
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jameel Singh | + 3291 word(s) | 3291 | 2021-07-05 11:22:20 | | | |
| 2 | Amina Yu | + 160 word(s) | 3451 | 2021-07-16 10:54:38 | | |
Video Upload Options
Cholangiocarcinoma (CCA) is a biliary malignancy that accounts for 3% of gastrointestinal malignancies and 15% of primary liver cancers. CCA can be subclassified based on its anatomical site of origin. These classifications include intrahepatic CCA (iCCA) (A–H), perihilar CCA (pCCA), and distal CCA (dCCA).
1. Cholangiocarcinoma
Cholangiocarcinoma (CCA) is a biliary malignancy that accounts for 3% of gastrointestinal malignancies and 15% of primary liver cancers [1]. CCA can be subclassified based on its anatomical site of origin. [2]. The overall incidence of CCA has increased over recent decades, and the percentage of patients who survive 5 years after diagnosis remains unchanged (approximately 10%) [3][4][5].
A US Surveillance, Epidemiology, and End Results (SEER) study reported an overall incidence of CCA of 11.98 [95% CI, 11.79–12.16] per 1,000,000 over the period from 2000 to 2015 [6]. The highest incidence rates were found among males (13.94 [95% CI, 13.64–14.25]), patients older than 65 years (63.43 [95% CI, 62.21–64.68]), and Asians (17.78 [95% CI, 17.00–18.58]) [6]. The overall mortality was shown to be 10.30 [95% CI, 10.118–10.47]. Furthermore, this report suggested that the highest risk of mortality was among males (hazard ratio (HR) 12.16 [95% CI 11.866–12.460]), patients older than 65 years (HR 57.85 [95% CI, 56.65–59.08]), and Asians (HR 14.96 [95% CI, 14.25–15.71]).
Globally, the incidence of CCA remains highest in northeast Thailand [7][8][9]. The age-standardized incidence rates (ASIR) suggest approximately 100 per 100,000 individuals for men and 50 per 100,000 for women [7][8][9]. In the West, the ASIR is 0.5–2.0 per 100,000 individuals [9][10]. These higher rates can be attributed to Opisthorchis viverrini infection, an endemic liver fluke [10].
The prognosis remains poor despite the advances made in recent decades to understand these complex malignancies and new treatment strategies. Liver resection (LR) is the definitive treatment, but only a few patients are candidates for surgery. For locally advanced CCA in patients who are not LR candidates, neoadjuvant therapies can be used to reduce the tumor burden and allow these patients to eventually be resection or transplantation candidates. These neoadjuvant therapies include transarterial chemoembolization (TACE), selective internal radiation therapy (SIRT), radiofrequency ablation (RFA), and photodynamic therapy (PDT), which can control local tumors and avoid systemic treatment side effects.
Chronic inflammation and cholestasis are the most common causes for developing cholangiocarcinoma. Increased exposure of cholangiocytes to inflammatory markers such as IL-6, tumor necrosis factor-a, cyclo-oxygenase-2, and Wnt can lead to mutations of tumor suppressor genes and proto-oncogenes and to DNA mismatch repairs. Accumulation of bile acids and the reduced pH can also lead to increased apoptosis via ERK1/2, Akt, and NF-KB pathways that increase cell proliferation.
Cholangiocarcinomas are anatomically classified; however, the cells of origin (cholangiocytes, peribiliary glands, hepatic progenitor cells or hepatocytes) allow for different forms of classifications which may allow for better prediction of tumor behavior [11]. Worldwide, the incidence of iCCA is rising, while the incidence of perihilar or distal CC is decreasing [12]. Incidence rates also vary significantly in different countries, with Switzerland having an incidence rate of 0.45 per 100,000, while Italy has an incidence rate of 3.36 per 100,000 [13]. However, the highest incidence rates occur in Asia due to the prevalence of parasitic liver infections (approximately 85 per 100,000 in northeast Thailand) [11].
Intraductal papillary neoplasms localized to the bile duct show a stepwise progression of increasing dysplasia which supports the adenoma–dysplasia–carcinoma sequence. Biliary intraepithelial neoplasia, which arises from flat lesions of the cholangiocytes and peribiliary glands of the bile duct, also supports this hypothesis [11].
Carcinogenesis of CCA can be attributed to transformed glucose metabolism. When pyruvate is generated, cell proliferation is also promoted, which has been supported by findings of higher lactate dehydrogenase in CCA Among risk factors for CCA include cholestatic liver diseases which increase inflammation and, ultimately, lead to overexposure of cholangiocytes to bile acids, which leads to abnormal cell proliferation and cholangiocarcinogenesis [11]. It is hypothesized that this is due to chronic inflammation as well as increased cell turnover and progressive fibrosis.
Biliary stone disease has also been shown to contribute to increased risks of both iCCA and eCCA. The proposed hypothesis is that the carcinogenesis stems from impaired biliary drainage and recurrent bacterial infections. Additionally, chronic infections such as hepatitis B and C as well as liver fluke infections in endemic areas also contribute to the increased risk of ICC which is attributed to sustained inflammation that either directly or indirectly affects the biliary tree and leads to mutagenesis and cancer development [11]. Additionally, non-alcoholic fatty liver disease (NAFLD) has been shown to have a 3-fold increase for the risk of IC (OR 3.52 95% CI 2.87–4.32) and ECC (2.93, 95% CI of 2.42–3.55)
In patients who carry a diagnosis of iCCA, surgical resection with histologically negative margins shows the best outcomes in regard to survival and is the preferred treatment [14][15]. Previous studies have reported delivery of RFA percutaneously through ultrasound guidance as well as the use of open or laparoscopic RFA techniques for ablation of large tumors [16]. The efficacy of RFA is based on whether or not complete tumor ablation is achieved. Of note, to achieve an optimal ablative field, a single electrode is normally used for smaller lesions (measuring up to 2–3 cm in diameter), and multiple or clustered electrodes are used for larger lesions (3–3.5 cm in diameter) and require ablative margins of 0.5–1 cm [17][18].
Han et al. also suggested that the overall hospital stay length, cost of treatment, and complication risk are less with RFA than with surgery [18]. The median survival time was reported to be 20–60 months (Table 1). Major complications include the possibility of liver abscess, biliary strictures, or bleeding. Minor complications included post-ablative syndrome and were controlled with conservative management [18].
The mechanism of action involves treatment with a photosensitive drug with affinity for the target tissue and irritation with light of a specific wavelength that causes death of tumor cells via production of oxygen free radicals. A recent meta-analysis compared the survival benefit and quality of life between patients who received PDT with biliary stenting vs. patients who received only biliary stenting (BS) [19]. ’s study also supported a high rate of successful biliary drainage in patients who had received PDT vs. those who received BS only, with an OR of 4.39 (95% CI: 2.35–8.19) It should be noted that all patients who received PDT also received biliary stenting, and further studies would be needed to clearly identify the risk of cholangitis with PDT.
Transarterial chemoembolization (TACE) has also been studied as a neoadjuvant approach in cholangiocarcinoma patients. TACE is an intra-arterial modality used in unresectable ICC that utilizes chemotherapeutics and an oil-based contrast agent in the tumor-supplying branch of the hepatic artery and subsequent embolizing agent [20]. A 2007 study by Herber et al. showed no change in disease in 60% of patients and a partial response in 7% of patients, with a median overall survival (OS) of 21.1 months; however, this study had a small sample size consisting of 15 patients in total [21]. Vogl’s 2013 study suggested stable disease in 57% of 115 iCCA patients receiving TACE, with a median OS of 13 months [22]
Radioembolization via yttrium-90, referred to as selective internal radiotherapy (SIRT), provides locoregional treatment for primary tumors and metastatic disease [10][23]. Prior studies have suggested a median response rate which ranged from 5% to 36% and a median OS of 9 to 22 months, which has been attributed to the heterogeneity of the study population [23]. TARE is an important modality with an overall good OS and low adverse events. However, future trials are needed to compare and assess its efficacy with other modalities [24].
Microwave ablation (MWA) evolved as an alternative to RFA that allows for larger ablation zones and can be used in tissues in which RFA would not be successful, such as charred desiccated tissue [25][26]. The largest retrospective study consisting of 107 patients with primary or recurrent iCCA who underwent MWA found an OS at 1, 3, and 5 years of 93.5%, 39.6%, and 7.9%, respectively [27]. A retrospective study found that MWA combined with transarterial chemoembolization in 26 patients showed a 6-, These studies suggest that ablative therapies may prolong survival in patients with iCCA, but further investigation is warranted [28].
Summary of studies on the use of locoregional therapy for cholangiocarcinoma.
Irreversible electroporation (IRE) is a newer form of ablative technology that uses a high electrical voltage rather than thermal-based ablation such as RFA, MWA, and cryoablation [26]. There is a lack of data for IRE due to the small amount of unresectable primary or recurrent iCCAs that meet criteria for IRE treatment. A systematic review of 9 studies including 21 patients with iCCA treated with IRE found a reduction in tumor size; however, the subtype of cholangiocarcinoma was not mentioned in the study [29]. IRE provides nonthermal technology which may be beneficial in primary or recurrent iCCA when there is proximity to sensitive structures [26].
Proton beam therapy (PBT) is increasingly used, has been shown to yield excellent dose localization to target tissues, and avoids irradiation of surrounding organs [30]. A total of 12 of these patients were treated for cure and 8 for palliation, and they found median survival rates of 27.5 months and 9.6 months, respectively [31]. A phase 2 clinical trial reported a median OS of 23 months and a median PFS of 10 months (Table 1) Despite the results of previous studies using PBT, further clinical trials are warranted to further evaluate the use of PBT in iCCA.
| Reference | Patients | Treatment | Responders | Median PFS (Months) | Median OS (Months) | Tumor Progression (%) |
|---|---|---|---|---|---|---|
| Han et al. (2015) [18] | 84 | Radiofrequency Ablation | - | - | 1-year survival rate—82% (95% CI 72–90%) 3-year survival rate—47% (95% CI 28–65%) 5-year survival rate—24% (95% CI 11–40% |
21% (CI 95% 13–30%) |
| Moole et al. (2017) [19] | 297 | Photodynamic Therapy | - | - | 13.6 months (95% CI 11.47–15.67) | - |
| Herber et al. (2007) [21] | 15 | TACE | - | - | 21.1 months (95% CI 9.4–32.5 months) | 4/15 patients—8.2% |
| Vogl et al. (2012) [22] | 115 | TACE | Partial Response 8.7% | 7 months | 13 months | - |
| Edeline et al. (2019) [23] | 41 | Selective Internal Radiotherapy | 41% (95% CI 28–55%) | 14 months (95% CI 8–17 months) | 22 months (95% CI 14–52 months) | - |
| Zhang et al. (2016) [27] | 107 | MWA | - | 8.9 months (95% CI 6.5–11.3 months) | 28.0 months (95% CI 23.7–32.2 months) | - |
| Yu et al. (2011) [32] | 15 | MWA | - | - | 10 months | 25% (6/24 nodules total in 15 patients) |
| Ohkawa et al. (2014) [31] | 20 | Proton Beam Therapy | - | - | 27.5 months in curative group 9.6 months in palliative group |
- |
| Hong et al. (2016) [33] | 37 | Proton Beam Therapy | - | 8.4 months (95% CI 5–15.7 months) | 22.5 (95% CI 12.4–49.7 months) | - |
| Frankulli et al. (2019) [34] | 182 | Stereotactic Body Radiation | - | - | 1-year survival rate of 57.1% (95% CI 45–58%) | - |
| Queen et al. (2014) [35] | 106 | Endoscopy | - | - | 2.89 months (95% CI 0.09–30 months) | - |
With the evolution of external beam radiotherapy, use of stereotactic body radiotherapy (SRBT) has been proposed for GI tumors. A 2019 systematic review consisting of nine studies suggested a 1-year pooled OS of 58.3% (CI 95% 50.2–66.1%), and the 1-year OS was 57.1% (CI 95% 45.0–58.0%) for iCCA Among the severe complications, cholangitis, abnormal LFTs, and duodenal obstruction, as well as transient biliary obstruction, were reported [36][37][38]. Due to the lack of data, further studies would be warranted to better define the role of SBRT in iCCA.
Patients who are not surgical candidates may benefit from palliative endoscopic stent therapy. The median survival of patients without biliary stent placement has been shown to be lower than that for those with biliary drainage [38]. Additionally, endoscopic biliary drainage improves the quality of life by relieving jaundice and associated symptoms of jaundice such as diarrhea, sleep disturbance, anorexia, and pruritis [39]. Among the complications of stent therapy includes clogging with stones or obstruction with tumor ingrowth or overgrowth [39].
2. Combined Hepatocellular Cholangiocarcinoma
Combined or mixed hepatocellular cholangiocarcinoma (CHC) is a class of liver cancer that shares characteristics of HCC and CC [40][41][42]. The earliest pathological classifications provided in 1949 by Allen and Lisa consisted of three subtypes: type A, characterized by synchronous but separate and individual epicenters of both HCC and CC in one liver; type B, composed of mixed distinguished foci of HCC and CC; and type C, consisting of both HCC and CC that stem from the same tumor Nonetheless, CHC and HCC can also coexist in the same liver simultaneously (Figure 1A–B). Recently, the WHO classification reported CHC as a distinct entity and identified two main subtypes: the classical type, and CHC with stem cell features [42].
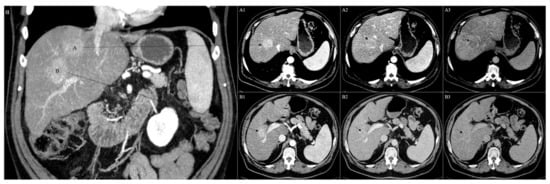
Mutations of TP53, TERT promoter, and ARID1A are common genetic anomalies in CHC. Definitive diagnosis of CHC requires biopsy. Surgical resection is the treatment of choice, while liver transplantation for early-stage CHC is shown to have favorable outcomes. Surgery has been effective in both younger as well as older patients, and resection margins of >10 mm have been associated with longer disease-free survival in patients [43].
Sorafenib monotherapy had a progression-free survival of only 4.8 months [43]. Portal and hepatic venous infiltration is observed in both CHC and HCC. There are no guidelines to suggest optimal management for CHC. Localized tumors are often managed with radical resection which has become the preferred treatment option, and advanced tumors are often treated with systemic therapies that overlap with treatment for iCCA and HCC [44].
In patients who have recurrent or inoperable CHC, it is nonsurgically treated with TACE, RFA, SIRT, and systemic chemotherapy. The data to suggest the benefit of loco-regional therapies are limited but have shown partial response rates and can allow for surgical resection and a possible survival benefit [45][46][47][48][49].
CHCs are a rare class of PLCs and can consist of clinicopathological and radiological features of both HCC and CC in the same tumor [50]. The primary hypothesis is that CHCs originate from bipotent hepatic progenitor cells (HPCs) with both hepatocellular and cholangiocellular differentiation [50]. An alternate hypothesis suggested is that there is redifferentiation or dedifferentiation of HCC to a biliary phenotype and vice versa, but this remains a controversial theory [51][52].
Spolverato et al. reported a doubling of the number of diagnosed CHC cases from 2004 to 2015, which was attributed to an increase in accurate diagnosis [53]. A SEER database analysis of 20,000 patients with primary liver cancer found the CHC had an incidence of 1.3% of total cases [54]. Additional retrospective population-based studies suggest an average age of diagnosis of 62.5 years and an overall incidence of 0.05 per 100,000 per year [55]. Western studies seemed to suggest a correlation between male sex and older age, which contradicted Eastern studies which showed a correlation with male sex but had an earlier age of diagnosis [49].
The second type contains stem cell features and consists of three different variants: (1) typical, with mature hepatocytes surrounded by HPC-like cells, (2) intermediate, with expression of both HCC and CC markers, and (3) cholangiolocellular (CLC), which consists of cells viewed as HPCs but organized into tubular cholangioles with desmoplastic stroma [51][56][57]. However, as of 2019, the WHO classification reclassified the CLC subtype to a subtype of small duct intrahepatic CC [58]. Molecular findings of CHC have not been clearly defined and carry a wide range of mutations which overlap between HCC and CC. demonstrated both LOH and TP53 mutations in more than 50% of CHC and CC cases, thus aligning CHC closer to CC [59].
Histologically, CHC commonly appears as a classical type which is characterized by variable merging of HCC and CC areas with areas of interface of unequivocal components (Figure 2) [60]. The HCC component is distinguished by the appearance of bile-producing cells (and canaliculi) with a granular eosinophilic cytoplasm and often contains intracytoplasmic fibrinogen, Mallory–Denk bodies, alpha1 antitrypsin, and fat globules [1]. Common immunohistochemical markers include HepPar1, glypican-3, CAM5.2, canalicular pCEA, and CD10. Typical stains for the biliary component include mucin/mucicarmine, pCEA, cytoplasmic CD10, AE1, MOC31, CK7, and CK19, the last two previously correlated with worse outcomes of HCC after liver transplantation [1][61].
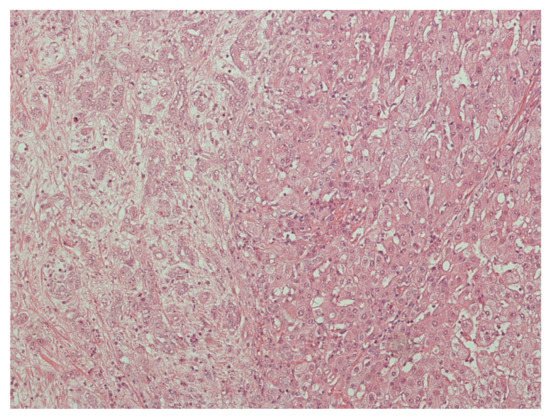
Risk factors for HCC and CC are similar to CHC, which include viral hepatitis, cirrhosis, and alcohol consumption. It is suggested that diabetes mellitus and obesity are possible confounders rather than risk factors for CHC as they contribute to non-alcoholic steatohepatitis and subsequent cirrhosis [62].
Ablative therapies are minimally invasive and destroy normal tissue that arises with different conditions. This can be performed by using chemical, thermal, and other techniques. It is low cost but has high recurrence rates which can limit its use for lesions smaller in size. Chemical ablation is mainly used for HCC and can also be applied to neuroendocrine tumors.
Surgical resection remains the only curative option for patients with CHC and is dictated by multiple factors including the patient’s overall condition, tumor invasion, and the tumor’s anatomical location. Despite the aim of liver resection, CHC has been shown to have high recurrence rates and 5-year survival rates of 30% [46]. Studies have shown worse outcomes for CHC patients after resection than HCC, which can be attributed to the difference in biological features [46]. However, the use of locoregional therapy to reduce the local tumor burden may provide patients with benefits prior to liver resection or transplantation.
TACE, percutaneous ethanol injections (PEI), and RFA are applied in cases of unresectable HCC and recurrence following surgical resection [63][64]. Conversely, CHC tends to be less vascular and more fibrotic. As a result of these features, CHC is less responsive to TACE or PEI. However, RFA or cryoablation may be beneficial in select cases (Table 2)
| Reference | Patients | Treatment | Responders | Median PFS (Months) | Median OS (Months) | Tumor Progression (%) |
|---|---|---|---|---|---|---|
| Fowler et al. (2015) [47] | 79 | TACE (6) | - | - | - | 2 (30%) |
| TARE (6) | 47% | 16 | 8.3 | 3 (50%) | ||
| HAI pump (6) | - | - | - | 0 | ||
| Systemic chemotherapy (28) | - | - | - | 15 (44%) | ||
| Surgery (33) | 35 (70%) | 16 | 12.3 | 0 | ||
| Kim et al. (2010) [65] | 50 | TACE | 35 (70%) | - | 12.3 | 15(30%) |
| Chan et al. (2017) [66] | 10 | TARE | 60% partial response and 40% stable disease | - | 10.2 from 1st RE treatment and 17.7 from initial diagnosis |
- |
| Na et al. (2018) [67] | 42 | TACE | Globally enhancing cHCC-CC—36% Peripherally enhancing cHCC-CC—0% HCC—35.6% |
Globally enhancing cHCC-CC—4.7 Peripherally enhancing cHCC-CC—2.1 HCC—9.7 |
Globally enhancing cHCC-CC—52.8 Peripherally enhancing cHCC-CC—12.4 HCC—67.5 |
cHCC-CC—37(88.1%) |
A small retrospective study showed that 18 patients who received liver-directed therapies out of a cohort of 79 patients [64] were more likely to have increased tumor size compared to those undergoing surgical resection (8.9 vs. 5.8 cm) and a higher incidence of metastatic disease (33% vs. 8%) [64]. However, liver-directed therapy yielded an overall partial response rate of 47% (50% with radioembolization, 20% with TACE, and 66% with hepatic arterial infusional chemotherapy). While the study was limited by the sample and retrospective design, it shows that liver-directed locoregional therapy may provide some patients with therapeutic benefits [64]. Furthermore, patients with down-sized tumors may be converted to surgical candidates.
In the future, as already demonstrated for HCC, the possible introduction of a subclassification of CHC stages could help in predicting the prognosis of patients and in choosing the more effective treatment option [68]. Overall, while the current data are very limited, the evidence appears to hint towards a therapeutic regimen which combines locoregional therapy such as RFA and platinum-containing drugs for nonresectable CHC. A small subgroup analysis revealed that patients treated with systemic chemotherapy compared to sorafenib and prior TACE or RFA tend to have better ECOG performance scores (63.9% vs. 24.3%,p< 0.001) Furthermore, therapies should include the integration of translational research, understand the genomic landscape of the tumor, and use molecular targeted treatments [46].
Summary of studies on the use of locoregional therapy for combined hepatocellular cholangiocarcinoma.
Chan et al. reported a small study of 10 patients (median age 59 years; 6 men, 4 women) with histologically confirmed unresectable CHC treated with radioembolization using resin (6 patients) or glass (4 patients) microspheres [69]. From initial diagnosis and from the first radioembolization treatment, the median overall survivals were 10.2 and 17.7 months, respectively (Table 2). The study shows that radioembolization may be a promising method for treating patients who are not initial candidates for resection (Figure 3A–H). Furthermore, radioembolization may be an option for patients with metastatic disease, though with a lower response rate [69][70].
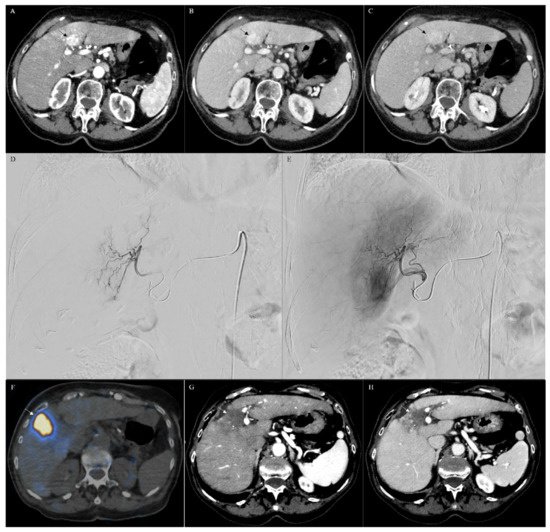
3. Conclusions
Locoregional therapies provide local cancer treatment and control. While advances in recent decades have developed strategic therapies, there is a paucity of large clinical trials, particularly with patients diagnosed with CHC. Current clinical trials are likely to impact clinical practice and offer patients an improved quality of life and survival.
References
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 577–588.
- Rizvi, S.; Gores, G.J. Pathogenesis, Diagnosis, and Management of Cholangiocarcinoma. Gastroenterology 2013, 145, 1215–1229.
- Khan, S.A.; Davidson, B.R.; Goldin, R.D.; Pereira, S.P.; Rosenberg, W.M.C.; Taylor-Robinson, S.D.; Thillainayagam, A.V.; Thomas, H.; Thursz, M.R.; Wasan, H. Guidelines for the diagnosis and treatment of cholangiocarcinoma: An update. Gut 2012, 61, 1657–1669.
- Everhart, J.E.; Ruhl, C.E. Burden of Digestive Diseases in the United States Part III: Liver, Biliary Tract, and Pancreas. Gastroenterology 2009, 136, 1134–1144.
- Tyson, G.L.; El-Serag, H.B. Risk factors for cholangiocarcinoma. Hepatology 2011, 54, 173–184.
- Gad, M.M.; Saad, A.M.; Faisaluddin, M.; Gaman, M.A.; Ruhban, I.A.; Jazieh, K.A.; Al-Husseini, M.J.; Simons-Linares, C.R.; Sonbol, M.B.; Estfan, B.N. Epidemiology of Cholangiocarcinoma; United States Incidence and Mortality Trends. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 885–893.
- Sripa, B.; Pairojkul, C. Cholangiocarcinoma: Lessons from Thailand. Curr. Opin. Gastroenterol. 2008, 24, 349–356.
- West, J.; Wood, H.; Logan, R.F.A.; Quinn, M.; Aithal, G.P. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971–2001. Br. J. Cancer 2006, 94, 1751–1758.
- Khan, S.A.; Taylor-Robinson, S.D.; Toledano, M.B.; Beck, A.; Elliott, P.; Thomas, H.C. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J. Hepatol. 2002, 37, 806–813.
- Bray, F. Cancer Incidence in Five Continents (CI5) Volume XI. IARC. 2017. Available online: (accessed on 1 June 2021).
- Labib, P.L.; Goodchild, G.; Pereira, S.P. Molecular Pathogenesis of Cholangiocarcinoma. BMC Cancer 2019, 19, 185.
- Razumilaza, N.; Gores, G. Cholangiocarcinoma. Lancet 2014, 383, 21–27.
- Bridgewater, J.; Galle, P.; Khan, S.; Llovet, J.; Park, J.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the diagnosis and management of intra-hepatic cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289.
- Aljiffry, M.; Abdulelah, A.; Walsh, M.; Peltekian, K.; Alwayn, I.; Molinari, M. Evidence-Based Approach to Cholangiocarcinoma: A Systematic Review of the Current Literature. J. Am. Coll. Surg. 2009, 208, 134–147.
- Ercolani, G.; Vetrone, G.; Grazi, G.L.; Aramaki, O.; Cescon, M.; Ravaioli, M.; Serra, C.; Brandi, G.; Pinna, A.D. Intrahepatic cholangiocarcinoma: Primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survival. Ann Surg. 2010, 252, 107–114.
- Simo, K.A.; Halpin, L.E.; McBrier, N.M.; Hessey, J.A.; Baker, E.; Ross, S.; Swan, R.Z.; Iannitti, D.A.; Martinie, J.B. Multimodality treatment of intrahepatic cholangiocarcinoma: A review. J. Surg. Oncol. 2016, 113, 62–83.
- Shindoh, J. Ablative therapies for intrahepatic cholangiocarcinoma. HepatoBiliary Surg. Nutr. 2017, 6, 2–6.
- Han, K.; Ko, H.K.; Kim, K.W.; Won, H.J.; Shin, Y.M.; Kim, P.N. Radiofrequency Ablation in the Treatment of Unresectable Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-Analysis. J. Vasc. Interv. Radiol. 2015, 26, 943–948.
- Moole, H.; Tathireddy, H.; Dharmapuri, S.; Moole, V.; Boddireddy, R.; Yedama, P.; Dharmapuri, S.; Uppu, A.; Bondalapati, N.; Duvvuri, A. Success of photodynamic therapy in palliating patients with nonresectable chol-angiocarcinoma: A systematic review and meta-analysis. World J. Gastroenterol. 2017, 23, 1278–1288.
- Rizzo, A.; Brandi, G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat. Res. Commun. 2021, 27, 100354.
- Herber, S.; Otto, G.; Schneider, J.; Manzl, N.; Kummer, I.; Kanzler, S.; Schuchmann, A.; Thies, J.; Düber, C.; Pitton, M. Transarterial Chemoembolization (TACE) for Inoperable Intrahepatic Cholangiocarcinoma. Cardiovasc. Interv. Radiol. 2007, 30, 1156–1165.
- Vogl, T.J.; Naguib, N.N.; Nour-Eldin, N.-E.A.; Bechstein, W.; Zeuzem, S.; Trojan, J.; Gruber-Rouh, T. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: Results and prognostic factors governing treatment success. Int. J. Cancer 2012, 131, 733–740.
- Edeline, J.; Touchefeu, Y.; Guiu, B.; Farge, O.; Tougeron, D.; Baumgaertner, I.; Ayav, A.; Campillo-Gimenez, B.; Beuzit, L.; Pracht, M.; et al. Radioemboliza-tion Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 6, 51–59.
- Mosconi, C.; Calandri, M.; Javle, M.; Odisio, B.C. Interventional radiology approaches for intra-hepatic cholan-giocarcinoma. Chin. Clin. Oncol. 2020, 9, 8.
- Andreano, A.; Brace, C.L. A Comparison of Direct Heating During Radiofrequency and Microwave Ablation in Ex Vivo Liver. Cardiovasc. Interv. Radiol. 2013, 36, 505–511.
- Sweeney, J.; Parikh, N.; El-Haddad, G.; Kis, B. Ablation of Intrahepatic Cholangiocarcinoma. Semin. Interv. Radiol. 2019, 36, 298–302.
- Zhang, K.; Yu, J.; Yu, X.; Han, Z.; Cheng, Z.; Liu, F.; Liang, P. Clinical and survival outcomes of percutaneous microwave ablation for intrahepatic cholangiocarcinoma. Int. J. Hyperth. 2018, 34, 292–297.
- Yousaf, A.; Kim, J.U.; Eliahoo, J.; Taylor-Robinson, S.D.; Khan, S.A. Ablative Therapy for Unresectable Intrahe-patic Cholangiocarcinoma: A Systematic Review and Meta-Analysis. J. Clin. Exp. Hepatol. 2019, 9, 740–748.
- Tian, G.; Zhao, Q.; Chen, F.; Jiang, T.; Wang, W. Ablation of hepatic malignant tumors with irreversible elec-troporation: A systematic review and meta-analysis of outcomes. Oncotarget 2017, 8, 5853–5860.
- Konstantinidis, I.T.; Arkadopoulos, N.; Ferrone, C.R. Surgical management of intrahepatic cholangiocarcinoma in the modern era: Advances and challenges. Chin. Clin. Oncol. 2016, 5, 9.
- Ohkawa, A.; Mizumoto, M.; Ishikawa, H.; Abei, M.; Fukuda, K.; Hashimoto, T.; Sakae, T.; Tsuboi, K.; Okumura, T.; Sakurai, H. Proton beam therapy for unresectable intrahepatic cholangiocarcinoma. J. Gastroenterol. Hepatol. 2014, 30, 957–963.
- Yu, M.A.; Liang, P.; Yu, X.L.; Cheng, Z.G.; Han, Z.Y.; Liu, F.Y.; Yu, J. Sonography-guided percutaneous micro-wave ablation of intrahepatic primary cholangiocarcinoma. Eur. J. Radiol. 2011, 80, 548–552.
- Hong, T.S.; Wo, J.Y.; Yeap, B.Y.; Ben-Josef, E.; McDonnell, E.I.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; Goyal, L.; et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J. Clin. Oncol. 2016, 34, 460–468.
- Frakulli, R.; Buwenge, M.; Macchia, G.; Cammelli, S.; Deodato, F.; Cilla, S.; Cellini, F.; Mattiucci, G.C.; Bisello, S.; Brandi, G.; et al. Stereotactic body radiation therapy in cholangiocarcinoma: A systematic review. Br. J. Radiol. 2019, 92, 20180688.
- Queen, T.; Adler, D. Stent placement in perihilar cholangiocarcinoma. Clin. Liver Dis. 2014, 3, 74–78.
- Welling, T.H.; Feng, M.; Wan, S.; Hwang, S.Y.; Volk, M.L.; Lawrence, T.S.; Zalupski, M.M.; Sonnenday, C.J. Neoadjuvant stereotactic body radiation therapy, capecitabine, and liver transplantation for unresectable hilar cholangiocarcinoma. Liver Transplant. 2014, 20, 81–88.
- Kopek, N.; Holt, M.I.; Hansen, A.T.; Høyer, M. Stereotactic body radiotherapy for unresectable cholangiocarci-noma. Radiother. Oncol. 2010, 94, 47–52.
- Tse, R.V.; Hawkins, M.; Lockwood, G.; Kim, J.J.; Cummings, B.; Knox, J.; Sherman, M.; Dawson, L.A. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholan-giocarcinoma. JCO 2008, 26, 657–664.
- Kim, J.H. Endoscopic Stent Placement in the Palliation of Malignant Biliary Obstruction. Clin. Endosc. 2011, 44, 76–86.
- Ramai, D.; Ofosu, A.; Lai, J.K.; Reddy, M.; Adler, D.G. Combined hepatocellular cholangiocarcinoma: A popula-tion-based retrospective study. Am. J. Gastroenterol. 2019, 114, 1496–1501.
- Goodman, Z.D.; Ishak, K.G.; Langloss, J.M.; Sesterhenn, I.A.; Rabin, L. Combined hepatocellu-lar-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer 1985, 55, 124–135.
- Bosman, F.T.; Carneiro, F.; Hruban, R.; Theise, N.D. WHO Classification of Tumours of the Digestive System; IARC Press: Lyon, France, 2010.
- Allen, R.A.; Lisa, J.R. Combined Liver Cell ahd Bile Duct Carcinoma*. Am. J. Pathol. 1949, 25, 647–655.
- Azizi, A.A.; Hadjinicolaou, A.V.; Goncalves, C.; Duckworth, A.; Basu, B. Update on the Genetics of and System-ic Therapy Options for Combined Hepatocellular Cholangiocarcinoma. Front Oncol. 2020, 10, 570958.
- Kassahun, W.T.; Hauss, J. Management of combined hepatocellular and cholangiocarcinoma. Int. J. Clin. Pr. 2008, 62, 1271–1278.
- Stavraka, C.; Rush, H.; Ross, P. Combined hepatocellular cholangiocarcinoma (CHC): An update of genetics, molecular biology, and therapeutic interventions. J. Hepatocell. Carcinoma 2018, 6, 11–21.
- Fowler, K.; Saad, N.E.; Brunt, E.M.; Doyle, M.B.M.; Amin, M.; Vachharajani, N.; Tan, B.; Chapman, W.C. Biphenotypic Primary Liver Carcinomas: Assessing Outcomes of Hepatic Directed Therapy. Ann. Surg. Oncol. 2015, 22, 4130–4137.
- Park, Y.-H.; Hwang, S.; Ahn, C.-S.; Kim, K.-H.; Moon, D.-B.; Ha, T.-Y.; Song, G.-W.; Jung, D.-H.; Park, G.-C.; Namgoong, J.-M.; et al. Long-Term Outcome of Liver Transplantation for Combined Hepatocellular Carcinoma and Cholangiocarcinoma. Transplant. Proc. 2013, 45, 3038–3040.
- Trikalinos, N.A.; Zhou, A.; Doyle, M.B.M.; Fowler, K.J.; Morton, A.; Vachharajani, N.; Amin, M.; Keller, J.W.; Chapman, W.C.; Brunt, E.M.; et al. Systemic Therapy for Combined Hepatocellular-Cholangiocarcinoma: A Single-Institution Experience. J. Natl. Compr. Cancer Netw. 2018, 16, 1193–1199.
- Schizas, D.; Mastoraki, A.; Routsi, E.; Papapanou, M.; Tsapralis, D.; Vassiliu, P.; Toutouzas, K.; Felekouras, E. Combined hepatocellular-cholangiocarcinoma: An update on epidemiology, classification, diagnosis and management. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 515–523.
- Zhao, Q.; Yu, W.-L.; Lu, X.-Y.; Dong, H.; Gu, Y.-J.; Sheng, X.; Cong, W.-M.; Wu, M.-C. Combined hepatocellular and cholangiocarcinoma originating from the same clone: A pathomolecular evidence-based study. Chin. J. Cancer 2016, 35, 82.
- Gera, S.; Ettel, M.; Acosta-Gonzalez, G.; Xu, R. Clinical features, histology, and histogenesis of combined hepa-tocellular-cholangiocarcinoma. World J. Hepatol. 2017, 9, 300–309.
- Spolverato, G.; Bagante, F.; Tsilimigras, D.I.; Ejaz, A.; Cloyd, J.; Pawlik, T.M. Management and outcomes among patients with mixed hepatocholangiocellular carcinoma: A population-based analysis. J. Surg. Oncol. 2018, 119, 278–287.
- Wachtel, M.S.; Zhang, Y.; Xu, T.; Chiriva-Internati, M.; Frezza, E.E. Combined hepatocellular cholangiocarci-nomas; analysis of a large database. Clin. Med. Pathol. 2008, 1, 43–47.
- Sasaki, M.; Sato, H.; Kakuda, Y.; Sato, Y.; Choi, J.H.; Nakanuma, Y. Clinicopathological significance of ‘subtypes with stem-cell feature’ in combined hepatocellular-cholangiocarcinoma. Liver Int. 2015, 35, 1024–1035.
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, M.K.; Carneiro, F.; Cree, I.A.; The WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188.
- Joseph, N.M.; Tsokos, C.G.; Umetsu, S.E.; Shain, A.H.; Kelley, R.K.; Onodera, C.; Bowman, S.; Talevich, E.; Ferrell, L.D.; Kakar, S.; et al. Genomic profiling of combined hepatocellular-cholangiocarcinoma reveals similar genetics to hepatocellular carcinoma. J. Pathol. 2019, 248, 164–178.
- Zhou, Y.M.; Zhang, X.F.; Wu, L.P.; Sui, C.J.; Yang, J.M. Risk factors for combined hepatocellu-lar-cholangiocarcinoma: A hospital-based case-control study. World J. Gastroenterol. 2014, 20, 12615–12620.
- Wang, A.-Q.; Zheng, Y.-C.; Du, J.; Zhu, C.-P.; Huang, H.-C.; Wang, S.-S.; Wu, L.-C.; Wan, X.-S.; Zhang, H.-H.; Miao, R.-Y.; et al. Combined hepatocellular cholangiocarcinoma: Controversies to be addressed. World J. Gastroenterol. 2016, 22, 4459–4465.
- Vasuri, F.; Golfieri, R.; Fiorentino, M.; Capizzi, E.; Renzulli, M.; Pinna, A.D.; Grigioni, W.F.; D’Errico-Grigioni, A. OATP 1B1/1B3 expression in hepatocellular carcinomas treated with orthotopic liver transplantation. Virchows Arch. 2011, 459, 141–146.
- Llovet, J.M.; Real, M.I.; Montaña, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Solà, R.; et al. Arterial embolisation or chemoembolisation versus sympto-matic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 2002, 359, 1734–1739.
- Okada, S. Local Ablation Therapy for Hepatocellular Carcinoma. Semin. Liver Dis. 1999, 19, 323–328.
- Na, S.K.; Choi, G.H.; Lee, H.C.; Shin, Y.M.; An, J.; Lee, D.; Shim, J.H.; Kim, K.M.; Lim, Y.S.; Chung, Y.H.; et al. The effectiveness of transarterial chemoembolization in recurrent hepatocellular-cholangiocarcinoma after resection. PLoS ONE 2018, 13, e0198138.
- Kim, J.H.; Yoon, H.K.; Ko, G.Y.; Gwon, D.I.; Jang, C.S.; Song, H.Y.; Shin, J.H.; Sung, K.B. Nonresectable combined hepatocellular carcinoma and cholangiocarcinoma: Analysis of the response and prognostic factors after transcatheter arterial chemoembolization. Radiology 2010, 255, 270–277.
- Giannini, E.G.; Moscatelli, A.; Pellegatta, G.; Vitale, A.; Farinati, F.; Ciccarese, F.; Piscaglia, F.; Rapaccini, G.L.; Di Marco, M.; Caturelli, E.; et al. Application of the Intermediate-Stage Subclassification to Pa-tients With Untreated Hepatocellular Carcinoma. Am. J. Gastroenterol. 2016, 111, 70–77.
- Chan, L.S.; Sze, D.Y.; Poultsides, G.A.; Louie, J.D.; Abdelrazek Mohammed, M.A.; Wang, D.S. Yttrium-90 Radi-oembolization for Unresectable Combined Hepatocellular-Cholangiocarcinoma. Cardiovasc. Intervent. Radiol. 2017, 40, 1383–1391.
- Tang, D.; Nagano, H.; Nakamura, M.; Wada, H.; Marubashi, S.; Miyamoto, A.; Takeda, Y.; Umeshita, K.; Dono, K.; Monden, M. Clinical and pathological features of Allen’s type C classification of resected combined hepato-cellular and cholangiocarcinoma: A comparative study with hepatocellular carcinoma and cholangiocellular carcinoma. J. Gastrointest. Surg. 2006, 10, 987–998.
- Kim, E.J.; Yoo, C.; Kang, H.J.; Kim, K.-P.; Ryu, M.-H.; Park, S.R.; Lee, D.; Choi, J.; Shim, J.H.; Kim, K.M.; et al. Clinical outcomes of systemic therapy in patients with unresectable or metastatic combined hepatocellu-lar-cholangiocarcinoma. Liver Int. 2020, 41, 1398–1408.
- Takada, T.; Kato, H.; Matsushiro, T.; Nimura, Y.; Nagakawa, T.; Nakayama, T. Comparison of 5-Fluorouracil, Doxorubicin and Mitomycin C with 5-Fluorouracil Alone in the Treatment of Pancreatic-Biliary Carcinomas. Oncology 1994, 51, 396–400.
- Connell, L.C.; Harding, J.J.; Lowery, M.A.; Kemeny, N.E.; Cercek, A.; Abdelgawad, M.I.; O’Reilly, E.M.; Saltz, L.; Abou-Alfa, G.K. Platinum-based combination therapy (PCT) and outcomes for patients (pts) with mixed hepa-tocellular carcinoma and intrahepatic cholangiocarcinoma (mHCC/ICC). J. Clin. Oncol. 2015, 33, e15146.




