| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ajaya Bhattarai | + 3087 word(s) | 3087 | 2020-06-27 19:49:38 | | | |
| 2 | Ajaya Bhattarai | + 3079 word(s) | 3079 | 2020-06-28 05:34:31 | | | | |
| 3 | Ajaya Bhattarai | + 623 word(s) | 3702 | 2020-06-30 11:04:02 | | | | |
| 4 | Catherine Yang | Meta information modification | 3702 | 2020-06-30 12:14:09 | | | | |
| 5 | Ajaya Bhattarai | + 656 word(s) | 4358 | 2020-06-30 18:46:09 | | | | |
| 6 | Ajaya Bhattarai | + 656 word(s) | 4358 | 2020-06-30 18:46:51 | | | | |
| 7 | Catherine Yang | -1490 word(s) | 2868 | 2020-08-31 06:23:28 | | | | |
| 8 | Ajaya Bhattarai | + 19 word(s) | 2887 | 2020-09-28 01:35:36 | | | | |
| 9 | Catherine Yang | Meta information modification | 2887 | 2020-10-20 07:48:19 | | | | |
| 10 | Vivi Li | -19 word(s) | 2868 | 2020-10-27 07:33:31 | | |
Video Upload Options
Polyelectrolytes are polymers of oppositely charged ions and their properties differ profoundly than their repeating units. Over recent years, much advancement has been made in the synthesis, characterization, and application of polyelectrolytes and polyelectrolyte complexes (PECs). It has many applications as flocculation agents, dispersant agents, and as super-plasticizers. In this article, the synthesis, types, characteristics, and application of PECs mainly for drug delivery and dynamics study are reviewed.
1. Introduction
Polymers that contain repeating electrolyte units i.e. polycations or polyanions, at near-neutral pH are called polyelectrolytes [1], which incorporate significant molecules, for example, DNA and other naturally available polymers. These groups charged the polymers after dissociation in aqueous solution and are physically and chemically similar to both electrolytes and polymers. Their solutions are conductive, like salts and viscous like polymer's solutions. Polymers are classified into various types, such as based on origin, composition, molecular architecture, and electrochemistry. Around a hundred years ago, the investigation of polyelectrolyte complexes started [2] but is being revived a short time ago on account of many different types of work that require immense knowledge of the field. One such line is the layer-by-layer (LbL) aggregation of electrolyte units, invented by Decher [3], which leads to many possible applications in the fields of catalysis, membranes, and biomedicine. The concept of polyelectrolyte complexes as a significant contributor to drug delivery vehicle design may be useful because of its improvements made a few decades back [4][5][6][7].
2. Chemistry of polyelectrolytes
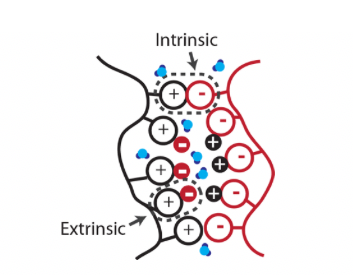
The properties of the starting materials and the reacting environment both affect the synthesis, shape and characteristics of polyelectrolyte complexes [8][9][10][11][12][13][14][15][16]. The formed shape of the PEC or for any charged group is usually depicted in figure 1. Oppositely charged polyelectrolytes get together by shaping inseparable ion pairs, and the rest unbonded charged particles are balanced by small counter-ions to makeup separable ion pairs. Water molecules enclose many ion pairs in the hydration layer because this phenomenon generally occurs in the aqueous phase.
Figure 1: Schematic of charged polyelectrolyte assembly
3. Polyelectrolytes in drug delivery
In the course of recent years, incredible improvements have been achieved in medicine transport systems. In recent times, the utilization of polyelectrolyte multilayers (PEMs) manufactured by LbL self-build of oppositely charged polyelectrolytes have been developed as an amazing and adaptable method to design surface layers for biofunctionalization and medicine conveyance [17][18][19].
Regarding on the synthesis and utilization of PECs in pharmacy, there are many papers reported. Daubine et al. [20] built up a new way of controlling bone metastasis using nanostructured PEMs. Poly-L-lysin covalently bound with β-cyclodextrin as a polycationic vector (PLL-CD) was utilized as the antitumor bisphosphonate drug, risedronate (RIS).
Anandhakumar et al. [21] proposed a nanoparticle stacking method to build up a transparent PEM with multiple functions that are used for remotely enabled medicine and protein conveyance. The complex film was engineered by alternate adsorption of poly(allylamine hydrochloride) (PAH) and dextran sulfate (DS) over a glass substrate and then by nanoparticle preparation by a polyol reduction technique.
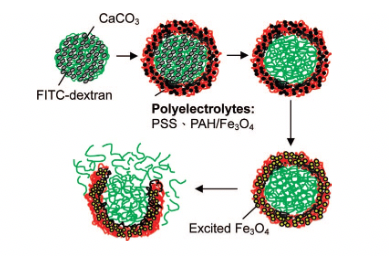
Hu et al. [22] investigated the controlled burst of magnetic polyelectrolyte capsules for drug transport. The microcapsules prepared by the LbL procedure utilizing Fe3O4/PAH are magnetic sensitive. Design quality, microstructural development, and related discharge characteristics of fluorescence dyes and doxorubicin were methodically researched. The test results indicated that the availability of the magnetic nanoparticles in the covered structure permitted the covered structure to advance from nanocavity improvement to a definite break of the cover under the influence of the given magnetic effect of various time spans.
Externally activated liposome discharge by NIR light absorption by means of hollow gold nanoparticles was recently developed by Wu et al. [23]. These liposomes can discharge medicines within seconds with 1X10-15 seconds pulses of NIR light stimulus.
Figure 2: Schematic diagram of encapsulation and release of substances in polyelectrolyte capsules.
Hydrophobic modification of the bio-adhesive polyelectrolyte hydrogel was done by Inoue et al. [24], which was manufactured by joining oligomers of methyl methacrylate (MMA) to the backbone of poly(acrylic acid) (PAA) hydrogel. Six different drugs were used to investigate the swelling nature and medication discharge profile of the hydrogel using phosphate-buffered saline (PBS) as a discharge medium. The pH-delicate, swelling-controlled release of caffeine was revealed by Siegel et al. [25] by developing hydrophobic polyelectrolyte random co-polymer hydrogels dependent on MMA and N, N-dimethyl aminoethyl methacrylate. The similar discharge nature of oxprenolol hydrochloride was observed by Kim and Lee [26] by synthesizing hydrogels utilizing MMA and methacrylic acid.
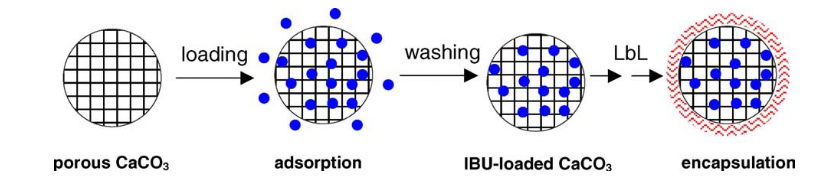
Wang et al. [27] studied the new combined effect of adsorption by porous CaCO3 microparticles and encapsulated PEM films for continued medication conveyance through LbL self-aggregation. The powdered ibuprofen (IBU) stacked in the pores of the CaCO3 microparticles had a faster discharge rate in the gastric fluid than in the intestinal fluid when compared with the crystalline uncovered IBU; however, the PEMs gathered on the drugs stacked particles by LbL decreased the rate of discharge in both liquids.
Figure 3: Combination of adsorption by microparticles and encapsulation by PEM films.
Hamman [28] described the complexation of chitosan-based PECs as potential drug carriers and view the stability of these PECs. From in vitro studies, he concluded that these complexes are significant ingredients with peculiar characteristics for effective dose from structural plans, which may be important in the advancement of altered medication conveyance systems.
Sawahata et al. [29] experimentally demonstrated electrically controlled medication transport systems utilizing polyelectrolyte gels and microparticles, whose principle depends on chemo-mechanical contraction and expansion of polymer gels under the influence of an electric field. The effective discharge of some bioactive substances like glucose and insulin from gel can be done by exchanging the electric field on and off alternately and it was reported that the sped and quantity of discharge can be constrained by the applied intensity of the electric field.
4. Types of polyelectrolytes
The following are the various types of polyelectrolytes:
|
Basis |
Types |
|
Origin |
Natural, synthetic, and chemically modified biopolymers. |
|
Composition |
Homo and co-polymer. |
|
Electrochemistry |
Polyacids/polyamines, polybase/polycations and polyampholytes. |
5. Synthesis of polyelectrolytes complexes
Despite the fact that PECs can be synthesized through connections between macromolecules because of a series of secondary binding forces, the often possible complexes to deal with are those synthesized by associations between macromolecules having inverse charges. Indeed, even in these complexes, the impact of different binding forces cannot be neglected.
Generally, the formation of polyelectrolytes includes 3 main steps: first, primary complex formation, and the formation of intracomplex, and the last one is intercomplex aggregation process [30]. Coulomb forces are the primary cause of the first phase, while the next involves the development of new bonds and the third one includes the collection of secondary complexes primarily via hydrophobic interactions.
- Polyelectrolytes using new synthetic methods
Late improvements in the manufacture of polyelectrolytes are featured, concerning the behavior of ionic gatherings, polymer backbones, manufacturing methods, and extra usefulness given to polyelectrolytes. Laschewsky [31] gives specific consideration to strong polyelectrolytes, and recent techniques for controlled polymerization (CP), called click reactions, which have empowered the novel specification of polyelectrolytes. Here, more and more developing strategies of the so-called CP, specifically of the controlled free-radical polymerization (CFRP) techniques, have given a significant push to making new polyelectrolytes recently.
Synthesis by 'click chemistry'
The term 'click chemistry' was first-authored by K.B. Sharpless in 1998. Click chemistry is another style of organic compound formation, or, perhaps more precisely, the revitalization of an old style of organic synthesis whose reason for existing is to quicken the revelation of substances with helpful properties, new medications being the limelight. It is not a specific reaction but describes the natural process of synthesis [32]. Click chemistry combines characteristics like modularity, insensitive to solvent parameters, high yield, insensitivity towards oxygen and water, regiospecificity, and enormous thermodynamic driving force to support a single reaction product [33].
Presently, the most popular “click” reaction is copper(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition [34]
Synthesis by controlled free radical polymerization (CFRP)
The CFRP strategies [35] have reformed the manufacture of polyelectrolytes in the past decades. Because of the greater resistance of radicals toward electrophiles just as nucleophiles, FRP is fated for manufacturing charged polymers, also in the aqueous medium, which does not require protecting groups or additional modification of polymers that change the well-structured neutral precursor polymer into the required polyelectrolyte. The CFRP methods have in this way given immediate access to polyelectrolytes with beforehand inconceivable characteristics, for example, predefined molar masses, limited molar mass conveyances, and very much characterized (and furthermore functional) end groups. Significantly, CFRP methods are different in permitting the preparation of block copolymers, in which each block is a statistical or even random copolymer, as shown for complex polyelectrolytes effectively, utilizing the reversible expansion discontinuity chain move polymerization (RAFT) strategy [36]. Actually, RAFT is seemingly the most adaptable strategy among the different CFRP procedures with regard to the manufacture of charged polymers.
6. Factors affecting the synthesis of PECs
The development of PECs is represented by the qualities of the individual polyelectrolyte (for example, characteristics of ionic locales, the strength of electrolytes, location of ionic destinations, charge density, stiffness of macromolecular chains) and compound conditions (for example, solvent, ionic strength, pH and temperature) [37][38]. PECs are either isolated from the solutions as solids or fluids or they are as yet in solution or may settle as gels because of the variety of the controlling components given previously. By altering the ionic quality, the number of polymers, their relative proportion, pH or temperature, the level of transformation of the interaction between the polycations and polyanions, and therefore the result of the final polymer complex, can be directed.
7. Structure of PECs
PECs are formed by the interaction of polycation and polyanions with one another in solution. The potential mixing includes strong polyacid-strong polybases, strong polyacid-weak polybases, weak polyacid-weak polybases, weak polyacid-weak polybases, or polyampholytes. The formed items are anticipated to show definite physicochemical characteristics because the other binding forces are weaker than the electrostatic interaction forces.
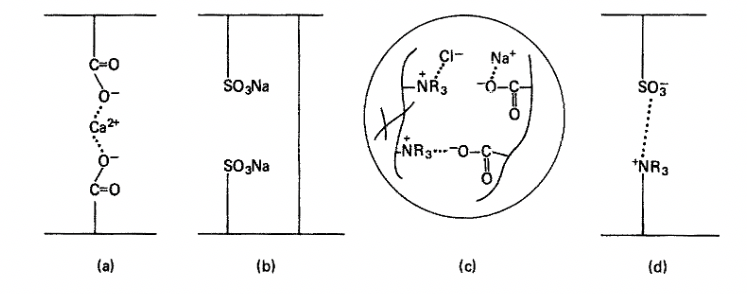
The shape of ion consisting of macromolecules such as ionomers, ion-exchange resins, and snake-cage resins contrasts with PECs [39]. There is only one type of polyelectrolyte in ionomers and ion-exchange resins, which are crosslinked by divalent counterions and covalent bonds, respectively. However, there are two types of polyelectrolytes in snake-cage resins [40]; however, they are excessively difficult as industrial materials.
Figure 4: (a) Ionomers; (b) Ion-exchange resins; (c) Snake-cage resins; (d) Polyelectrolyte complex
8. Properties of polyelectrolytes
Since the characteristics of the beginning, materials are basically unique to those of PECs, and this encourages the investigation of their production. In spite of many ways, some of the methods that identify such contrasts are potentiometry, conductometry, turbidimetry, viscometry, calorimetry, sedimentation, dynamic flow birefringence, light scattering, NMR spectroscopy, electronic spectroscopy, chromatography, and even electron microscopy.
To accomplish a more extensive business of PECs, the number of nitty-gritty information on their characteristics is required. It is commonly accepted that PECs have special features on the grounds that the principle interaction forces are strong Coulomb forces and their electrostatic nature can undoubtedly be altered by varying just their composition [41]:
- Physicochemical properties
- Good transparency
- Selectivity for ion sorption
- ion-exchange properties
- Electrical properties
- Transport properties
- Good anti-coagulant properties
PECs, which are made out of a strong polyacid and a strong polybase, are not soluble in regular organic and inorganic solvents. They are just dissolvable in a definite medium, for example, water/water-soluble organic solvents/micro salts, such as water/acetone/NaBr [41][42][43][44].
PECs have remarkably large and governable penetrability in water and low-molecular-weight solutes. The commercial cellophane membrane shows lower permeability towards low-molecular-weight solutes than that of complex membranes [45]. The permeability of membranes made from PECs derived by mixing polyacids and polybases with equimolar composition is generally more to water and urea [46].
Biologically derived solids show a closer relation to PECs on the basis of electrical characteristics. Not for many artificial polymers, incredibly high relative dielectric constants at low frequencies, and the dispersion qualities of salt-containing PECs are not observed [47].
At 22⁰C, the refractive index of homogeneous PECs containing 40-80% gel water is expressed by
n = 1.294 + 0.336 (1 - α)
where α refers to the gel-water content as a weight fraction [42].
The dynamics of polyelectrolytes can likewise be examined utilizing rheological and scattering methods. Di Cola [48] studied the structure and dynamic characteristics of sodium maleate copolymers utilizing light scattering and rheological methods and showed great agreement with conventional rheological and micro-rheological methods.
Hoffmann et al. [49] introduced the dynamics, structure, and characteristics of JR 400 blended with SDBS or SDES by using rheological, dynamic light scattering (DLS) and small-angle neutron scattering (SANS) methods.
By uniting neutron scattering and fluorescence methods, Hoffmann et al. [50] depicted the process of changing the flow behavior in oppositely charged mixtures of polycations JR 400 and the anionic surfactant SDS in a surfactant excess system.
Marciel et al. [51] presented the noticeable similar structural properties of the semidilute solution of polyelectrolytes and liquid PECs. The correlation length in coacervates was found to be nearly the same as the semidilute polyelectrolyte solution of polymer concentration (ξ~C-1/2). The effects of adding salt on the scattering from coacervates were also consistent with the proposed structural description of coacervates.
Tsitsilianis et al. [52] characterized the polystyrene end-capped polyelectrolytes by rheological behavior. The gel properties at concentration 0.2 %, which is much lower than that found for other end-capped polymers. Moreover, the system seems to have a very long relaxation time, greater than 100 seconds.
Lopez et al. [53] presented viscosity data of sodium carboxymethyl cellulose (NaCMC) solution as a function of molecular weight and concentration of added salt (Cs). He found entanglement crossover is independent of Cs with the help of rheological data. The study of NaCMC with the degree of substitution 1.2 by SANS and rheology [54] indicated that the solution is molecularly dispersed in water. According to the rheology study, NaCMC shoes identical characteristics as other polyelectrolyte systems and the polymer chain has a cross-sectional radius of ≃ 3.4 Å. Another study [55] showed that, in comparison to NaCMC, viscosities of divalent salts is lower at low concentration in the non-entangled regime but above C ≃ 0.07 M, the viscosities of solutions with divalent counterions are up to an order of magnitude larger than NaCMC. However, the dynamics are largely unaffected by the counterion type.
The data of viscosity of non-entangled sodium polystyrene sulfonate in salt-free solution was reported by Lopez et al. [56]. They observed the deviation from Fuoss' law at a high polymer concentration of greater than equal to 0.02 M. Further, the study of entanglement properties of polyelectrolyte's in salt-free and salt-excess solutions [57] found out entanglement density and entanglement crossover is independent of solvent's ionic strength; which contradicts current models.
Adamo et al. [58] performed a contrast variation and matching with the model system: H2O/D2O, SDS, Pluronic F127, Ludox in isotropic aqueous mixtures by microfluid SANS. The effects of flow dispersion and neutron beam over-illumination of the systems in terms of the composition resolution and precision were determined.
For a PNIPM based microgel containing 2% ionic group Lopez et al. [59] presented DLS data and this result fit with former papers, the hydrodynamic radius changes as RH α Cs-0.05 in rich solvent and RH α Cs-0.15 in the bad solvent. But this outcome does not agree with scaling models of polyelectrolyte gel swelling.
Yang et al. [60] revealed the entangled characteristics for the broad and narrow molecular weight for longer polyelectrolytes by time-temperature superposition (TTS). While time-temperature-salt doping superposition (TTSS) was obtained by taking accounts of the impacts of the rising amount of salt on PECs.
Lopez et al. [61] presented the shear rheology data for NaPSS in semidilute salt-free aqueous solution. The scaling theory of Dobryn et al. [62] fit good with the variation of electrostatic Kuhn Length (LK,e ≃ 41C-1/2 ≃ 1.3 ξ). Also, the calculated terminal modulus fits with the scaling prediction of G ≃ KBTcN/N.
9. Other applications of PECs
As indicated by the fact that polyelectrolytes are broadly utilized as flocculants [63], they might be practically used in protein fractionation, which is significant in medication and clinical cure [64][65].
In addition, intermolecular complexes are used in particular extraction of organic and metallic ions; for example; Cu2+ ions are more successfully precipitated by the PECs than by one of its components [66]
PECs can be utilized to manufacture ultra-filtration films that can be utilized in artificial kidneys, artificial lungs whose proficiency is restricted by previously existing membrane materials that give significant protection from mass exchange [46].
10. Problems of PECs
The manufacture of PECs, in spite of the specific level of development, still faces various uncertain issues. There are difficulties and still numerous new compounds that are yet to be found. In the near future, polyelectrolytes will not be explored by the desire of their own, but will be generally inspired by material angles; these results not only new ionic structures but progressively complex all around molecular structures and geometry. Subsequently, there will be numerous chances, and furthermore, require an unstoppable expansion of the extent of available polyelectrolyte structures.
11. Conclusion
The development of enthusiasm for the study of polyelectrolytes is expected essentially to the expanding utilization of these polymers as flocculants, stabilizers of scatterings, definite absorbents or adsorbents, and as medication bearers. In a significant number of these applications, the creation of amphiphilic polymers, materials containing both hydrophilic and hydrophobic units with extraordinary morphology are of crucial significance. In this unique circumstance, the interaction between macromolecules offers broad uses and their capacity has not yet been completely achieved. Proceeding with research polyelectrolyte collaborations will undoubtedly advance the connections between the polymer and different sciences, specifically the connections with biochemistry.
References
- Kubisa P. Terminology of polymers Containing Ionizable or Ionic Groups and of Polymers Containing Ions, IUPAC Recommendations. 2004. (Draft 23 December 2004).
- Bungenberg de Jong H. G., Kruyt H. R. Coacervation (Partial Miscibility in Colloid Systems). Proc. K. Ned. Akad. Wet. 1929, 32, 849−856.
- Decher G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science. 1997, 277, 1232−1237.
- Kabanov VA. In: Macromolecular Complexes in Chemistry and Biology. Dubin P, Bock J, Davis R, Schulz DN, Thies C, editor New York: Springer 1994.
- Leclercq L, Boustta M, Vert M. A Physico-chemical approach of polyanion-polycation interactions aimed at better understanding the in vivo behavior of polyelectrolyte based drug delivery and gene transfection. J Drug Targeting. 2003, 11, 129-138.
- Kekkonen J, Lattu H, Stenius P, Adsorption kinetics of complexes formed by oppositely charged polyelectrolytes. J Colloid Interface Sci. 2001, 234, 384-392.
- Shojaei AH. Buccal mucosa as route for systematic drug delivery: A review. J pharm pharmaceut Sci. 1998, 1, 15-36.
- Dautzenberg H. Polyelectrolyte complex formation in highly aggregating systems. 1. Effect of salt: polyelectrolyte complex formation in the presence of NaCl. Macromolecules. 1997, 30 (25), 7810.
- Perry S. L., Sing C. E. PRISM-Based Theory of Complex Coacervation: Excluded Volume versus Chain Correlation. Macromolecules. 2015, 48 (14), 5040.
- Qin J., de Pablo J. J. Criticality and Connectivity in Macromolecular Charge Complexation. Macromolecules. 2016, 49 (22), 8789.
- Perry S. L., Li Y., Priftis D., Leon L., Tirrell M. The effect of salt on the complex coacervation of vinyl polyelectrolytes. Polymers. 2014, 6 (6), 1756.
- Zhang Y., Yildirim E., Antila H. S., Valenzuela L. D., Sammalkorpi M., Lutkenhaus J. L. The influence of ionic strength and mixing ratio on the colloidal stability of PDAC/PSS polyelectrolyte complexes. Soft Matter. 2015, 11 (37), 7392.
- Zhang H., Wang C., Zhu G., Zacharia N. S. Self-Healing of Bulk Polyelectrolyte Complex Material as a Function of pH and Salt. ACS Appl. Mater. Interfaces. 2016, 8 (39), 26258.
- Shah N. J., Hyder M. N., Quadir M. A., Dorval Courchesne N. M., Seeherman H. J., Nevins M., Spector M., Hammond P. T. Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (35), 12847.
- Kremer T., Kovacević, Salopek J., Pozar J. Conditions Leading to Polyelectrolyte Complex Overcharging in Solution: Complexation of Poly (acrylate) Anion with Poly (allylammonium) Cation. Macromolecules. 2016, 49 (22), 8672.
- Laaser J. E., Lohmann E., Jiang Y., Reineke T. M., Lodge T. P. Architecture-Dependent Stabilization of Polyelectrolyte Complexes between Polyanions and Cationic Triblock Terpolymer Micelles. Macromolecules. 2016, 49 (17), 6644.
- Decher G. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science 1997, 277, 1232–7.
- Peyratout CS, Dahne L. Tailor-made polyelectrolyte microcapsules: from multilayers to smart containers. Angew Chem Int Ed. 2004, 43, 3762–83.
- Volodkin A, Skirtach A, Mohwald H. LbL films as reservoirs for bioactive molecules. Adv Polym Sci. 2011, 240, 135–61.
- Dubaine F., Cortial D., Ladam G., Atmani H., Haikel Y., Voegel J.C., Clezardin P., Benkirane-Jessel N. Nanostructured polyelectrolyte multilayer drug delivery systems for bone metastasis prevention. Bio materials. 2009 30, 6367-6373.
- Anandhakumar S., Raichur A.M. Polyelectrolyte/silver nanocomposite multilayer films as multifunctional thin film platforms for remote activated protein and drug delivery. Acta Biomaterilia. 2013, 9, 8864-8874.
- Hu S-H, Tsai C-H, Liao C-F, Liu D-M, Chen S-Y. Controlled Rupture of Magnetic Polyelectrolyte Microcapsules for Drug Delivery. Langmuir. 2008, 24, 11811-11818.
- Wu G., Mikhailovsky A., Khant H.A., Fu C., Chiu W., Zasadzinski J.A. Remotely Triggered Liposome Release by Near-Infrared Light Absorption via Hollow Gold Nanoshells. J. Am. Chem. Soc. 2008, 130, 8175-8177.
- Inoue T., Chen G., Nakamae K., Hoffmann A.S. A hydrophobically-modified bioadhesive polyelectrolyte hydrogel for drug delivery. J. Control Release. 1997, 49, 167-176.
- Siegel R.A., falamarzian M., Firestone B.A., Moxley B.C. pH-Controlled release from hydrophobic/ polyelectrolyte copolymer hydrogel, polymer hydrogels. J. control Release. 1988, 8, 179-182.
- Kim C.J., Lee P.I. Hydrophobic anionic gel beads for swelling-controlled drug delivery. pharm. Res. 1992, 9, 195-199.
- Wang C., He C., Tong Z., Liu X., Ren B., Zeng F. Combination of adsorption by porous CaCO3 microparticles and encapsulation by polyelectrolyte multilayer films for sustained drug delivery. Intl. J. Pharm. 2006, 308, 160-167.
- Hamman J.H. Chitosan Based Polyelectrolyte Complexes as Potential Carrier Materials in Drug Delivery Systems. Drugs. 2010, 8, 1305-1322.
- Sawahata K., Hara M., Yansunaga H., Osada Y. Electrically controlled drug delivery system using polyelectrolyte gels. Control Releasr. 1990, 14, 253-262.
- Kokufuta E. Colloid titration behavior of poly(ethyleneimine). Macromolecules. 1979, 12, 350-403.
- Laschewsky A. Recent trends in the synthesis of polyelectrolyte's. Current opinion in Col. Inter. Sci. 2012, 17, 56-63.
- Kolb H.C., Finn M.G., Sharpless K.B. Click Chemistry: Diverse chemical function from a few good reactions. Angew Chem. Int. Ed., 2001, 40, 2004-2021.
- Wang X., Schmidt F., Hanaor D., Kamm P.H., Li S., Guvlo A. Additive manufacturing of ceramics from preceramic polymers: A versatile stereolithographic approach assisted by thiol-ene click chemistry. Additive manufacturing, 2019, 27, 80-90.
- Kolb H.C., Sharpless K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today. 2003, 8, 1128-1137.
- Matyjaszewski K, Müller AHE, editors. Controlled and living polymerizations.From mechanisms to applications. Weinheim: Wiley-VCH; 2009.
- Kotzev A., Laschewsky A., Rakotoaly R. Polymerizable surfactants and micellar polymers bearing fluorocarbon hydrophobic chains based on styrene. Macromol. Chem. Phys., 2001, 202, 3257-3267.
- Marteen P., Biesheuvel, Martein A., Cohen S. Cylindrical Cell Model for the Electrostatic Free Energy of Polyelectrolyte Complexes. 2004, 20, 4764-4770.
- Fredheim G.E., Christensen B.E. Polyelectrolyte complexes: Interactions between lignosulfonate and chitosan. 2003,4, 232-309.
- Rembaum A. Biological activity of ionene polymers. Polym. Symp. 1973, 22, 299.
- Hatch M.J., Dillon J.A., Smith H.B. Preparation and Use of Snake-Cage Polyelectrolytes. Eng. Chem. 1957, 44, 1812.
- Michaels A. S., Falkenstein G. L., Schneider N. S. Dielectric properties of polyanion–polycation complexes. Phys. Chem., 1965, 69, 1456.
- Michaels A. S., Mir L., Schneider N. S. A Conductometric Study of Polycation-Polyanion Reactions in Dilute Aqueous Solution. Phys. Chem. 1965, 69, 1447.
- Michaels, A. S. Polyelectrolyte Complexes. Eng. Chem. 1965, 57, 32.
- Bixler H. J., Michaels A. S. Polyelectrolyte Complexes. Encycl. Polym. Sci. Technol. 1969, 10, 765.
- Smolen V. F., Hahman D. E. A water membrane hypothesis: Behavior of hydrated polycation-polyanion salt complexed membranes as apparent lipoidal barriers to solute transport. Colloid Interface Sci. 1973, 42, 70.
- Kalyuzhnaya R.I., Volynskii A.L., Rudman A.R. Vengerova N.A., Razvodovskii Ye.F., El'tsefon B.S., Zezin A.B. Investigation of the mechanical properties of membranes of polyelectrolyte complexes based on weak polyelectrolytes. Vysokomol. Soyed. 1976, 18, 71.
- Kurokawa Y., Shirakawa N., Terada M., Yui N. Formation of polyelectrolyte complex and its adsorption properties. Technol. Rep., Tohoku Univ., 1979, 49, 129.
- Di cola E., Waigh T.A., Colby R.H. Dynamic Light Scattering and Rheology Studies of Aqueous Solutions of Amphiphilic Sodium Maleate Containing Copolymers. Polymer Physics, 2006, 45, 774-785.
- Hoffmann J., Heunemann P., Prevost S., Schweins R., Wagner N.J., Gradzeielski M. Self-Aggregation of Mixtures of Oppositely Charged Polyelectrolyte's and Surfactants Studied by Rheology, Dynamic Light Scattering and Small-Angle Neutron Scattering. Langmuir. 2011, 27, 4386-4396.
- Hoffmann J., Simon M., Frago B., Schweins R., Falus P., Halderer O., Gradzeielski M. Structure and dynamics of polyelectrolyte surfactant mixtures under conditions of surfactant excess. Chem. Phys. 2016, 145, 1-11.
- Marciel A.B., Srivastava S., Tirell M.V. Structure and Rheology of Polyelectrolyte Complex Coacervates. Soft Matter. 2018, 14, 2454-2464.
- Tistsilionis C., Iliopoulos I., Ducouret G. An associative polyelectrolyte End-Capped with short polystyrene chains. Synthesis and rheological behavior. 2000, 33, 2936-2943.
- Lopez C.G. Entanglement of semiflexible polyelectrolytes: Crossover concentrations and entanglement density of sodium carboxymethyl cellulose. Rheol. 2020, 64, 191.
- Lopez C.G., Rogers S.E., Colby R.H., Graham P., Cabral J.T. Microfluidic-SANS: flow processing of complex fluids. Sci. 2015, 53, 492-501.
- Lopez C.G., Richtering W. Influence of divalent counterions on the solution rheology and supramolecular aggregation of carboxymethyl cellulose. 2019, 26, 1517-1534.
- Lopez C.G., Richtering W. Viscosity of Semidilute and Concentrated Nonentangled Flexible Polyelectrolytes in Salt-Free Solution. J. Phys. Chem. 2019, 123, 5626-5634.
- Lopez C.G. Entanglement Properties of Polyelectrolytes in Salt-Free and Excess-Salt Solutions. ACS Macro Lett. 2019, 8, 979-983.
- Adamo M., Poulos A.S., Miller R.M., Lopez C.G., Martel A., Porcar L., Cabral J.T. Rapid contrast matching by microfluidic SANS. Lab Chip. 2017, 17, 1559-1569.
- Lopez C.G., Lohmeir T., Wong J.E., Richtering W. Electrostatic expansion of polyelectrolyte microgels: Effect of solvent quality and added salt. Colloid Inter. Sci. 2020, 558, 200-210.
- Yang M., Shi J., Schlenoff J.B. Control of Dynamics in Polyelectrolyte Complexes by Temperature and Salt. 2019, 52, 1930-1941.
- Lopez C.G., Richtering W. Conformation, and dynamics of flexible polyelectrolytes in semidilute salt-free solutions. Chem. Phys. 2018, 148, 244902.
- Dobrynin A. V., Colby R. H., Rubinstein M. Scaling theory of polyelectrolyte solutions. Macromolecules. 1995, 28, 1859–1871.
- Grutsh, J. F. Wastewater treatment: the electrical connection. Environ. Sci. Technol. 1978, 12, 1022.
- Ohno, H., Abe, K., Tsuchida, E. Interaction of human serum proteins with synthetic polymers inhomogeneous systems. Makromol. Chem. 1981 182, 1253.
- Ohno, H., Abe, K., Tsuchida, E. Interaction of human serum proteins with synthetic polymers in heterogeneous systems. Makromol. Chem. 1981, 182, 1407.
- Tsuchida E., Abe K. interactions between macromolecules in solution and intermacromolecular complexes. Adv. Poly. Sci. 1982, 45, 1-119.