1000 population in 2013 to only 6.7 cases per 1000 population in 2019
[67]. Furthermore, malaria-related deaths also decreased from 4.7 deaths per 1,000,000 persons annually in 2013 to zero in 2015. The data also showed that
P. falciparum, P. vivax and mixed infections accounted for 69.2%, 30.6% and 0.2% of all malaria cases, respectively, in the Harari Region. Ethiopia is now determined to eliminate malaria by 2030. In this regard, the country is actively pursuing artemether/lumefantrin treatment as one of the cornerstone strategies for uncomplicated
P. falciparum malaria control
[68]. Previous studies from GCC countries have shown that travelers or returning travelers from Ethiopia have contributed to imported malaria cases in all GCC countries
[47][27][28][29][48][30][50].
2.8. Characterization of Malaria Cases from Sudan
Sudan, a sub-Saharan African country, belongs to the WHO Eastern Mediterranean Region. Sudan reported nearly 2.4 million malaria cases in 2019, the highest number reported by any country in this WHO region
[5]. Recent reports of the development of drug resistance in the malarial parasite is a major threat to malaria control programs in Sudan. One recent study based on molecular makers of antimalarial drug resistance in
P. falciparum isolates was carried out during 2015–2017
[69]. The authors reported failure of artemisinin-based combination therapy for the treatment of uncomplicated malaria in Sudan. This was attributed to the high prevalence of mutations in
P. falciparum drug resistance genes. In another study, the authors reported evolution of drug resistance in
P. falciparum following artemisinin combination therapy
[70]. The study reported high frequency of mutations in
Pfcrt,
Pfdhfr and
Pfdhps, which are associated with chloroquine and sulfadoxine/pyrimethamine (SP) resistance in
P. falciparum. Travelers or returning travelers from Sudan have contributed to the number of imported malaria cases in all GCC countries
[47][27][28][29][48][30][50].