
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrew FG Quest | + 4187 word(s) | 4187 | 2021-07-12 08:22:52 | | | |
| 2 | Amina Yu | Meta information modification | 4187 | 2021-07-14 04:04:33 | | |
Video Upload Options
Extracellular vesicles (EVs) are mainly featured as a heterogeneous population of membrane-enclosed, non-replicating, and sub-micron sized structures, which are actively secreted by wide variety of eukaryotic and prokaryotic organisms. In addition, EVs are mediators of communication between cells in physiological and pathological settings, and they transport a diverse array of biomolecules, including lipids, nucleic acids, carbohydrates and proteins. This article discusses the role of EVs in cancer drug resistance and the literature proposing the use of EVs for therapeutic and prognostic purposes in cancer.
1. Introduction
Extracellular vesicles (EVs) were initially identified in the 1950s as a type of particle derived from platelets present in plasma [1]. It took several years before the role of EVs was revealed to be very much the opposite of meaningless cell debris, as their fundamental role in regulating homeostasis at the local and systemic level became apparent [2][3]. Moreover, EVs can be found in biological fluids, such as serum, plasma, urine, saliva, and breast milk, amongst others [4][5][6][7]. In general terms, EVs can be separated into three subtypes according to their biogenesis and biophysical properties [8], namely exosomes, microvesicles, and other small membrane-limited fragments, such as apoptotic bodies, which are generally thought to be less relevant to cell-to-cell communication [9][10].
Specifically in cancer, EVs secreted by tumor cells promote the development of tumor-related features in recipient cells and the acquisition of the cancer hallmarks described in the literature [11]. Furthermore, several studies have documented that cancer cells secrete increased levels of EVs when compared to normal cells [12][13]. Considering the aforementioned data and the fact that EVs play an important role in cancer progression, EVs can also be envisioned as appealing targets for developing non-invasive liquid biopsy strategies in patients with cancer. Thus, this review will discuss literature relating to the role of EVs in promoting acquisition of the hallmarks of cancer and also the use of these vesicles in cancer therapy.
To facilitate communication between research groups and comparison of results, a consensus in nomenclature needed to be established based on the type of isolation procedure used to purify EVs and the techniques used to distinguish between EV subtypes according to their biogenesis or release. This effort gave rise to the development of guidelines, which now permit distinguishing between EVs according to their size, density, molecular cargo, or information regarding the cell of origin. In addition, these guidelines also determined that the terms “exosomes” or “microvesicles” should only be used, for example, when imaging techniques were included to confirm a specific biogenesis pathway [14][15]. Alternatively, when such data are unclear or lacking, the term “EVs” will be used instead.
2. Extracellular Vesicles
Extracellular vesicles (EVs) are mainly featured as a heterogeneous population of membrane-enclosed, non-replicating, and sub-micron sized structures, which are actively secreted by a wide variety of eukaryotic and prokaryotic organisms [16][17]. In addition, EVs are mediators of communication between cells in physiological and pathological settings, and they transport a diverse array of biomolecules, including lipids, nucleic acids, carbohydrates and proteins [17][18]. Finally, EVs can be sorted into three different subtypes according to their biogenesis and biophysical properties (Figure 1) [8].
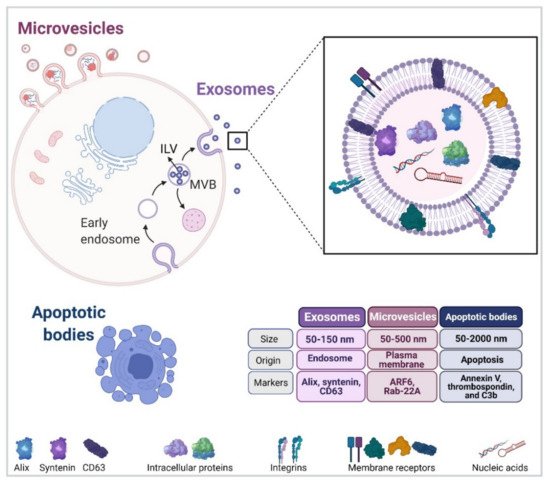
Interestingly, exosome biogenesis and cargo sorting are closely related processes. In addition, ESCRT-I and II are held responsible for the binding of specific cargoes to the aforementioned microdomains. The second mechanism, also considered as being independent of ESCRT, requires the presence of Alix and transmembrane proteins, such as syntenin and syndecan, which are responsible for recruiting specific molecular cargoes (adhesion molecules, growth factors, integrins, etc.) Recent evidence points towards the existence of a third mechanism of ILV biogenesis, which does not depend on components of the ESCRT complexes but rather involves the participation of membrane lipid microdomains or lipid rafts.
MVBs can either fuse with the plasma membrane for release of their content or with lysosomes for their subsequent destruction [17][19]. Several reports are available indicating that the final destination depends on factors, such as the interaction with microtubules or the actin cytoskeleton, as well as the engagement of specific members of the Rab GTPase family of proteins [19]. Examples in the latter case include Rab27b, Rab11, and Rab35, which promote MVB motility and fusion with the plasma membrane in HeLa, K562 (bone marrow chronic myelogenous leukaemia cells) and Oli-neu (oligodendroglial) cells, respectively [20][21][22].
The second subtype of vesicles ranging in size 50–500 nm (up to 1000 nm), also known as microvesicles (MVs), ectosomes, oncosomes, or microparticles, are described to be released from the cell surface by blebbing from the plasma membrane and subsequent membrane fission [17]. Interestingly, MVs are formed by phospholipid redistribution, positioning phosphatidylserines to the outer leaflet followed by actin–myosin contraction [23][24]. In addition, MV biogenesis requires the participation of small GTPases, such as ADP-ribosylation factor 6 (ARF6) [25][26] and Ras-related proteins, e.g., Rab-22A [26][27]. Importantly, ESCRT complexes also participate in MV formation [24], increasing the level of complexity in EV subtype studies when evaluating vesicle biogenesis.
Apoptotic bodies, referred to as the third subtype of EVs in the literature, vary widely in size ranging from 50 to 2000 nm in diameter and are ultimately produced by an essential physiological process, which is known as programmed cell death or apoptosis. One of the main features of apoptotic bodies is that mechanisms for specific sorting of organelles, RNA and DNA fragments can be detected, which are absent in other EV subtypes [24][28].
The discovery of exomeres was made possible by the development of new technologies to isolate and visualize EVs. In addition, they were shown to be highly enriched in calreticulin, argonaute proteins, amyloid precursor proteins, proteins associated with coagulation (for instance, factors VIII and X), and enzymes involved in metabolism (e.g., glycolysis), especially glycolysis, and mammalian target of rapamycin complex 1 (mTORC1) metabolic pathways [29][30]. Moreover, several recent reports have shown that exomeres can carry nucleic acids, such as DNA, RNA, and miRNAs along with lipids, such as ceramide, esterified cholesterol triglycerides, and phosphatidylcholine [31]. Recently, a novel role for exomeres has been proposed in cancer, since they were shown to promote tumor organoid growth in recipient cells [29].
Interestingly, a novel role for exomeres in the COVID-19 pandemic was suggested, as full-length angiotensin-converting enzyme 2 (ACE2) was reported to be contained in EVs from colorectal cancer cells. Specifically, these cells were able to shed ectodomain fragments of ACE2 that were enriched in exomeres [32]. Given that ACE2 can bind to SARS-CoV-2 [33], the binding of SARS-CoV-2 S protein to ACE2 fragments in EVs and exomeres may play an important role in controlling the infection [32]. A relevant question at this point is whether the ability to shed ACE2 fragments is limited to cancer cells and if so, thinking of treatments for SARS-CoV-2 infection, why this might be the case.
EVs have emerged as essential players in cell-to-cell communication, because they represent a complex type of “biological package” capable of transporting a wide variety of molecules from one cell to another.
EVs can elicit cellular responses without the need to be internalized into a cell by two mechanisms referred to as soluble and juxtacrine signaling. Soluble signaling involves the proteolysis of an EV surface ligand and its subsequent binding to a cell membrane receptor, whereas juxtacrine signaling requires the juxtapositioning of ligands and receptors on opposing surfaces of the EVs and the target cell [34].
On the other hand, EV internalization by recipient cells involves at least four mechanisms: membrane fusion, phagocytosis, micropinocytosis, and endocytosis. For membrane fusion, the EV membrane directly merges with the cell plasma membrane and transfers cargo molecules to recipient cells. Macropinocytosis is characterized by plasma membrane ruffling induced by growth factors or other signals. CME is produced by the interaction between ligands on the EV surface and specific receptors present on the plasma membrane that utilize clathrin and adaptor protein 2
In summary, soluble signaling, juxtacrine signaling, and membrane fusion are more likely to culminate in a cellular response, since EV components do not enter the endosomal-lysosomal degradation pathway directly.
EVs can modify the behavior of recipient cells depending on the biological message or cargo that is being transferred from the donor cell or tissue [35]. Specifically in cancer, EVs have been shown to play a critical role in cell-to-cell communication in the tumor microenvironment that permits the acquisition and maintenance of cell traits, which are referred to as the hallmarks of cancer.
Using scanning electron microscopy, normal human ovarian cells were found to release EVs from a few select areas of the plasma membrane, while ovarian serous adenocarcinoma cells release EVs from the entire cell surface [36]. Elevated EV release in cancer cells has been proposed to occur via a Ca2+-Munc13-4-Rab11-dependent pathway. Rab-binding protein, is elevated in cancer cells, which combined with the increased Ca2+ levels enhances exosome release from cancer cells [37]. This was accompanied by an increase in Rab GTPase expression, which could represent another mechanism that permits increased exosome release from more malignant cells [38].
3. EVs in Cancer Drug Resistance
Chemotherapy is widely used to treat cancer, but the effectivity of such therapies is reduced in several types of cancer due to the development of drug resistance, which can be attributed to the activation of intrinsic or acquired mechanisms. Intrinsic resistance refers to the presence of resistance factors in tumor cells prior to chemotherapy that render the treatment ineffective. Moreover, tumors are extremely heterogeneous, so drug resistance can arise through the therapy-induced selection of a minor resistant subpopulation of cells that was present in the original tumor [39]. Alternatively, drug resistance can also be acquired by drug-sensitive cells via communication with drug-resistant cells (cancer or stromal) through EV-mediated transfer of resistance factors.
Regardless of the route of anticancer drug administration, these drugs generally need to be taken up by cancer cells because they target an intracellular process. The uptake of such drugs by cancer cells may involve active transport mechanisms or rely on simple diffusion because of high membrane permeability. However, cancer cells are known to express multidrug resistance (MDR)–ATP binding-cassette (ABC) proteins that export drugs to the extracellular space. This phenomenon results in decreased intracellular anticancer drug accumulation, which decreases or even abolishes drug effects.
Shedden et al., (2003) were the first to report that anticancer drug resistance and the release of EVs could be mechanistically linked. In cancer cell lines, the expression of vesicle shedding-related genes is associated with chemosensitivity profiles. Furthermore, in the breast cancer cell line MCF7, the fluorescent chemotherapeutic agent doxorubicin was incorporated into EVs and released to the culture media [40]. Similarly, in vitro B-cell lymphoma cell lines efficiently extrude doxorubicin in exosomes [41].
Early studies suggested that cisplatin, once inside tumor cells, may be sequestered into acidic vesicles belonging to a secretory pathway. The treatment of human ovarian carcinoma cells with cisplatin showed that the exosomes released from cisplatin-resistant cells contained more than 2-fold higher platinum levels than those released from cisplatin-sensitive cells [42]. Moreover, exosomes released by drug-resistant melanoma cells that were previously treated with a fixed dose of cisplatin in culture contained varying amounts of the drug depending on the pH of the medium, and the level of cisplatin in the exosomes was higher in acidic culture medium [43]. Additionally, it was reported that mouse leukemia cell-derived exosomes can include paclitaxel and, interestingly, that the paclitaxel-containing exosomes reduced the proliferation of a human pancreatic cell line.
The first evidence for the transfer of ABC transporters between cancer cells was obtained studying human acute lymphoblastic leukemia cells. The results revealed that P-gp protein transfer coincided with reduced drug accumulation in recipient cells, confirming that the transfer of functional P-gp was mediated by EVs [44]. Later studies showed that exosomes from docetaxel-resistant human prostate cancer cell lines conferred resistance to previously sensitive target cells. The treatment of doxorubicin-sensitive (DXS) osteosarcoma cells with exosomes derived from DXR cells reduced the sensitivity of the recipient cells to doxorubicin.
P-gp is the best studied drug efflux pump; however, other members of the ABC transporter family have been identified in cancer cell-derived EVs/exosomes, too. GAIP interacting protein C terminus (GIPC) is a protein regulator of autophagy and the exocytotic pathways in cancer. The knockdown of GIPC in pancreatic cancer cells induces the overexpression and incorporation into exosomes of the ATP-binding cassette sub-family G member 2 (ABCG2). This finding opens up the possibility that horizontal transfer of ABCG2 via exosomes mediates drug resistance in pancreatic cancer [45].
In addition, exposure to the chemotherapeutic drug vincristine increases the secretion of ATP-binding cassette sub-family B member 1 (ABCB1)-enriched EVs by inducing dysregulation of the Ras-related proteins Rab8B and Rab5. The transfer of ABCB1 via exosomes helps sensitive cancer cells develop a drug-resistant phenotype [46].
EV cargoes also include pro-survival factors, which decrease apoptosis sensitivity and increase cell viability, thus leading to resistance to anticancer drugs. Components of the PI3K/AKT pathway, an oncogenic signaling axis involved in cancer cell proliferation and survival, have been reported in EVs. Exosomes derived from HCC cells induced sorafenib resistance in vitro and in vivo by activating the HGF/c-Met/AKT signaling pathway and inhibiting sorafenib-induced apoptosis [47]. Triple negative breast cancer cell lines, resistant to docetaxel and doxorubicin, release EVs that induced resistance to the same drugs in recipient non-tumorigenic breast cells.
BRAF is a component of the MAPK pathway involved in cell differentiation and survival. BRAF kinase inhibitors, such as vemurafenib and dabrafenib, are used in advanced melanoma treatment. Platelet-derived growth factor receptor β (PDGFRβ) is a receptor tyrosine kinase that induces activation of the PI3K/AKT pathway. Vella et al. showed that PDGFRβ can be transferred to recipient melanoma cells in EVs, resulting in a dose-dependent activation of PI3K/AKT signaling and escape from MAPK pathway inhibition by BRAF [48].
In addition, resistance to apoptosis is an escape mechanism by which cancer cells acquire drug resistance and thus contribute to cancer progression. Cancer-associated fibroblast (CAF)-EVs induced the drug resistance of gastric cancer cells by decreasing cisplatin-induced apoptosis. The proteomics analysis of CAF-derived EVs identified that annexin A6 plays a pivotal role in the drug resistance of gastric cancer cells via the activation of β1 integrin and the downstream intracellular signaling pathways, involving focal adhesion kinase (FAK) and the yes-associated protein (YAP). Consistently, the inhibition of FAK or YAP efficiently attenuated gastric cancer drug resistance in vitro and in vivo [49].
Survivin is a pro-survival protein member of the inhibitor of apoptosis (IAP) family that is present in EVs derived from different tumor types [50]. Paclitaxel treatment of triple negative breast cancer cells induces the secretion of EVs enriched in survivin, which increased the survival of serum-starved, as well as paclitaxel-treated fibroblasts and breast cancer cells [51].
MicroRNAs (miRs) are well-established components of EVs and their horizontal transfer favors the development of drug resistance. Sorafenib is a kinase inhibitor drug approved for the treatment of primary kidney cancer, advanced primary liver cancer, and advanced thyroid carcinoma. Elevated miR-31-5p in EVs derived from SR cells downregulated the expression of MLH1, which is a gene commonly associated with hereditary nonpolyposis colorectal cancer in SS cells and thus promoted sorafenib resistance in vitro. Experiments in mice also confirmed that miR-31-5p could regulate drug sensitivity in vivo.
Exosomes isolated from gemcitabine (GEM)-resistant human pancreatic cancer stem cells (R-CSCs) inhibited GEM-induced cell cycle arrest and apoptosis as well as promoted tube formation and cell migration in drug-sensitive human pancreatic cancer stem cells (S-CSCs). Elevated miR-210 levels were detected in R-CSC exosomes compared to S-CSCs exosomes, and MiR-210 levels in exosomes were dependent on the GEM doses used to treat cells. Moreover, treatment with R-CSC-derived exosomes increased miR-210 levels in recipient cells [52].
The aforementioned studies are only a few recently published examples of the increasing evidence linking cancer drug resistance to the presence of specific miRNA cargos in EVs. A more comprehensive summary of related information can be found in a recent article by Maacha et al. [50].
Specific EV surface antigens can be targeted by immunotherapy where they act as a “hunter” in monoclonal antibody-based therapies by diminishing antibody bioavailability. For instance, rituximab (anti-CD20 antibody) binds to CD20 on the surface of EVs and protects targeted lymphoma cells from rituximab-induced toxicity [53]. EVs secreted either by HER2-overexpressing breast carcinoma cells or present in the serum of breast cancer patients bind to trastuzumab. In vitro studies showed that HER2-containing EVs, but not EVs lacking HER2, prevent the reduction in cell proliferation induced by trastuzumab treatment, although no change in HER2 activation status was detected in EV-treated cells by Western blotting [54].
The detection of immune checkpoint ligand (PD-L1) on EVs early after therapy is indicative of whether the patients will respond or not to anti-PD-1 therapy. PD-L1 binds to PD-1 receptors on the surfaces of effector T cells, preventing their ability to target tumor cells for destruction. In addition, EVs from glioblastoma stem cells were found to contain PD-L1 and inhibit T cell proliferation and antigen-specific T cell responses [55]. These results suggest that by capturing the anti-PD-1 antibodies on their surface, EVs prevent this antibody from accessing the tumor, thereby permitting PD-L1 to bind to PD-1 on T cells and attenuate anti-tumor immune responses.
These findings further extend our understanding of the implications of EVs in the development of the disease. The composition of cancer-derived EVs can regulate patient responses to chemotherapy using one or more of the aforementioned mechanisms. With this in mind, one may predict that EVs will serve to predict or evaluate therapy efficacy, and as such will likely become powerful tools to improve cancer treatment. However, the clinical application of new techniques for rapid EV detection and characterization remains a pending issue.
4. EVs in Organ Tropism, Drug Delivery, Imaging and Theranosis
The intrinsic organ tropism of EVs and their potential physiological benefits, combined with drug loading and targeting strategies, provide multiple therapeutic benefits for drug delivery, such as greater cellular uptake and focalization, prolonged circulation time, immunomodulation, biocompatibility, and stability. Furthermore, EVs can be used as biological nanocarriers with the inclusion of active principles, nanoparticles, or imaging agents. As such, they can significantly improve the therapeutic efficacy and selectivity, as well as facilitate the early detection of multiple diseases, including cancer [56]. However, to consider the use of EVs in potential clinical applications, the effects discussed previously relating to the role of EVs in cancer and other pathologies need to be kept in mind.
For instance, EVs from melanoma cells predominantly accumulate in the lungs, while EVs from dendritic cells tend to accumulate in the spleen [57]. Interestingly, EVs derived from tumor cells reportedly also show selective tropism toward the tumor tissue from which they originated. EVs from brain endothelial cells can cross the blood–brain barrier and accumulate in the brain and brain tumor tissue, while EVs from melanoma cells preferentially target metastatic melanoma tumors [58][59]. [60] observed the in vitro and in vivo targeting and accumulation of lung cancer cell-derived EVs in colon carcinoma cells and vice versa.
EVs have become novel biological delivery vehicles for several cargoes, due to the variety of natural properties that they possess. EV tropism is determined by the presence on their surface of different adhesion and immunoregulatory molecules, as well as specific cell receptors, which contribute to enhancing their accumulation in specific tissues [61][62]. EVs have been widely studied as drug delivery nanocarriers in cancer research, so recent and representative studies for each application of these vesicles in the delivery of proteins, genetic material, and chemotherapeutics drugs will be described. In this field, Kim et al. developed a formulation of paclitaxel-loaded exosomes by the sonication and conjugation of an aminoethilanisamide–polyethylene glycol (AA-PEG) vector moiety to target the sigma receptor, which is overexpressed by lung cancer cells.
With respect to protein delivery, Aspe et al. [63] engineered EVs from melanoma cells to overexpress survivin-T34A, which is a dominant-negative mutant variant of the inhibitor of apoptosis protein survivin that blocks the protein’s function. and radiotherapy in pancreatic cancer. The authors observed that EVs containing either survivin-T34A alone or in combination with gemcitabine increased apoptosis in multiple pancreatic cancer cell lines, as well as enhanced the sensitivity of these cells to gemcitabine.
Beyond such applications, the use of EVs in site-specific drug delivery can be improved by protein engineering and modifying the vesicle surface by attaching additional ligands to improve EV targeting properties and their interaction with tumor cells [64]. For instance, glycosylphosphatidylinositol (GPI) anchored EV proteins such as decay-accelerating factor (known as CD55) [65] to attach anti-epidermal growth factor receptor (EGFR) nanobodies to EVs and thereby improve targeting to EGFR overexpressing epidermoid carcinoma A431 cells. They showed that the GPI-linked nanobodies were successfully displayed on EV surfaces and greatly improved EV binding to tumor cells in a manner dependent on EGFR density.
On the other hand, EVs readily transfer nucleic acids, such as DNA or RNA, to cells where they can cause specific genetic changes. Regarding genetic drug delivery, Kamerkar et al. [66] engineered EVs known as iExosomes derived from fibroblast-like mesenchymal cells loaded by electroporation with siRNA or shRNA specific for the oncogenic GTPase KrasG12D, which is a common mutation in pancreatic cancer. Subsequently, the treatment with iExosomes was shown to inhibit tumor growth and significantly increase the overall survival in multiple mouse models of pancreatic cancer.
Fluorescence is generally used for exosome tracking and imaging because of its great versatility and simple application by incubation of EVs with a variety of lipophilic fluorescent markers. In this field, generally small lipophilic fluorescent dyes, such as DiR, DiD and PKH67, have been used to label the membranes of EVs. Additionally, EV membranes have been labeled with fluorescent proteins, such as green fluorescent protein (GFP) or tandem dimer tomato (td Tomato) [67]. This type of labeling is considered more stable and suitable for evaluation in clinical applications.
Another alternative is the use of semiconductor quantum dots as optical reporters. They are more stable and have tunable optical properties that can be used for a wide range of applications, including in vivo imaging and diagnosis. For instance, Zong et al. [68] obtained high-resolution images of breast tumor cells or their metastatic activity by loading either silicon or gold-carbon quantum dots, respectively, onto the outer membrane of the exosomes secreted by SKBR3 cells.
Superparamagnetic iron oxide nanoparticles (SPIONs) represent another interesting system for imaging. They have been effectively incorporated into EVs and then tracked in vivo by magnetic particle imaging and MRI, as has been shown for breast cancer [69] and melanomas [70].
Another type of nanomaterial that can be used for EV imaging is gold nanoparticles (AuNPs), which are highly versatile due to their tunability, biocompatibility, and unique optical properties [71]. On the other hand, gold exhibits a high absorption coefficient of X-rays, which make AuNPs useful as contrast agents for computerized tomography. AuNPs can be efficiently incorporated into EVs and then used for imaging, as well as tumor ablation in cancer therapy. This combination permitted analyzing the vesicle biodistribution and detecting the presence of small metastatic foci in the animal lungs by neutron activation analysis, NIR fluorescence, CT imaging and gold-enhanced microscopy imaging.
The use of EVs in imaging applications has been made possible by exploiting some of their natural properties. In particular, EVs have a mean size of 50–200 nm and can evade clearance by the mononuclear phagocytes, as well as favor passive extravasation in inflamed tissues [72]. The presence of immunomodulatory molecules, such as CD47 and PD-L1 ligand, on the EV surface aids significantly in avoiding phagocytosis and suppressing T cell activation, respectively [73][66]. For these reasons, EVs are nowadays considered very appealing nanoscale tools for use as diagnostic sensors, as well as therapeutic vehicles in oncology.
With the advances in nanotechnology and thanks to their unique properties, nanoparticles have become a promising tool in many areas in recent years, including theranosis. Existing evidence points towards the great potential of EVs both as diagnostic biomarkers and therapeutic tools. Such tumor-derived EVs have characteristic proteomic and genomic signatures, indicating that they represent suitable vectors for cancer diagnosis and prognosis [13][74]. In addition, because EVs can transfer various therapeutic compounds, as well as imaging agents, some researchers have proposed to exploit these vesicles as a tool for simultaneous therapy and active diagnosis.
Then, the isolated exosomes (RGD-Exos-SH) were functionalized with AuNRs by the formation of Au-S bonds and coupling folic acid (FA) to improve the uptake efficiency by tumor cells. Furthermore, doxorubicin (DOX) was loaded into exosomes by electroporation. Such designer exosomes showed effective accumulation at target tumor sites via dual ligand-mediated endocytosis, which was monitored in nude mice bearing tumor cell xenografts, using non-invasive near-infrared optical imaging. Moreover, the localized hyperthermia induced by the conjugated AuNRs during near-infrared irradiation increases the permeability of exosome membranes to enhance drug release, thereby preventing tumor relapse in a programable manner.
References
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946, 166, 189–197.
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066.
- Aheget, H.; Mazini, L.; Martin, F.; Belqat, B.; Marchal, J.A.; Benabdellah, K. Exosomes: Their Role in Pathogenesis, Diagnosis and Treatment of Diseases. Cancers 2020, 13, 84.
- Caby, M.-P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005, 17, 879–887.
- Gonzales, P.A.; Zhou, H.; Pisitkun, T.; Wang, N.S.; Star, R.A.; Knepper, M.A.; Yuen, P.S.T. Isolation and Purification of Exosomes in Urine. Methods Mol. Biol. 2010, 641, 89–99.
- Gomez, C.D.L.T.; Goreham, R.V.; Serra, J.J.B.; Nann, T.; Kussmann, M. “Exosomics”—A Review of Biophysics, Biology and Biochemistry of Exosomes with a Focus on Human Breast Milk. Front. Genet. 2018, 9, 92.
- Ding, X.-Q.; Wang, Z.-Y.; Xia, D.; Wang, R.-X.; Pan, X.-R.; Tong, J.-H. Proteomic Profiling of Serum Exosomes From Patients With Metastatic Gastric Cancer. Front. Oncol. 2020, 10, 1113.
- Mir, B.; Goettsch, C. Extracellular Vesicles as Delivery Vehicles of Specific Cellular Cargo. Cells 2020, 9, 1601.
- Roy, S.; Lin, H.-Y.; Chou, C.-Y.; Huang, C.-H.; Small, J.; Sadik, N.; Ayinon, C.M.; Lansbury, E.; Cruz, L.; Yekula, A.; et al. Navigating the Landscape of Tumor Extracellular Vesicle Heterogeneity. Int. J. Mol. Sci. 2019, 20, 1349.
- Xavier, C.P.R.; Caires, H.R.; Barbosa, M.A.G.; Bergantim, R.; Guimarães, J.E.; Vasconcelos, M.H. The Role of Extracellular Vesicles in the Hallmarks of Cancer and Drug Resistance. Cells 2020, 9, 1141.
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674.
- Meng, X.; Pan, J.; Sun, S.; Gong, Z. Circulating exosomes and their cargos in blood as novel biomarkers for cancer. Transl. Cancer Res. 2018, 7, S226–S242.
- Huang, T.; Deng, C.-X. Current Progresses of Exosomes as Cancer Diagnostic and Prognostic Biomarkers. Int. J. Biol. Sci. 2019, 15, 1–11.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal Experimental Requirements for Definition of Extracellular Vesicles and their Functions: A Position Statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913.
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950.
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228.
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312.
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell. Vesicles 2020, 9, 1703244.
- Savina, A.; Vidal, M.; Colombo, M.I. The exosome pathway in K562 cells is regulated by Rab11. J. Cell Sci. 2002, 115, 2505–2515.
- Hsu, C.; Morohashi, Y.; Yoshimura, S.-I.; Hoyos, N.M.; Jung, S.; Lauterbach, M.A.; Bakhti, M.; Grønborg, M.; Möbius, W.; Rhee, J.; et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A–C. J. Cell Biol. 2010, 189, 223–232.
- Ostrowski, M.; Carmo, N.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2009, 12, 19–30.
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro Oncol. 2013, 113, 1–11.
- Petrovčíková, E.; Vičíková, K.; Leksa, V. Extracellular vesicles–biogenesis, composition, function, uptake and therapeutic applications. Biologia 2018, 73, 437–448.
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-Regulated Shedding of Tumor Cell-Derived Plasma Membrane Microvesicles. Curr. Biol. 2009, 19, 1875–1885.
- Tan, C.F.; Teo, H.S.; Park, J.E.; Dutta, B.; Tse, S.W.; Leow, M.K.-S.; Wahli, W.; Sze, S.K. Exploring Extracellular Vesicles Biogenesis in Hypothalamic Cells through a Heavy Isotope Pulse/Trace Proteomic Approach. Cells 2020, 9, 1320.
- Wang, T.; Gilkes, D.M.; Takano, N.; Xiang, L.; Luo, W.; Bishop, C.J.; Chaturvedi, P.; Green, J.J.; Semenza, G.L. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc. Natl. Acad. Sci. USA 2014, 111, E3234–E3242.
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738.
- Zhang, Q.; Higginbotham, J.N.; Jeppesen, D.; Yang, Y.-P.; Li, W.; McKinley, E.T.; Graves-Deal, R.; Ping, J.; Britain, C.M.; Dorsett, K.A.; et al. Transfer of Functional Cargo in Exomeres. Cell Rep. 2019, 27, 940–954.e6.
- Zijlstra, A.; Di Vizio, D. Size matters in nanoscale communication. Nat. Cell Biol. 2018, 20, 228–230.
- Anand, S.; Samuel, M.; Mathivanan, S. Exomeres: A New Member of Extracellular Vesicles Family. In Prokaryotic Cytoskeletons; Springer Science and Business Media LLC: Berlin, Germany, 2021; Volume 97, pp. 89–97.
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Franklin, J.L.; Crowe, J.E.; Coffey, R.J. Angiotensin-converting Enzyme 2–containing Small Extracellular Vesicles and Exomeres Bind the Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein. Gastroenterology 2021, 160, 958–961.e3.
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado Del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913.e7.
- McKelvey, K.J.; Powell, K.L.; Ashton, A.W.; Morris, J.M.; McCracken, S.A. Exosomes: Mechanisms of Uptake. J. Circ. Biomark. 2015, 4, 7.
- Simons, M.; Raposo, G. Exosomes–vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581.
- Giusti, I.; D’Ascenzo, S.; Dolo, V. Microvesicles as Potential Ovarian Cancer Biomarkers. BioMed Res. Int. 2013, 2013, 1–12.
- Messenger, S.W.; Woo, S.S.; Sun, Z.; Martin, T.F.J. A Ca2+-stimulated exosome release pathway in cancer cells is regulated by Munc13-4. J. Cell Biol. 2018, 217, 2877–2890.
- Augimeri, G.; La Camera, G.; Gelsomino, L.; Giordano, C.; Panza, S.; Sisci, D.; Morelli, C.; Győrffy, B.; Bonofiglio, D.; Andò, S.; et al. Evidence for Enhanced Exosome Production in Aromatase Inhibitor-Resistant Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 5841.
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726.
- Shedden, K.; Xie, X.T.; Chandaroy, P.; Chang, Y.T.; Rosania, G.R. Expulsion of small molecules in vesicles shed by cancer cells: Association with gene expression and chemosensitivity profiles. Cancer Res. 2003, 63, 4331–4337.
- Koch, R.; Aung, T.; Vogel, D.; Chapuy, B.; Wenzel, D.; Becker, S.; Sinzig, U.; Venkataramani, V.; Von Mach, T.; Jacob, R.; et al. Nuclear Trapping through Inhibition of Exosomal Export by Indomethacin Increases Cytostatic Efficacy of Doxorubicin and Pixantrone. Clin. Cancer Res. 2016, 22, 395–404.
- Safaei, R.; Larson, B.J.; Cheng, T.C.; Gibson, M.A.; Otani, S.; Naerdemann, W.; Howell, S.B. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol. Cancer Ther. 2005, 4, 1595–1604.
- Federici, C.; Petrucci, F.; Caimi, S.; Cesolini, A.; Logozzi, M.; Borghi, M.; D’Ilio, S.; Lugini, L.; Violante, N.; Azzarito, T.; et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS ONE 2014, 9, e88193.
- Bebawy, M.; Combes, V.; Lee, E.; Jaiswal, R.; Gong, J.; Bonhoure, A.; Grau, G.E.R. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia 2009, 23, 1643–1649.
- Bhattacharya, S.; Pal, K.; Sharma, A.; Dutta, S.K.; Lau, J.S.; Yan, I.K.; Wang, E.; Elkhanany, A.; Alkharfy, K.M.; Sanyal, A.; et al. GAIP Interacting Protein C-Terminus Regulates Autophagy and Exosome Biogenesis of Pancreatic Cancer through Metabolic Pathways. PLoS ONE 2014, 9, e114409.
- Wang, X.; Qiao, D.; Chen, L.; Xu, M.; Chen, S.; Huang, L.; Wang, F.; Chen, Z.; Cai, J.; Fu, L. Chemotherapeutic drugs stimulate the release and recycling of extracellular vesicles to assist cancer cells in developing an urgent chemoresistance. Mol. Cancer 2019, 18, 1–18.
- Qu, Z.; Wu, J.; Wu, J.; Luo, D.; Jiang, C.; Ding, Y. Exosomes derived from HCC cells induce sorafenib resistance in hepatocellular carcinoma both in vivo and in vitro. J. Exp. Clin. Cancer Res. 2016, 35, 1–12.
- Vella, L.J.; Behren, A.; Coleman, B.; Greening, D.W.; Hill, A.; Cebon, J. Intercellular Resistance to BRAF Inhibition Can Be Mediated by Extracellular Vesicle–Associated PDGFRβ. Neoplasia 2017, 19, 932–940.
- Uchihara, T.; Miyake, K.; Yonemura, A.; Komohara, Y.; Itoyama, R.; Koiwa, M.; Yasuda, T.; Arima, K.; Harada, K.; Eto, K.; et al. Extracellular Vesicles from Cancer-Associated Fibroblasts Containing Annexin A6 Induces FAK-YAP Activation by Stabilizing β1 Integrin, Enhancing Drug Resistance. Cancer Res. 2020, 80, 3222–3235.
- Maacha, S.; Bhat, A.A.; Jimenez, L.; Raza, A.; Haris, M.; Uddin, S.; Grivel, J.-C. Extracellular vesicles-mediated intercellular communication: Roles in the tumor microenvironment and anti-cancer drug resistance. Mol. Cancer 2019, 18, 1–16.
- Kreger, B.T.; Johansen, E.R.; Cerione, R.A.; Antonyak, M.A. The Enrichment of Survivin in Exosomes from Breast Cancer Cells Treated with Paclitaxel Promotes Cell Survival and Chemoresistance. Cancers 2016, 8, 111.
- Yang, Z.; Zhao, N.; Cui, J.; Wu, H.; Xiong, J.; Peng, T. Exosomes derived from cancer stem cells of gemcitabine-resistant pancreatic cancer cells enhance drug resistance by delivering miR-210. Cell. Oncol. 2020, 43, 123–136.
- Aung, T.; Chapuy, B.; Vogel, D.; Wenzel, D.; Oppermann, M.; Lahmann, M.; Weinhage, T.; Menck, K.; Hupfeld, T.; Koch, R.; et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc. Natl. Acad. Sci. USA 2011, 108, 15336–15341.
- Ciravolo, V.; Huber, V.; Ghedini, G.C.; Venturelli, E.; Bianchi, F.; Campiglio, M.; Morelli, D.; Villa, A.; Della Mina, P.; Menard, S.; et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J. Cell. Physiol. 2012, 227, 658–667.
- Lubin, A.J.; Zhang, R.R.; Kuo, J.S. Extracellular Vesicles Containing PD-L1 Contribute to Immune Evasion in Glioblastoma. Neurosurgery 2018, 83, E98–E100.
- Lara, P.; Chan, A.; Cruz, L.; Quest, A.; Kogan, M. Exploiting the Natural Properties of Extracellular Vesicles in Targeted Delivery towards Specific Cells and Tissues. Pharmaceutics 2020, 12, 1022.
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316.
- Lara, P.; Palma-Florez, S.; Salas-Huenuleo, E.; Polakovicova, I.; Guerrero, S.; Lobos-Gonzalez, L.; Campos, A.; Muñoz, L.; Jorquera-Cordero, C.; Varas-Godoy, M.; et al. Gold nanoparticle based double-labeling of melanoma extracellular vesicles to determine the specificity of uptake by cells and preferential accumulation in small metastatic lung tumors. J. Nanobiotechnol. 2020, 18, 1–17.
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome Delivered Anticancer Drugs Across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm. Res. 2015, 32, 2003–2014.
- Garofalo, M.; Villa, A.; Crescenti, D.; Marzagalli, M.; Kuryk, L.; Limonta, P.; Mazzaferro, V.; Ciana, P. Heterologous and cross-species tropism of cancer-derived extracellular vesicles. Theranostics 2019, 9, 5681–5693.
- Escrevente, C.; Keller, S.; Altevogt, P.; Costa, J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 2011, 11, 108.
- Matsumoto, A.; Takahashi, Y.; Nishikawa, M.; Sano, K.; Morishita, M.; Charoenviriyakul, C.; Saji, H.; Takakura, Y. Accelerated growth of B16 BL 6 tumor in mice through efficient uptake of their own exosomes by B16 BL 6 cells. Cancer Sci. 2017, 108, 1803–1810.
- Aspe, J.R.; Osterman, C.D.; Jutzy, J.M.; Deshields, S.; Whang, S.; Wall, N.R. Enhancement of Gemcitabine sensitivity in pancreatic adenocarcinoma by novel exosome-mediated delivery of the Survivin-T34A mutant. J. Extracell. Vesicles 2014, 3, 1–9.
- Hood, J.L. Post isolation modification of exosomes for nanomedicine applications. Nanomedicine 2016, 11, 1745–1756.
- Kooijmans, S.A.A.; Aleza, C.G.; Roffler, S.R.; Van Solinge, W.W.; Vader, P.; Schiffelers, R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles 2016, 5, 31053.
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.; Melo, S.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nat. Cell Biol. 2017, 546, 498–503.
- Lai, C.P.-K.; Kim, E.Y.; Badr, C.E.; Weissleder, R.; Mempel, T.R.; Tannous, B.A.; Breakefield, X.O. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat. Commun. 2015, 6, 7029.
- Jiang, X.; Zong, S.; Chen, C.; Zhang, Y.; Wang, Z.; Cui, Y. Gold–carbon dots for the intracellular imaging of cancer-derived exosomes. Nanotechnology 2018, 29, 175701.
- Jung, K.O.; Jo, H.; Yu, J.H.; Gambhir, S.S.; Pratx, G. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials 2018, 177, 139–148.
- Hu, L.; Wickline, S.A.; Hood, J.L. Magnetic resonance imaging of melanoma exosomes in lymph nodes. Magn. Reson. Med. 2015, 74, 266–271.
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979.
- Li, Y.; Wang, J.; Wientjes, M.G.; Au, J.L.-S. Delivery of nanomedicines to extracellular and intracellular compartments of a solid tumor. Adv. Drug Deliv. Rev. 2012, 64, 29–39.
- Xie, F.; Xu, M.; Lu, J.; Mao, L.; Wang, S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol. Cancer 2019, 18, 146.
- Choi, D.-S.; Kim, D.-K.; Kim, Y.-K.; Gho, Y.S. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom. Rev. 2015, 34, 474–490.




