Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Goran Šimić | + 2187 word(s) | 2187 | 2021-06-13 06:36:00 | | | |
| 2 | Vicky Zhou | Meta information modification | 2187 | 2021-07-13 13:31:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Šimić, G. Amygdala. Encyclopedia. Available online: https://encyclopedia.pub/entry/12021 (accessed on 07 February 2026).
Šimić G. Amygdala. Encyclopedia. Available at: https://encyclopedia.pub/entry/12021. Accessed February 07, 2026.
Šimić, Goran. "Amygdala" Encyclopedia, https://encyclopedia.pub/entry/12021 (accessed February 07, 2026).
Šimić, G. (2021, July 13). Amygdala. In Encyclopedia. https://encyclopedia.pub/entry/12021
Šimić, Goran. "Amygdala." Encyclopedia. Web. 13 July, 2021.
Copy Citation
The amygdala is one of the areas in the brain involved in the development of PTSD as the starting point for the process of activation of the hypothalamo–pituitary axis and the cascade of physiological responses to acute stress. An appropriate response to acute stress is a vital adaptive mechanism, but its prolongation causes various biopsychosocial (previously, psychosomatic) disorders. Chronic stress leads to higher expression of CRH/CRF in the CE and BLA, which has an anxiogenic effect.
amygdala
emotion
evolution
fear
anxiety
1. Introduction
The amygdala is formed by several nuclei and cortical fields located bilaterally in the anteromedial part of temporal lobes of the cerebrum (Figure 1). There are several concepts about what the term amygdala should encompass as well as whether it is a single structure or a set of extensions from different parts of the brain [1].
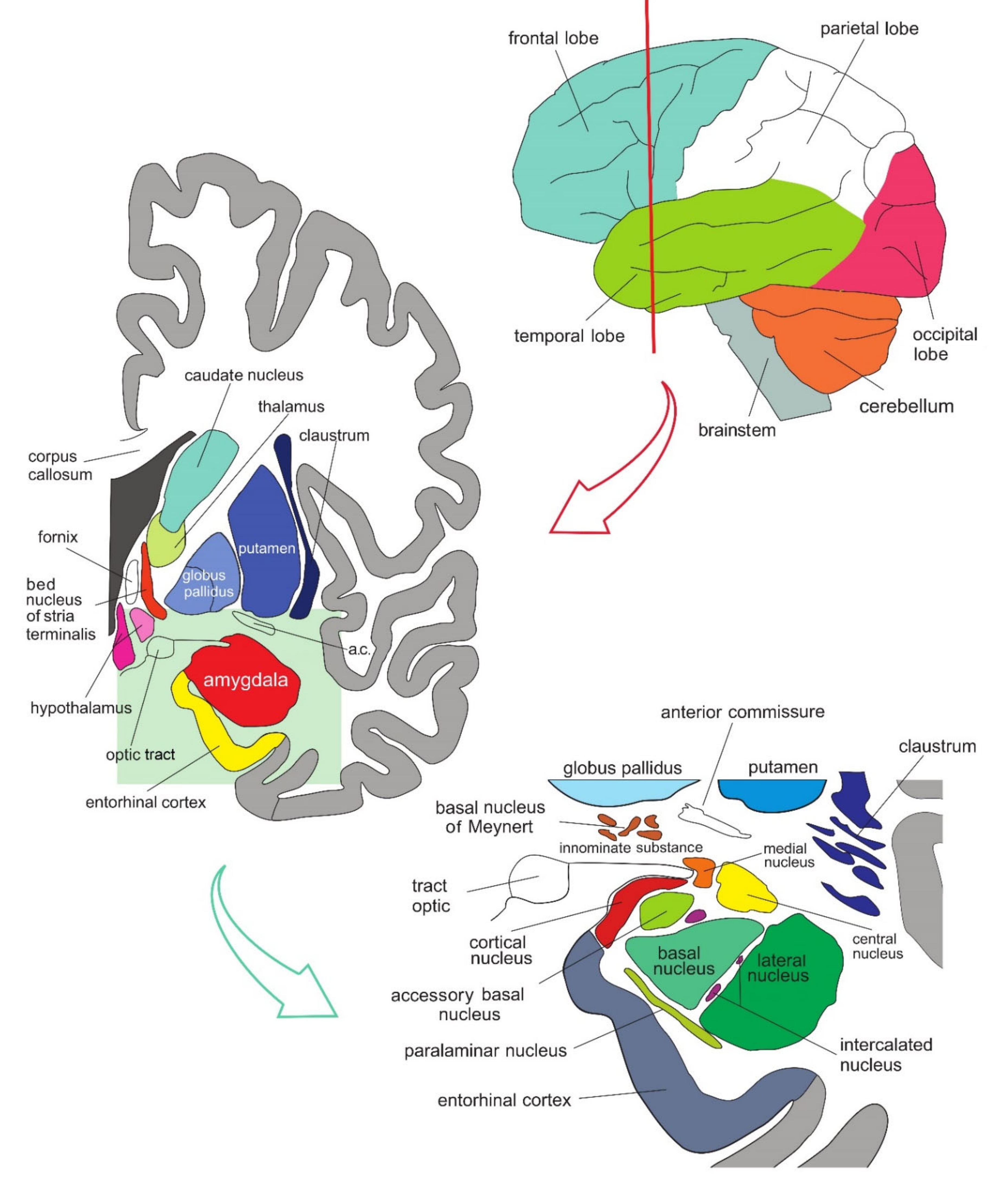 Figure 1. Simplified representation of the structure and location of the amygdala. The upper part of the schematic shows the human brain when viewed from the lateral side, where the brainstem, cerebellum, and four lobes of the cerebrum can be seen. The middle part of the schematic shows the structures present on the coronal plane through the temporal lobe of the cerebrum on which the position of the amygdala can be observed. The lower part of the schematic shows an enlarged amygdala with its individual nuclei. a.c.—anterior commissure. See text for details.
Figure 1. Simplified representation of the structure and location of the amygdala. The upper part of the schematic shows the human brain when viewed from the lateral side, where the brainstem, cerebellum, and four lobes of the cerebrum can be seen. The middle part of the schematic shows the structures present on the coronal plane through the temporal lobe of the cerebrum on which the position of the amygdala can be observed. The lower part of the schematic shows an enlarged amygdala with its individual nuclei. a.c.—anterior commissure. See text for details.In primates, the amygdala is usually divided into 13 nuclei and cortical fields [2][3][4][5]. Most agree that the amygdala can be divided into several groups of nuclei, as some nuclei show certain anatomical and functional similarities. The deep or basolateral group contains the lateral, basal, accessory basal and the paralaminar nucleus. The superficial or corticomedial group includes the cortical nucleus in contact with the relatively thin periamygdaloid paleocortex, the central and medial nuclei as two functionally similar nuclei, and the nucleus of the lateral olfactory tract, which some authors do not include as a part of the amygdala. The BNST might be added to this group although most do not consider it a part of the amygdala. It should be noted that the central nucleus (CE) has a more specific functional role and connections, so it can be observed separately. Additional nuclei include the anterior amygdaloid area, the amygdalohippocampal area, and groups of inserted neuronal clusters (Figure 2).
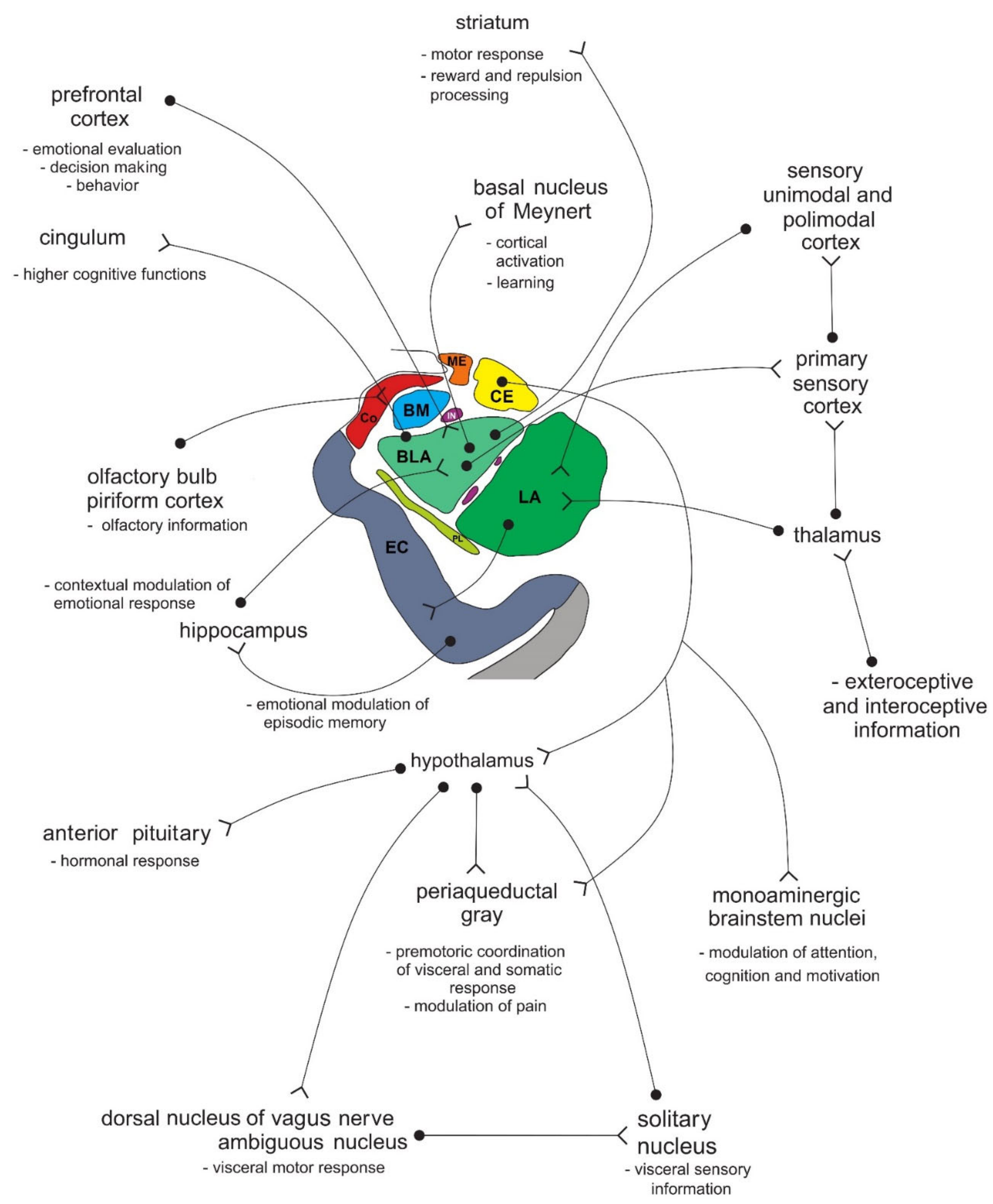 Figure 2. Simplified schematic representation of the connections of individual amygdala nuclei with numerous cortical and subcortical structures, and their role in processing functionally different types of information. Amygdala nuclei are marked in colors as shown in Figure 1. BLA—basolateral (basal) nucleus; BM—basomedial (accessory basal) nucleus; CE—central nucleus; Co—cortical nucleus; EC—entorhinal cortex; IN—intercalated neurons; ME—medial nucleus; LA—lateral nucleus; PL—paralaminar nucleus. See text for details.
Figure 2. Simplified schematic representation of the connections of individual amygdala nuclei with numerous cortical and subcortical structures, and their role in processing functionally different types of information. Amygdala nuclei are marked in colors as shown in Figure 1. BLA—basolateral (basal) nucleus; BM—basomedial (accessory basal) nucleus; CE—central nucleus; Co—cortical nucleus; EC—entorhinal cortex; IN—intercalated neurons; ME—medial nucleus; LA—lateral nucleus; PL—paralaminar nucleus. See text for details.2. The Role of the Amygdala in Sensation Seeking, Psychosis, Major Depression and Other Psychiatric Disorders
Distinct morphological and functional features of the amygdala have been reported across psychiatric disorders. The amygdala plays a key role in both emotional processing and stress response; alterations in amygdala neural activation on emotional tasks were reported in patients with disorders associated with stress and disturbed emotional perception, such as affective disorders. However, amygdala reactivity on specific cues was not uniform across the affective disorders spectrum, given the different amygdala activation patterns during emotion processing in unipolar depression and bipolar disorder. Of note, the majority of fMRI studies showed greater amygdala activation on negative emotional stimuli in unipolar depression than in bipolar disorder, while the opposite was reported for positive stimuli [6]. While increased amygdala activation was observed in patients with bipolar disorder across all illness phases, similar findings were also observed during attention tasks that had no emotional components, suggesting the additional role of the amygdala in cognition [7]. A recent meta-analysis reported smaller amygdala volumes in participants with major depressive disorder (MDD) compared to healthy controls, although greater differences between groups were observed for hippocampal volume [8]. Interestingly, amygdala volumes in bipolar patients did not differ from healthy controls [9].
Negative emotions that are induced by telling a subject that a painful stimulation will be delivered shortly may result in either amplification of pain if a mild pain stimulus is delivered (hyperalgesia) or in the perception of pain when a tactile stimulus is applied (allodynia) [10]. In other words, anxiety about pain activates brain circuits that may increase or decrease the feeling of pain. Using this paradigm, neuroimaging studies in patients with MDD compared with healthy controls showed significantly lateralized perception of pain in depressed patients, as thermal pain tolerance and electrical pain tolerance were significantly increased on the right hand side [11], and impaired ability to modulate pain experience in MDD patients, due to increased emotional reactivity during the anticipation of pain. Subjects with MDD compared with healthy controls showed increased activation in the right anterior insula, dorsal part of the ACC, and right amygdala during anticipation of painful, relative to nonpainful, stimuli, increased activation in the right amygdala and decreased activation in the PAG, rostral ACC and PFC during painful stimulation relative to nonpainful stimulation, and greater activation in the right amygdala during anticipation of pain, which was associated with greater levels of perceived helplessness [12].
A recent metaanalysis comprising 1141 patients and 1242 healthy controls in 54 studies showed that both young and adult patients with MDD showed abnormal neural activities in the ACC, insula, superior and middle temporal gyrus, and occipital cortex during emotional processing. However, hyperactivities in the superior and mid frontal gyrus, amygdala, and hippocampus were observed only in adult patients, while hyperactivity in the striatum was only found in young patients compared to the controls [13]. Apart from the fact that both young and adult patients with MDD have the negative processing bias during emotional processing, these findings suggest that adult patients with MDD are more subject to impaired appraisal and emotional reactivity, while young patients with MDD are more prone to an impaired perception process [13]. After comparing 313 MDD patients with 283 healthy controls, another metaanalysis of the resting-state functional activity in medication-naïve patients with their first episode of MDD revealed that MDD patients had significant and robust resting-state hyperactivity, mainly in the left amygdala and the left hippocampus [14]. These results confirmed the earlier notion that the left hyperactive amygdala in depression affects both the onset and maintenance of emotional dysfunction by eliciting dysfunctional negative biases at automatic stages of affective information processing [15].
Real-time fMRI coupled with neurofeedback allows a person to see and regulate the localized hemodynamic signal from his or her own brain. Using this method, an applied neurofeedback training was given to healthy and depressed individuals with the amygdala as the neurofeedback target to increase the hemodynamic response during positive autobiographical memory recall. The initial results of this approach are encouraging and suggest its clinical potential in alleviating symptoms of depression [16], especially stress-induced depression [17].
In sharp contrast to MDD, patients with schizophrenia, even in the early phase, had smaller amygdala volumes relative to both healthy groups and bipolar patients [9]. Patients with schizophrenia had also decreased structural connectivity between the amygdala and orbitofrontal cortex and abnormal resting-state functional connectivity with the medial prefrontal cortices [9]. Such findings may be related to specific symptoms of schizophrenia. For example, increased amygdala activity may have a role in distress and the perception of threat, related to auditory hallucinations [18]. There are also important differences in the nature of motivational deficits associated with psychosis vs. depression. Namely, depressive individuals, particularly those who experience anhedonia, have the presence of impairments in in-the-moment hedonics (“liking”), and such deficits may propagate forward to impairments in other constructs that are dependent on reward responses, such as anticipation, learning, effort, and action selection, which could reflect alterations in dopaminergic and opioid signaling in the striatum related to depression or specifically to anhedonia in depressed people [19]. In contrast, there is relatively intact in-the-moment hedonic processing in psychosis, but there are impairments in other components involved in translating reward to action selection. In particular, psychotic individuals exhibit altered reward prediction and associated striatal and prefrontal activation, impaired reward learning, and impaired reward-modulated action selection [19].
Individuals with sensation-seeking traits have generally higher thresholds for threat detection, which may arise from amygdala—inferior frontal gyrus interaction. Inferior frontal gyrus suppresses amygdala activity, resulting in feeling less fear, which may result in reckless behavior of drug abuse [20]. Sensation seeking is associated with an initial blunted amygdala response [21], which may result in pursuing more stimulating rewards, using risky and reckless behavior. Sensation (novelty) seeking is defined as the motivation to seek out novel, complex, and arousing experiences and is one of the three main independent dimensions of temperament (the other two being reward dependence and harm avoidance) and one of the four main independent dimentions of impulsivity (the other three being lack of premeditation, lack of persistence, and urgency) [22]. Impulsivity is considered a major endophenotype associated with disorders of behavioral control, such as substance use and pathological gambling, as well as co-morbid neuropsychiatric disorders, such as bipolar disorder and borderline personality disorder [23].
Adolescents endorse greater sensation- and novelty-seeking motivation and reduced behavioral markers of anxiety than adults (with the peak of sensation seeking coming and going earlier in females than in males). From an evolutionary perspective, orientation toward novelty seeking and risky actions could represent an advantageous mode of interacting with the environment during adolescence, given the heightened demands on adolescents to find novel territories, mates, and resources [24]. Sensation seeking is closely related to the extent to which adolescents utilize emotionally relevant information in decision-making, e.g., concerning the gain and loss of territories, mates, and resources.
Using the Iowa Gambling Task to quantify approach vs. avoidance-based decision-making in children, adolescents, and young adults, Cauffman and colleagues (2010) [25] found that levels of approach toward potential reward took on a curvilinear function, with the maximal sensitivity to positive feedback and risky choices (including risky [unprotected] sexual behavior) occurring during the adolescent years (peaks in late adolescence around ages 18–20; in contrast, use of negative feedback to avoid negative outcomes strengthen with age in a linear manner, not showing full maturity until the adult years). This age trend of sensation seeking has been replicated across many cultures [26][27] and confirms the conventional wisdom saying that people become more cautious and conservative with age. However, adolescents do not reveal these tendencies in all situations, but only in the arousing, thrilling contexts [28][29], when they tend to disregard information about the odds of gain and loss and report greater reliance on “gut-level” and “excitement” cues to shape their choices, ultimately impairing their performance. The social context has been shown also to propel adolescents’ decision-making in the direction of risk. Adolescents are more likely to make dangerous moves while driving in the presence of peers [30] and are more prone to deviant behavior when with others than when alone [31]. It still needs to be clarified which of the proposed potential mechanisms predominantly underlie peer influence: enhanced desire to impress, peers introducing a “cognitive load”, the capacity for peers to shift orientation toward reward, or heightened physiological and emotional arousal in the context of peer evaluation [32].
There is substantial evidence that some alleles in the dopaminergic system (such as those for COMT, DAT1, MAOA, and genes for dopamine receptors, especially DRD4 and DRD2) and the serotonin-transporter-linked polymorphic region (5-HTTLPR) gene variants are related to executive attention, temperament, attachment, psychosis risk, and sensation seeking [33][34]. One of these genes, the gene for the dopamine receptor 4 (DRD4) in chromosome 11, was found to influence sensation-seeking behavior as early as 18–20 months in interaction with the quality of parenting [35]: when the 7-repeat allele was present, relatively low-quality parenting produced higher sensation-seeking ratings, but when the 7-repeat allele was absent, sensation seeking was moderate and low, regardless of parenting quality. This finding of the susceptibility of children and adults with the 7-repeat allele to parental and other environmental influences has been replicated many times [36][37][38], supporting the view that reward processing in appetitive motivation has an important role in sensation seeking. Besides sensation seeking in toddlers when combined with poor parenting, DRD4 gene polymorphisms have been associated with several other phenotypes, including an increased risk of attention deficit hyperactivity disorder (ADHD), impulsivity, and lower levels of response inhibition [35][39].
On a number of occasions, patient S. M. reported a high level of excitement and enthusiasm while riding a rollercoaster and also wanted to try skydiving [40]. While these observations suggest a high level of “sensation seeking”, in everyday life S.M. rarely engaged in purposeful risk-taking behavior, perhaps due to her inability to afford such activities [40].
Altogether, these results suggest that damage of the amygdala causes behavioral disinhibition that may interact with unemotional traits in a number of ways. Low levels of fear may result in unresponsiveness to parental discipline, ambivalence about parental or peer disproval, and low levels of anxiety in response to one’s own misbehavior [41]. These factors conceivably combine to produce a child who is unafraid of being disciplined, unmotivated to behave appropriately, and unable to feel remorse for his or her misbehavior. Therefore, disinhibition may represent a risk factor for reactive aggression as well as for sensation seeking and a lack of empathy and remorse. Reactive aggression and psychopathology both implicate hypoactivity of both the amygdala and OFC [42][43].
References
- Swanson, L.W.; Petrovich, G.D. What is the amygdala? Trends Neurosci. 1998, 21, 323–331.
- Heimer, L.; De Olmos, J.; Alheid, G.; Pearson, J.; Sakamoto, N.; Shinoda, K.; Marksteiner, J.; Switzer, R. The human basal forebrain. Part II. In Handbook of Chemical Neuroanatomy; Elsevier: Amsterdam, The Netherlands, 1999; pp. 57–226.
- Amaral, D.G.; Price, J.L.; Pitkänen, A.; Carmichael, S.T. Anatomical organization of the primate amygdaloid complex. In The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction; Aggleton, J.P., Ed.; Wiley-Liss: New York, NY, USA, 1992; pp. 1–66.
- Price, J.L.; Russchen, F.T.; Amaral, D.G. The limbic region: II. The amygdaloid complex. In Handbook of Chemical Neuroanatomy; Vol. Integrated Systems of the CNS (Part, I); Bjorklund, A., Hokfelt, T., Swanson, L.W., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 279–388.
- Gloor, P. The amygdaloid system. In The Temporal Lobe and Limbic System; Gloor, P., Ed.; Oxford University Press: New York, NY, USA, 1997; pp. 591–721.
- Han, K.-M.; De Berardis, D.; Fornaro, M.; Kim, Y.-K. Differentiating between bipolar and unipolar depression in functional and structural MRI studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 91, 20–27.
- Sepede, G.; Spano, M.C.; Lorusso, M.; De Berardis, D.; Salerno, R.M.; Di Giannantonio, M.; Gambi, F. Sustained attention in psychosis: Neuroimaging findings. World J. Radiol. 2014, 6, 261–273.
- Nolan, M.; Roman, E.; Nasa, A.; Levins, K.J.; O’Hanlon, E.; O’Keane, V.; Roddy, D.W. Hippocampal and Amygdalar Volume Changes in Major Depressive Disorder: A Targeted Review and Focus on Stress. Chronic Stress 2020, 4, 1–19.
- Ho, N.F.; Chong, P.L.H.; Lee, D.R.; Chew, Q.H.; Chen, G.; Sim, K. The Amygdala in Schizophrenia and Bipolar Disorder: A Synthesis of Structural MRI, Diffusion Tensor Imaging, and Resting-State Functional Connectivity Findings. Harv. Rev. Psychiatry 2019, 27, 150–164.
- Colloca, L.; Sigaudo, M.; Benedetti, F. The role of learning in nocebo and placebo effects. Pain 2008, 136, 211–218.
- Bär, K.-J.; Brehm, S.; Boettger, M.; Boettger, S.; Wagner, G.; Sauer, H. Pain perception in major depression depends on pain modality. Pain 2005, 117, 97–103.
- Strigo, I.A.; Simmons, A.N.; Matthews, S.C.; Craig, A.D. (Bud); Paulus, M.P. Association of Major Depressive Disorder With Altered Functional Brain Response During Anticipation and Processing of Heat Pain. Arch. Gen. Psychiatry 2008, 65, 1275–1284.
- Li, X.; Wang, J. Abnormal neural activities in adults and youths with major depressive disorder during emotional processing: A meta-analysis. Brain Imaging Behav. 2021, 15, 1134–1154.
- Ma, X.; Liu, J.; Liu, T.; Ma, L.; Wang, W.; Shi, S.; Wang, Y.; Gong, Q.; Wang, M. Altered Resting-State Functional Activity in Medication-Naive Patients With First-Episode Major Depression Disorder vs. Healthy Control: A Quantitative Meta-Analysis. Front. Behav. Neurosci. 2019, 13, 89.
- Dannlowski, U.; Ohrmann, P.; Bauer, J.; Kugel, H.; Arolt, V.; Heindel, W.; Suslow, T. Amygdala reactivity predicts automatic negative evaluations for facial emotions. Psychiatry Res. Neuroimaging 2007, 154, 13–20.
- Young, K.D.; Zotev, V.; Phillips, R.; Misaki, M.; Drevets, W.C.; Bodurka, J. Amygdala real-time functional magnetic resonance imaging neurofeedback for major depressive disorder: A review. Psychiatry Clin. Neurosci. 2018, 72, 466–481.
- Lee, E.-H.; Han, P.-L. Reciprocal interactions across and within multiple levels of monoamine and cortico-limbic systems in stress-induced depression: A systematic review. Neurosci. Biobehav. Rev. 2019, 101, 13–31.
- Larøi, F.; Thomas, N.; Aleman, A.; Fernyhough, C.; Wilkinson, S.; Deamer, F.; McCarthy-Jones, S. The ice in voices: Under-standing negative content in auditory-verbal hallucinations. Clin. Psychol. Rev. 2019, 67, 1–10.
- Barch, D.M.; Pagliaco, D.; Luking, K. Mechanisms underlying motivational deficits in psychopathology: Similarities and differences in depression and schizophrenia. Curr. Top. Behav. Neurosci. 2016, 27, 411–449.
- Mujica-Parodi, L.R.; Cha, J.; Gao, J. From Anxious to Reckless: A Control Systems Approach Unifies Prefrontal-Limbic Regulation Across the Spectrum of Threat Detection. Front. Syst. Neurosci. 2017, 11, 18.
- Tapia León, I.; Kruse, O.; Stark, R.; Klucken, T. Relationship of sensation seeking with the neural correlates of appetitive con-ditioning. Soc. Cogn. Affect. Neurosci. 2019, 14, 769–775.
- Congdon, E.; Canli, T. The Endophenotype of Impulsivity: Reaching Consilience Through Behavioral, Genetic, and Neuroimaging Approaches. Behav. Cogn. Neurosci. Rev. 2005, 4, 262–281.
- Weiland, B.J.; Heitzeg, M.M.; Zald, D.; Cummiford, C.; Love, T.; Zucker, R.A.; Zubieta, J.-K. Relationship between impulsivity, prefrontal anticipatory activation, and striatal dopamine release during rewarded task performance. Psychiatry Res. Neuroimaging 2014, 223, 244–252.
- Ellis, B.J.; Del Giudice, M.; Dishion, T.J.; Figueredo, A.J.; Gray, P.B.; Griskevicius, V.; Hawley, P.H.; Jacobs, W.J.; James, J.; Volk, A.A.; et al. The evolutionary basis of risky adolescent behavior: Implications for science, policy, and practice. Dev. Psychol. 2012, 48, 598–623.
- Cauffman, E.; Shulman, E.P.; Steinberg, L.; Claus, E.; Banich, M.T.; Graham, S.; Woolard, J. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Dev. Psychol. 2010, 46, 193–207.
- Chan, W.; McCrae, R.R.; De Fruyt, F.; Jussim, L.; Löckenhoff, C.E.; De Bolle, M.; Costa, P.T.; Sutin, A.R.; Realo, A.; Allik, J.; et al. Stereotypes of age differences in personality traits: Universal and accurate? J. Person. Soc. Psychol. 2012, 103, 1050–1066.
- Steinberg, L.; Albert, D.; Cauffman, E.; Banich, M.; Graham, S.; Woolard, J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Dev. Psychol. 2008, 44, 1764–1778.
- Figner, B.; Mackinlay, R.J.; Wilkening, F.; Weber, E.U. Affective and deliberative processes in risky choice: Age differences in risk taking in the Columbia Card Task. J. Exp. Psychol. Learn. Mem. Cogn. 2009, 35, 709–730.
- Casey, B.J.; Caudle, K. The teenage brain: Self control. Curr. Dir. Psychol. Sci. 2013, 22, 82–87.
- Simons-Morton, B.; Lerner, N.; Singer, J. The observed effects of teenage passengers on the risky driving behavior of teenage drivers. Accid. Anal. Prev. 2005, 37, 973–982.
- Zimring, F.E. American Youth Violence; NYU Press: New York, NY, USA, 2014; pp. 7–36.
- Sommerville, L.H. Emotional Development in Adolescence. In Handbook of Emotions, 4th ed.; Feldman Barrett, L., Lewis, M., Haviland-Jones, J.M., Eds.; The Guilford Press: New York, NY, USA, 2016; pp. 350–365.
- Posner, M.I.; Rothbart, M.K.; Sheese, B.E.; Voelker, P. Control networks and neuromodulators of early development. Dev. Psychol. 2012, 48, 827–835.
- Gothelf, R.; Law, A.J.; Frisch, A.; Chen, J.; Zarchi, O.; Michaelovsky, E.; Ren-Patterson, R.; Lipska, B.K.; Carmel, M.; Kolachana, B.; et al. Biological Effects of COMT Haplotypes and Psychosis Risk in 22q11.2 Deletion Syndrome. Biol. Psychiatry 2014, 75, 406–413.
- Sheese, B.E.; Voelker, P.M.; Rothbart, M.K.; Posner, M.I. Parenting quality interacts with genetic variation in dopamine receptor DRD4 to influence temperament in early childhood. Dev. Psychopathol. 2007, 19, 1039–1046.
- Belsky, J.; Pluess, M. Beyond diathesis stress: Differential susceptibility to environment stress. Psychol. Bull. 2009, 135, 895–908.
- Sheese, B.E.; Rothbart, M.K.; Voelker, P.M.; Posner, M.I. The Dopamine Receptor D4 Gene 7-Repeat Allele Interacts with Parenting Quality to Predict Effortful Control in Four-Year-Old Children. Child. Dev. Res. 2012, 2012, 1–6.
- Larsen, H.; van der Zwaluw, C.S.; Overbeek, G.; Granic, I.; Franke, B.; Engels, R.C. A variable-number-of-tandem-repeats polmorphism in the dopamine D4 receptor gene affects social adaptation of alcohol use: Investigation of a gene—environment interaction. Psychol. Sci. 2010, 21, 1064–1068.
- Holmboe, K.; Nemoda, Z.; Fearon, R.M.P.; Csibra, G.; Sasvari-Szekely, M.; Johnson, M.H. Polymorphisms in dopamine system genes are associated with individual differences in attention in infancy. Dev. Psychol. 2010, 46, 404–416.
- Amaral, D.G.; Adolphs, R. (Eds.) Living without an Amygdala; The Guilford Press: New York, NY, USA, 2016; p. 12.
- Frick, P.J.; Barry, C.T.; Bodin, S.D. Applying the concept of psychopathy to children: Implication for the assessment of antisocial youth. In The Clinical and Forensic Assessment of Psychopathy; Gacono, C.B., Ed.; Erlbaum: Mahway, NJ, USA, 2000; pp. 3–25.
- Davidson, R.J.; Putnam, K.M.; Larson, C.L. Dysfunction in the neural circuitry of emotion regulation—A possible prelude to violence. Science 2000, 289, 591–594.
- Blair, R.J.R. Neurological basis of psychopathy. Br. J. Psychiatry 2003, 182, 5–7.
More
Information
Subjects:
Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
5.7K
Revisions:
2 times
(View History)
Update Date:
13 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




