
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Giulia Lionetto | + 3076 word(s) | 3076 | 2021-07-06 10:37:02 | | | |
| 2 | Beatrix Zheng | Meta information modification | 3076 | 2021-07-15 04:09:56 | | |
Video Upload Options
Marine biodiversity is threatened by several anthropogenic pressures. Pollution deriving from the discharge of chemical contaminants in the sea represents one of the main threats to the marine environment, influencing the health of organisms, their ability to recover their homeostatic status, and in turn endangering biodiversity. Molecular and cellular responses to chemical pollutants, known as biomarkers, are effect-based methodologies useful for detecting exposure and for assessing the effects of pollutants on biota in environmental monitoring. Pollution biomarkers can be useful tools for monitoring and assessment of pollution threats to marine biodiversity, both in the environmental quality monitoring of protected areas and the assessment of the health status of species at risk.
1. Introduction
Chemical pollution derived from the discharge of chemical contaminants in the sea, from both point and non-point pollution sources, represents one of the main threats to the marine environments and their resources and services and remains a great environmental challenge [1][2]. Shipping is a source of pollutants through accidental spillages, operational discharges, and antifouling paint leaching; mariculture accounts for medicinal product, biocide, and food additive release; offshore activities produce drill cuttings and hydrocarbon release; dredging of sediment and dumping at sea contribute to water column contaminant level increase [3]. Moreover, the release of chemical contaminants into the sea from land-based activities, such as urban wastewater discharge, industrial and agricultural activities, mining, and runoff from coastal areas, contributes dramatically to the contamination of the seas. Several of them are contaminants of emerging concern (CECs), which include a wide array of anthropogenic chemicals that have no regulatory standards yet [4][5].
Chemicals absorbed by the organisms through the gills, the gastrointestinal tract, and the tegument can interact with biological macromolecules, producing several toxicological effects at the cellular and molecular levels. This includes enzyme inhibition, alterations of transport properties, alteration of the functioning of membrane and intracellular receptors, alterations of intracellular signaling pathways, oxidative stress, and DNA damage [6][7][8][9][10]. These primary effects at the molecular and cellular levels can produce integrated toxicity effects over time, including impairment of organ and systems functioning such as neurotoxic effects, immunological responses, hepatotoxicity, behavioral changes, reproductive and developmental alterations, endocrine disruption, and genotoxicity [2]. For example, some persistent marine pollutants can exacerbate the adverse effects of certain pesticides as well as other persistent organic pollutants (POPs) in marine organisms [11].
Healthy oceans are among the main objectives of the EU by 2030. To reach these goals, water quality monitoring and assessment assume a fundamental role. The study of the molecular, cellular, and physiological alterations in the organism in relation to the exposure to chemical pollutants has contributed to developing several markers (biomarkers) of exposure and toxicological responses to chemical pollutants [12][13]. The application of the biomarker approach in marine environment monitoring and assessment, integrated into the physicochemical analysis of the environmental matrices, has greatly increased in recent years. This is mainly due to the fact that the assessment of the entity of the organism exposure to pollutants in a certain environment and the extent of the suffered toxicological effects is of fundamental importance for decision making related to habitat and species protection, ecosystem services provision, adoption of remediation procedures, or impacted area monitoring [14].
Recently, the application of the biomarker approach in biomonitoring is considered with great interest in the field of biodiversity conservations. Considering that chemical pollution is recognized as one of the major pressures driving biodiversity reduction loss worldwide [15], the study of the responses of the organisms to the anthropogenic alterations of the environment that may cause or contribute to population decline can support biodiversity conservation strategies. Biomarkers have been recently applied to several research areas of the biodiversity conservation field, including environmental quality monitoring of protected areas and the assessment of the health status of species at risk.
2. Pollution Biomarkers in Biomonitoring of Marine Protected Areas
| Protected Areas | Bioindicator Species | Bioindicator Class | Endpoint | Biomarkers Analyzed | Ref. |
|---|---|---|---|---|---|
| Europe | |||||
| Egadi Islands Marine Protected Area (Italy) |
Coris julis, Patella caerulea, Paracentrotus lividus |
Osteichthyes Gastropoda Echinoidea |
Detoxification of organic pollutants | EthoxyresorufinO-deethylase, BaPMO, NADH ferry red, and NADH cyt c | [23] |
| Tremiti Islands Marine Protected Area (Italy) | Paracentrotus lividus | Echinoidea | Coelomocytes alterations | Coelomocytes subpopulations ratio, heat-shock protein 70 | [24] |
| National Park of La Maddalena Arcipelago (Italy) | Mytilus galloprovincialis | Bivalvia | Lysosomal alterations | Lysosomal membrane stability, lipofuscin content, neutral lipid contents, lysosomal structural changes | [25] |
| Capo Peloro Natural Reserve (Italy) | Atherina boyeri | Osteichthyes | Detoxification of organic pollutants, neurotoxicity, genotoxicity |
Acetylcholinesterase, benzo(a)pyrene-monooxygenase, polycyclic aromatic hydrocarbons metabolites in bile, erythrocytic nuclear abnormalities assay |
[26] |
| The Pelagos Sanctuary (International Sanctuary for the Protection of Mediterranean Marine Mammals) (Italy, France) |
Meganyctiphanes norvegica | Malacostraca | Detoxification of organic pollutants, neurotoxicity, response to xenoestrogens | Cytochrome P450, BaPMO activity, NADPH cytochromec reductase, NADH-ferricyanide reductase, esterases, porphyrins, vitellogenin, zona radiata proteins, acetylcholinesterase |
[27] |
| Stenella coeruleoalba | Mammalia | Detoxification of organic pollutants, oxidative stress |
Cytochrome P4501A, cytochrome P4502B, catalase | [28] | |
| Balaenoptera physalus | Mammalia | Detoxification of organic chemical pollutants, oxidative stress |
Cytochrome P4501A, cytochrome P4502B, lipoperoxidation | [29] | |
| Balaenoptera physalus, Physeter macrocephalus |
Mammalia | Metal excretion | Metals in the fecal material | [30] | |
| North America | |||||
| Florida Keys National Marine Sanctuary (U.S.A.) |
Montastraea annularis | Anthozoa | Oxidative stress, stress protein multidrug resistance induction |
Superoxide dismutase, glutathione peroxidase, glutathione-s-transferase, heat-shock proteins, metabolic condition, multixenobiotic resistance proteins | [31] |
| Veracruz Coral Reef System National Park (Mexico) | Haemulon Aurolineatum, Ocyurus chrysurus |
Osteichthyes | Detoxification of organic chemical pollutants, response to xenoestrogens | Cytochrome P4501A, vitellogenin, glutathione-S-transferase, PAH metabolites in fish bile |
[32] |
| Natural protected area of Laguna Madre in the Gulf of Mexico (Mexico) | Chione elevata | Bivalvia | Neurotoxic effects, oxidative stress, metabolic alterations |
Acetylcholinesterase, butyrylcholinesterase, carboxylesterase, alkaline phosphatase, glutathione s-transferase, oxygen radical absorbance capacity |
[33] |
| South America | |||||
| Morrocoy National Park (Venezuela) | Siderastrea sidereal | Anthozoa | Detoxification of organic chemical pollutants, oxidative stress | Cytochrome P450 I, cytochrome P450 II, NADPH reductase, glutathione S-transferase, catalase, superoxide dismutase | [34] |
| Parque Nacional Archipielago Los Roques (Venezuela) | Siderastrea sidereal | Anthozoa | Detoxification of organic chemical pollutants, oxidative stress | Cytochrome P450 I, cytochrome P450 II, NADPH reductase, glutathione S-transferase, catalase, superoxide dismutase | [34] |
| Fernando de Noronha Archipelago protected area (Brazil) | Amphistegina lessonii | Foraminifera | Oxidative stress, metal detoxification | Antioxidant capacity against peroxyl radicals, lipid peroxidation, protein carbonylation, metallothionein-like proteins | [35] |
| Paranaguá Bay protected areas (Brazil) | Atherinella brasiliensis | Osteichthyes | Neurotoxicity, detoxification of organic chemical pollutants, oxidative stress | Cholinesterase, ethoxyresorufinO-deethylase, glutathione S-transferase, catalase | [36] |
| Cananéia–Iguape–Peruíbe Environmental Protected Area (Brazil) | Cathorops spixii | Osteichthyes | Detoxification of organic pollutants, oxidative stress, genotoxicity, metal detoxification | Glutathione S-transferase, glutathione peroxidase, GSH levels, lipid peroxidation, DNA strand breaks, metallothonein |
[37] |
| Cathorops spixii | Osteichthyes | Genotoxicity | Comet assay, micronucleus test (MN), and nuclear abnormalities test (NA) in peripheral blood |
[38] | |
| Natural Protected Area San Antonio Bay (Argentina) | Neohelice granulata | Malacostraca | Detoxification of organic pollutants, oxidative stress, metal detoxification | Catalase, lipid radical content, lipid peroxidation, α-tocopherol, catalase, glutathione-S-transferases, metallothioneins | [39] |
| Cananéia–Iguape–Peruíbe Protected Area (Brazil) | Callinectes danae | Malacostraca | Genotoxicity, detoxification of organic pollutants, oxidative stress, metal detoxification, neurotoxicity | Glutathione S-transferase, glutathione peroxidase, intracellular glutathione, acetylcholinesterase, lipid peroxidation, metallothionein, DNA strand breaks |
[40] |
| Estuarine Lagoon Complex of Iguape–Cananéia (Brazil) | Gobioides broussonnetii | Osteichthyes | Oxidative stress, genotoxicity, metal detoxification, histopathological alterations |
Superoxide dismutase, catalase, glutathione peroxidase activity, glutathione S-transferase, glutathione, metallothionein, lipoperoxidation, micronuclei, histological alterations | [41] |
| Australia | |||||
| Great Barrier Reef (Australia) | Plectropomus leopardus | Osteichthyes | Detoxification of organic chemical pollutants, neurotoxicity | EROD, cholinesterase | [42] |
| Acropora millepora | Anthozoa | Oxidative stress | Genetic loci involved in environmental stress tolerance and antioxidant capacity | [43] |
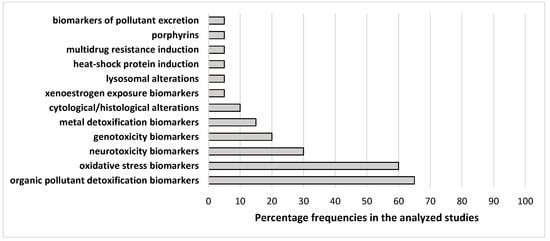
3. Conclusions
In conclusion, the analyses and discussion of the recent literature suggest that pollution biomarkers have proved to be useful tools for monitoring and assessment of pollution threat to marine biodiversity, both in the environmental quality monitoring of protected areas and the assessment of the health status of species at risk.References
- Rios, L.M.; Moore, C.; Jones, P.R. Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar. Pollut. Bull. 2007, 54, 1230–1237.
- Mearns, A.J.; Morrison, A.M.; Arthur, C.; Rutherford, N.; Bissell, M.; Rempel-Hester, M.A. Effect of pollution on marine organisms. Water Environ. Res. 2020, 92, 1510–1532.
- Tornero, V.; Hanke, G. Chemical contaminants entering the marine environment from sea-based sources: A review with a focus on European seas. Mar. Pollut. Bull. 2016, 112, 17–38.
- Salimi, M.; Esrafili, A.; Gholami, M.; Jafari, A.J.; Kalantari, R.R.; Farzdakia, M.; Kermani, M.; Sobhi, H.R. Contaminants of emerging concern: A review of new approach in AOP technologies. Environ. Monit. Assess. 2017, 189, 414.
- Torres-Padrón, M.E.; Montesdeoca-Esponda, S.; Santana-Viera, S.; Guedes-Alonso, R.; Herrera-Melián, J.A.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. An Update of the Occurrence of Organic Contaminants of Emerging Concern in the Canary Islands (Spain). Water 2020, 12, 2548.
- Lionetto, M.G.; Vilella, S.; Trischitta, F.; Cappello, M.S.; Giordano, M.E.; Schettino, T. Effects of CdCl2 on electrophysiological parameters in the intestine of the teleost fish, Anguilla Anguilla. Aquat. Toxicol. 1998, 41, 251–264.
- Lionetto, M.G.; Giordano, M.E.; Vilella, S.; Schettino, T. Inhibition of eel enzymatic activities by cadmium. Aquat. Toxicol. 2000, 48, 561–571.
- Calisi, A.; Lionetto, M.G.; Caricato, R.; Giordano, M.E.; Schettino, T. Morphometric alterations in Mytilus galloprovincialis granulocytes: A new biomarker. Environ. Toxicol. Chem. 2008, 27, 1435–1441.
- Bolognesi, C.; Cirillo, S. Genotoxicity biomarkers in aquatic bioindicators. Curr. Zool. 2014, 60, 273–284.
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117.
- Rodea-Palomares, I.; Makowski, M.; Gonzalo, S.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F. Effect of PFOA/PFOS pre-exposure on the toxicity of the herbicides 2,4-D, Atrazine, Diuron and Paraquat to a model aquatic photosynthetic microorganism. Chemosphere 2015, 139, 65–72.
- Owen, R.; Galloway, T.S.; Hagger, J.A.; Jones, M.B.; Depledge, M.H. Biomarkers and environmental risk assessment: Guiding principles from the human health field. Mar. Pollut. Bull. 2008, 56, 613–619.
- Hook, S.H.; Gallagher, E.P.; Batley, G.E. The role of biomarkers in the assessment of aquatic ecosystem health. Integr. Environ. Assess. Manag. 2014, 10, 327–341.
- Schettino, T.; Caricato, R.; Calisi, A.; Giordano, M.E.; Lionetto, M.G. Biomarker Approach in Marine Monitoring and Assessment: New Insights and Perspectives. Open Environ. Sci. 2012, 6, 20–27.
- Backhaus, T.; Snape, J.; Lazorchak, J. The impact of chemical pollution on biodiversity and ecosystem services: The need for an improved understanding. Integr. Environ. Assess. Manag. 2012, 8, 575–576.
- Klein, C.; Brown, C.; Halpern, B.; Segan, D.B.; McGowan, J.; Beger, M.; Watson, J.E.M. Shortfalls in the global protected area network at representing marine biodiversity. Sci. Rep. 2015, 5, 17539.
- O’Leary, B.C.; Winther-Janson, M.; Bainbridge, J.M.; Aitken, J.; Hawkins, J.P.; Roberts, C.M. Effective Coverage Targets for Ocean Protection. Conserv. Lett. 2016, 9, 398–404.
- Selig, E.R.; Turner, W.R.; Troëng, S.; Wallace, B.P.; Halpern, B.S.; Kaschner, K.; Lascelles, B.G.; Carpenter, K.E.; Mittermeier, R.A. Global Priorities for Marine Biodiversity Conservation. PLoS ONE 2014, 9, e82898.
- Edgar, G.; Stuart-Smith, R.; Willis, T.; Kininmonth, S.; Baker, S.C.; Banks, S.; Barrett, N.S.; Becerro, M.A.; Bernard, A.T.F.; Berkhout, J.; et al. Global conservation outcomes depend on marine protected areas with five key features. Nature 2014, 506, 216–220.
- Abessa, D.M.S.; Albuquerque, H.C.; Morais, L.G.; Araújo, G.S.; Fonseca, T.G.; Cruz, A.C.F.; Campos, B.G.; Camargo, J.B.D.A.; Gusso-Choueri, P.K.; Perina, F.C.; et al. Pollution status of marine protected areas worldwide and the consequent toxic effects are unknown. Environ. Pollut. 2018, 243, 1450–1459.
- Pozo, K.; Lazzerini, D.; Perra, G.; Volpi, V.; Corsolini, S.; Focardi, S. Levels and spatial distribution of polychlorinated biphenyls (PCBs) in superficial sediment from 15 Italian Marine Protected Areas (MPA). Mar. Pollut. Bull. 2009, 58, 773–776.
- Chatwin, A. Priorities for Coastal and Marine Conservation in South America; The Nature Conservancy: Arlington, TX, USA, 2007.
- Bonacci, S.; Iacocca, A.; Fossi, S.; Lancini, L.; Caruso, T.; Corsi, I.; Focardi, S. Biomonitoring Aquatic Environmental Quality in a Marine Protected Area: A Biomarker Approach. AMBIO 2007, 36, 308–315.
- Pinsino, A.; Della Torre, C.; Sammarini, V.; Bonaventura, R.; Amato, E.; Matranga, V. Sea urchin coelomocytes as a novel cellular biosensor of environmental stress: A field study in the Tremiti Island Marine Protected Area, Southern Adriatic Sea, Italy. Cell Biol. Toxicol. 2008, 24, 541–552.
- Moschino, V.; Schintu, M.; Marrucci, A.; Marras, B.; Nesto, N.; Da Ros, L. An ecotoxicological approach to evaluate the effects of tourism impacts in the Marine Protected Area of La Maddalena (Sardinia, Italy). Mar. Pollut. Bull. 2017, 122, 306–315.
- Caliani, I.; Rodríguez, L.P.; Casini, S.; Granata, A.; Zagami, G.; Pansera, M.; Querci, G.; Minutoli, R. Biochemical and genotoxic biomarkers in Atherina boyeri to evaluate the status of aquatic ecosystems. Reg. Stud. Mar. Sci. 2019, 28, 100566.
- Fossi, M.C.; Borsani, J.F.; Di Mento, R.; Marsili, L.; Casini, S.; Neri, G.; Mori, G.; Ancora, S.; Leonzio, C.; Minutoli, R.; et al. Multi-trial biomarker approach in Meganyctiphanes norvegica: A potential early indicator of health status of the Mediterranean “whale sanctuary”. Mar. Environ. Res. 2002, 54, 761–767.
- Fossi, M.C.; Panti, C.; Marsili, L.; Maltese, S.; Spinsanti, G.; Casini, S.; Caliani, I.; Gaspari, S.; Muñoz-Arnanz, J.; Jimenez, B.; et al. The Pelagos Sanctuary for Mediterranean marine mammals: Marine Protected Area (MPA) or marine polluted area? The case study of the striped dolphin (Stenella coeruleoalba). Mar. Pollut. Bull. 2013, 70, 64–72.
- Fossi, M.C.; Marsili, L.; Baini, M.; Giannetti, M.; Coppola, D.; Guerranti, C.; Caliani, I.; Minutoli, R.; Lauriano, G.; Finoia, M.G.; et al. Fin whales and microplastics: The Mediterranean Sea and the Sea of Cortez scenarios. Environ. Pollut. 2016, 209, 68–78.
- Marangi, M.; Airoldi, S.; Beneduce, L.; Zaccone, C. Wild whale faecal samples as a proxy of anthropogenic impact. Sci. Rep. 2021, 11, 5822.
- Downs, C.A.; Fauth, J.E.; Robinson, C.E.; Curry, R.; Lanzendorf, B.; Halas, J.C.; Halas, J.; Woodley, C.M. Cellular diagnostics and coral health: Declining coral health in the Florida Keys. Mar. Pollut. Bull. 2005, 51, 558–569.
- Gold-Bouchot, G.; Rubio-Piña, J.; Montero-Muñoz, J.; Ramirez-Miss, N.; Echeverría-García, A.; Patiño-Suarez, V.; Puch-Hau, C.A.; Zapata-Pérez, O. Pollutants and biomarker responses in two reef fish species (Haemulon aurolineatum and Ocyurus chrysurus) in the Southern Gulf of Mexico. Mar. Pollut. Bull. 2017, 116, 249–257.
- Aguilera, C.; Leija, A.; Torres, M.; Mendoza, R. Assessment of Environmental Quality in the Tamaulipas Laguna Madre, Gulf of Mexico, by Integrated Biomarker Response Using the Cross-Barred Venus Clam Chione elevata. Water Air Soil Pollut. 2019, 230, 27.
- Ramos, R.; Bastidas, C.; Debrot, D.; García, E. Phase I and II biotransformation and antioxidant enzymes in the coral Siderastrea siderea act as biomarkers for reproductive condition and habitat quality. Mar. Biol. Res. 2011, 7, 398–406.
- De Freitas Prazeres, M.; Eslava Martins, S.; Bianchini, A. Assessment of water quality in coastal waters of Fernando de Noronha, Brazil: Biomarker analyses in Amphistegina lesson. J. Foramin. Res. 2012, 42, 56–65.
- De Oliveira Ribeiro, C.A.; Katsumiti, A.; França, P.; Maschio, J.; Zandoná, E.; Cestari, M.M.; Vicari, T.; Roche, H.; de Assis, H.C.S.; Neto, F.F. Biomarkers responses in fish (Atherinella brasiliensis) of Paraganuá Bay, Southern Brazil, for assessment of pollution effects. Braz. J. Oceanogr. 2013, 61, 1–11.
- Gusso-Choueri, P.K.; Choueri, R.B.; de Araújo, G.S.; Cruz, A.C.F.; Stremel, T.; Campos, S.; de Sousa Abessa, D.M.; Oliveira Ribeiro, C.A. Assessing pollution in marine protected areas: The role of a multi-biomarker and multi-organ approach. Environ. Sci. Pollut. Res. 2015, 22, 18047–18065.
- Gusso-Choueri, P.K.; Choueri, R.B.; Santos, G.S.; de Araújo, G.S.; Cruz, A.C.F.; Stremel, T.; de Campos, S.X.; Cestari, M.M.; Oliveira Ribeiro, C.A.; de Sousa Abessa, D.M. Assessing genotoxic effects in fish from a marine protected area influenced by former mining activities and other stressors. Mar. Pollut. Bull. 2016, 104, 229–239.
- Giarratano, E.; Gil, M.N.; Marinho, C.H.; Malanga, G. Metals from mine waste as potential cause of oxidative stress in burrowing crab Neohelice granulata from San Antonio bay. Ecotoxicol. Environ. Saf. 2016, 132, 68–76.
- Araujo, G.S.; Gusso-Choueri, P.K.; Favaro, D.I.T.; Rocha, R.C.C.; Saint’Pierre, T.D.; Hauser-Davis, R.A.; Braz, B.; Santelli, R.E.; Freire, A.S.; Machado, W.T.V.; et al. Metal-Associated Biomarker Responses in Crabs from a Marine Protected Area in Southeastern Brazil. Arch. Environ. Contam. Toxicol. 2020, 78, 463–477.
- Salgado, L.D.; Meister Luz Marques, A.E.; Kramer, R.D.; de Oliveira, F.G.; Moretto, S.L.; de Lima, B.A.; Prodocimo, M.M.; Cestari, M.M.; de Azevedo, J.C.R.; de Assis, H.C.S. Sediment contamination and toxic effects on Violet Goby fish (Gobioides broussonnetii—Gobiidae) from a marine protected area in South Atlantic. Environ. Res. 2021, 195, 110308.
- Klumpp, D.; Humphrey, C.; Codi King, S. Biomarker Responses in Coral Trout (Plectropomus leopardus) as an Indicator of Exposure to Contaminants in a Coral Reef Environment. Australas J. Ecotoxicol. 2007, 13, 9–17.
- Jin, Y.K.; Lundgren, P.; Lutz, A.; Raina, J.-B.; Howells, E.J.; Paley, A.S.; van Oppen, M.J.H. Genetic markers for antioxidant capacity in a reef-building coral. Sci. Adv. 2016, 2, e1500842.
- Lionetto, M.G.; Caricato, R.; Giordano, M.E.; Pascariello, M.F.; Marinosci, L.; Schettino, T. Integrated use of biomarkers (acetylcholinesterase and antioxidant enzymatic activities) in Mytilus galloprovincialis and Mullus barbatus in an Italian coastal marine area. Mar. Pollut. Bull. 2003, 46, 324–330.
- Bacchetta, C.; Rossi, A.; Aleb, A.; Campana, M.; Parma, M.J. Combined toxicological effects of pesticides: A fish multi-biomarker approach. Ecol. Indic. 2014, 36, 532–538.
- Burkina, V.; Zlabek, V.; Zamaratskaia, G. Effects of pharmaceuticals present in aquatic environment on Phase I metabolism in fish. Environ. Toxicol. Pharmacol. 2015, 40, 430–444.
- Lionetto, M.G.; Giordano, M.E.; Caricato, R.; Pascariello, M.F.; Marinosci, L.; Schettino, T. Biomonitoring of heavy metal contamination along Salento coast (Italy) by metallothionein evaluation in Mytilus galloprovincialis and Mullus barbatus. Aquat. Conserv. 2001, 11, 305–310.
- Lionetto, M.G.; Caricato, R.; Giordano, M.E.; Schettino, T. Biomarker application for the study of chemical contamination risk on marine organisms in the Taranto marine coastal area. Chem. Ecol. 2004, 20, S333–S343.
- Caricato, R.; Giordano, M.E.; Schettino, T.; Maisano, M.; Mauceri, A.; Giannetto, A.; Cappello, T.; Parrino, V.; Ancora, S.; Caliani, I.; et al. Carbonic anhydrase integrated into a multimarker approach for the detection of the stress status induced by pollution exposure in Mytilus galloprovincialis: A field case study. Sci. Total Environ. 2019, 690, 140–150.
- Maisano, M.; Cappello, T.; Natalotto, A.; Vitale, V.; Parrino, V.; Giannetto, A.; Oliva, S.; Mancini, G.; Cappello, S.; Mauceri, A.; et al. Effects of petrochemical contamination on caged marine mussels using a multi-biomarker approach: Histological changes, neurotoxicity and hypoxic stress. Mar. Environ. Res. 2017, 128, 114–123.
- Micheletti, C.; Critto, A.; Marcomini, A. Assessment of ecological risk from bioaccumulation of PCDD/Fs and dioxin-like PCBs in a coastal lagoon. Environ. Int. 2007, 33, 45–55.
- Ricciardi, F.; Matozzo, V.; Binelli, A.; Marin, M.G. Biomarker responses and contamination levels in crabs (Carcinus aestuarii) from the Lagoon of Venice: An integrated approach in biomonitoring estuarine environments. Water Res. 2010, 44, 1725–1736.




