Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carla Moura | + 1340 word(s) | 1340 | 2021-07-02 11:45:33 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Moura, C. Temporomandibular Joint Disc Disorders. Encyclopedia. Available online: https://encyclopedia.pub/entry/11982 (accessed on 07 February 2026).
Moura C. Temporomandibular Joint Disc Disorders. Encyclopedia. Available at: https://encyclopedia.pub/entry/11982. Accessed February 07, 2026.
Moura, Carla. "Temporomandibular Joint Disc Disorders" Encyclopedia, https://encyclopedia.pub/entry/11982 (accessed February 07, 2026).
Moura, C. (2021, July 12). Temporomandibular Joint Disc Disorders. In Encyclopedia. https://encyclopedia.pub/entry/11982
Moura, Carla. "Temporomandibular Joint Disc Disorders." Encyclopedia. Web. 12 July, 2021.
Copy Citation
Temporomandibular joint disorder (TMD) is a type of musculoskeletal pain that affects the orofacial region, like masticatory muscles, temporomandibular joint and other surrounding structures. Chronic musculoskeletal pain refers to a persistent pain, felt for more than 3 months, arising in bones, joints, and tissues. A subset of disc-related TMD involves disc displacement, disc thinning and perforation. Biomechanical unbalance or extreme loading can also lead to damage in the articular disc.
temporomandibular joint disc
fibrocartilage
disc dysfunctions
tissue engineering
decellularization
1. Introduction
The temporomandibular joint (TMJ) is a synovial joint between the temporal bone and the mandibular condyle, located bilaterally in the face. This joint is composed of bony articular surfaces, articular disc, fibrous capsule and synovial membrane, ligaments and muscles. It is responsible for basic functions, such as talking, chewing, swallowing, eating, yawning, smiling, laughing, screaming and kissing [1]. The articular disc is a crucial element present in the TMJ, as it softens and absorbs shocks between the articular structures. It separates the joint cavity in the upper and lower compartments, and it is surrounded by attachments that sustain its position [2].
This joint is surrounded by a synovial capsule, whose main function is to produce synovial fluid. This fluid plays a major role in joint lubrication and acts as a medium for nutrient and waste exchanges. As part of the joint, the disc is an avascular structure that relies heavily on nutrients and oxygen from the synovial fluid to survive [3]. Lubrication plays an essential role in the rotational and translational movements of the TMJ. These two types of movements take place between the condyle and the articular disc and between the mandibular fossa and the articular disc, respectively [4], it is therefore considered a ginglymoarthrodial joint [5].
Temporomandibular joint disorder (TMD) is a type of musculoskeletal pain that affects the orofacial region, like masticatory muscles, temporomandibular joint and other surrounding structures [6]. Chronic musculoskeletal pain refers to a persistent pain, felt for more than 3 months, arising in bones, joints, and tissues [7]. Statistics for 2002 indicate that, in Europe, about 95 million adults report having musculoskeletal pain associated with arthritis or rheumatism, corresponding to over 3 million Portuguese [8].
Symptoms of TMD include headache, neuralgia, pain and discomfort, clicking sounds and muscle spasms [9][10], affecting patients’ quality of life and daily/work functions [11]. TMD is considered the second most common musculoskeletal disorder affecting the general population in 5–12% [12].
In a study conducted by AlShaban and Gul Abdul Waheed (2018), 41 patients out of 100 revealed the presence of TMD. Of this percentage, the clicking sound appeared as the symptom with the highest prevalence, affecting 89% of the patients. Of these 89% patients, clicking sound was 32% from the right side, 24% from the left side, 32% on both sides and the remaining 12% were absent [13].
Just as disorders affect the TMJ and surrounding tissue, clinical options also solve problems in these tissues, including the disc. These treatments can vary according to the stage and severity. They can be classified into three categories: (i) non-invasive; (ii) minimally invasive; or (iii) invasive. Non-invasive procedures are the first option of therapy for TMD patients and include medications, such as anti-inflammatory drugs and muscle relaxants, physical therapy and acupuncture. Minimally invasive treatments can be divided into intra-articular injections, which involve the injection of medications and/or sodium hyaluronate. Arthrocentesis and arthroscopy are also in this category and are used to lubricate the joint and eventually reposition the articular disc. Invasive treatments are the last option to be performed and include open joint surgery, where there can be a discopexy (disc repositioning), discectomy (total removal of the articular disc), condylectomy (excision of the condyle), or a total joint replacement with compatible materials [14][15][16][17][18]. Recent strategies have improved the surgical cosmesis of the open surgery incision [19][20].
Regarding TMJ disc clinical possibilities, minimally invasive procedures do not restore damaged disc, and discectomy leads to condylar remodeling [21]. Considering this, the demand for a possible material to replace the disc has been investigated. The first materials used for TMJ disc replacement after discectomy were Silicone rubber and Proplast-Teflon [22][23]. After that, the field of Tissue Engineering (TE) became active to find a solution for disc pathologies. Through the use of natural or synthetic materials, it is possible to produce bioengineered scaffolds for the repair of the entire or only a portion of the disc [24]. Despite the search for a promising bioengineered scaffold-based TE strategy, the need for a successful TMJ disc remains. The use of decellularized tissue could be a potential substitute to synthetic materials since its three-dimensional architecture and biological composition are the same as the native one [25].
2. Forefront Approaches for Temporomandibular Disc Replacement: Native Decellularized Extracellular Matrices
Decellularized tissues are a well-known matter in the biomedical field and by analysing Figure 1, it is possible to observe that this area is being increasingly explored and evolving over the years.
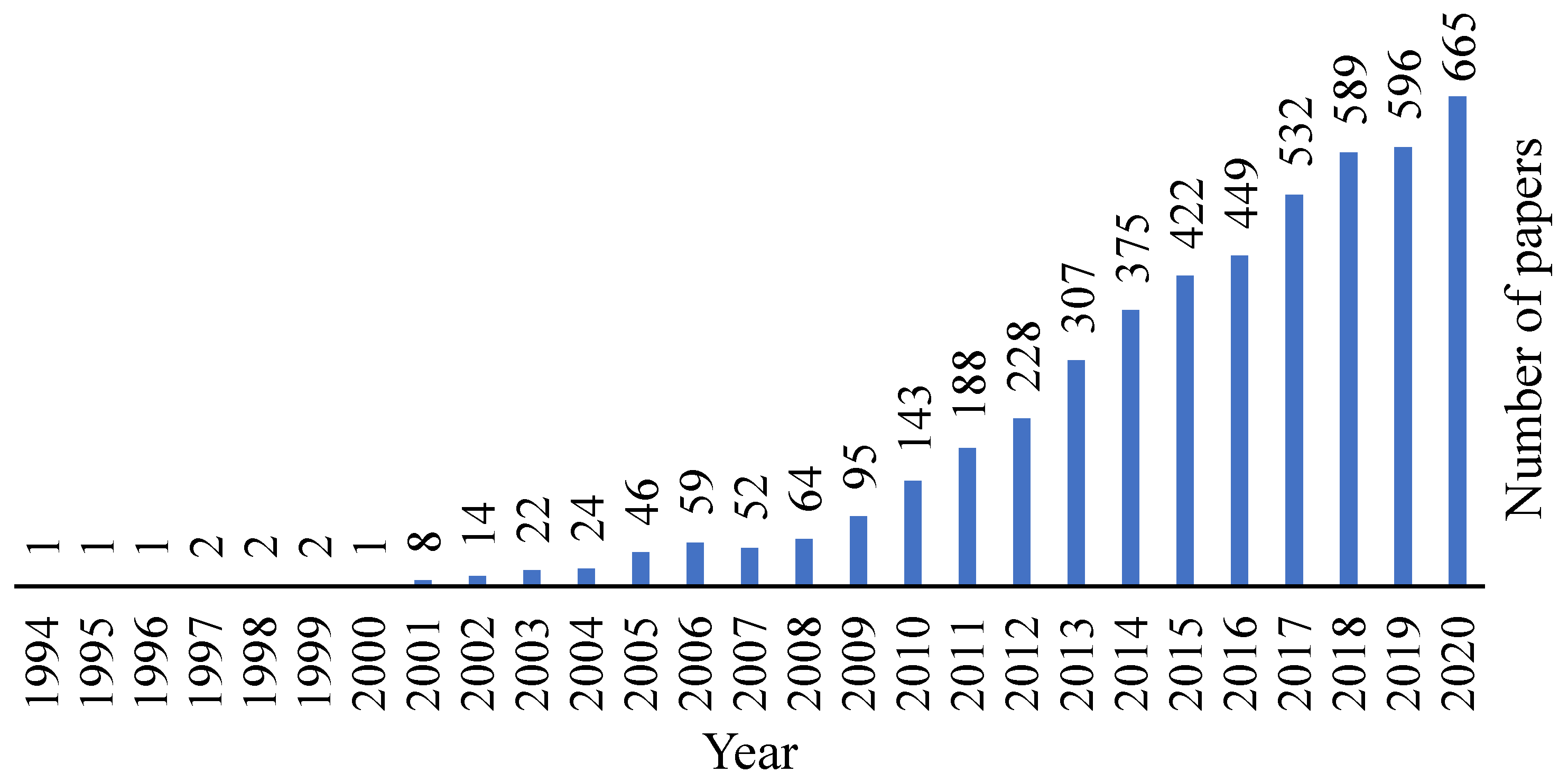 Figure 1. Evolution of the decellularization area over the years. Retrieved from PubMed.org with the research designation “decellularization”, where 4140 results were found.
Figure 1. Evolution of the decellularization area over the years. Retrieved from PubMed.org with the research designation “decellularization”, where 4140 results were found.Cartilage has a low regeneration capacity and therefore, different substitutes have been the focus of research in order to repair cartilage defects. Cartilage matrix can be collected from different sources, but access to allogeneic or autologous donor tissue is restricted, so the interest in using xenogeneic tissues for cartilage constructions has been increasing, where the TE field can offer a positive alternative [26][27]. Regardless, for these tissue types, decellularization and sterilization methods are required with the aim of removing the immunogenic components that lead to infection and disease transmission [26][28].
The decellularized extracellular matrix (dECM) has immense potential to serve as a beneficial material for tissue damage repair as it preserves the native environment by providing cells with the necessary elements, such as support and biochemical components, that are needed to provide their proliferation and differentiation. Extracellular matrix (ECM) organization and compounds differ from tissue to tissue [29], but in terms of cartilage, the two major components are collagen and proteoglycans, which include bioactive factors, such as growth factors, integrins, and functional peptides. The main benefits of using dECM are related to its ability to preserve native tissue growth factors (e.g., transforming growth factor beta (TGF-β), fibroblast growth factor (FGF) and insulin-like growth factor (IGF) for cartilage tissue), unlimited access to obtain ECM and the relationship between cost and effectiveness [27]. Still, there are problems associated with it and that may create undesired responses. The remaining cell contents, heterogeneous cell distribution, and the difficulty of obtaining an intact ECM are some of the cautions to pay attention to [30].
Choosing the right animal model for any given tissue is a critical step, and the decellularization method depends on the tissue choice [28]. Decellularization methods can be divided into (i) chemical agents, such as acids and bases, detergents, hypotonic and hypertonic solutions, and solvents, (ii) biological agents, such as enzymes and chelating agents; and (iii) physical agents, such as freeze-thaw, force and pressure, electroporation and sonication [25][31].
The aim of decellularization (Figure 2) is to preserve the organic and mechanical properties, such as the architecture of the collagen network of the tissue, as the immunogenic components are removed to allow cell adhesion and proliferation. After obtaining the xenogeneic scaffold, there are two possible ways of application: direct implantation or cell culture in the decellularized scaffold [26][28].
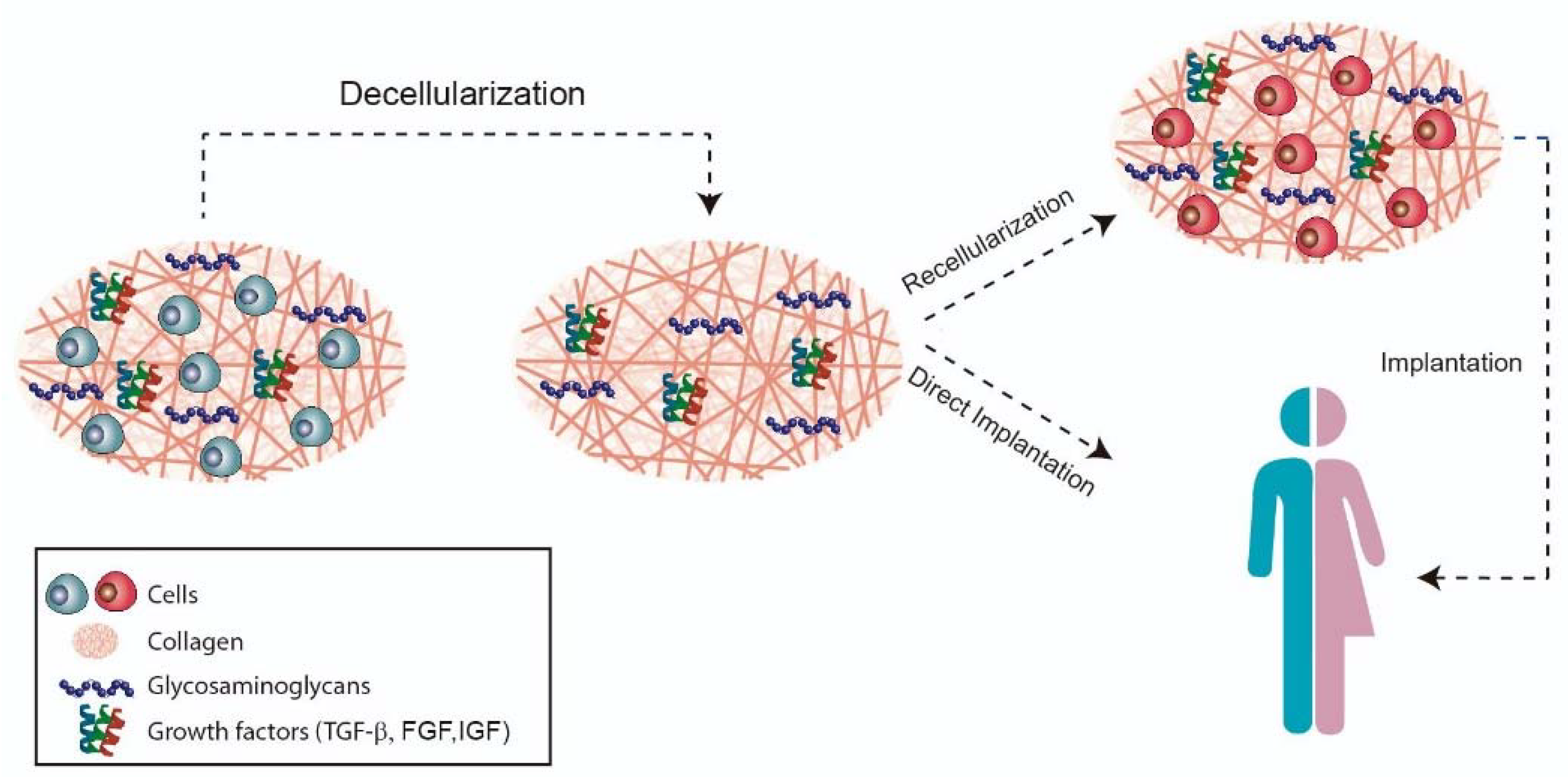 Figure 2. Decellularization strategy.
Figure 2. Decellularization strategy.3. Conclusion and Future Strategies
The temporomandibular disc is a complex structure, with specific collagen and GAG distribution. It is a fibrocartilaginous disc with no vascularization and remodeling capacities. This combined with its dynamic properties in the normal function of the TMJ, makes this tissue to be highly predisposed to suffer pathologies.
The TE field has actively contributed to the possibility of bringing new outcomes to the search for a new and long-lasting tissue that effectively substitute/regenerates the disc. Still, the production of a suitable material that best imitates the native properties of the disc, such as mechanical, physical, and biological, with no negative reactions, has not been achieved.
Decellularized xenogeneic tissues may be the next step in the development of a native-equivalent disc. Since, in a simplified way, it is possible to obtain the necessary characteristics (physical and biochemical) to successfully obtain one engineered-disc for the treatment of disc pathologies.
References
- Riera-Punet, N.; Martinez-Gomis, J.; Willaert, E.; Povedano, M.; Peraire, M. Functional limitation of the masticatory system in patients with bulbar involvement in amyotrophic lateral sclerosis. J. Oral. Rehabil. 2017, 45, 204–210.
- Hargitai, I.A.; Hawkins, J.M.; Ehrlich, A.D. The Temporomandibular Joint. In Temporomandibular Disorders; Gremillion, H.A., Klasse, G.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 91–107.
- Willard, V.P.; Zhang, L.G.; Athanasiou, K.A. Tissue Engineering of the Temporomandibular Joint. In Comprehensive Biomaterials; Ducheyne, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 221–235.
- Demerjian, G.G.; Barkhordarian, A.; Chiappelli, F. Neuroanatomy of the Trigeminal Nerve and Proximal Innervation of the TMJ. In Temporomandibular Joint and Airway Disorders; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–15.
- Donahue, R.P.; Hu, J.C.; Athanasiou, K.A. Remaining Hurdles for Tissue-Engineering the Temporomandibular Joint Disc. Trends Mol. Med. 2019, 25, 241–256.
- Fernandes, G.; Gonçalves, D.; Conti, P. Musculoskeletal Disorders. Dent. Clin. North Am. 2018, 62, 553–564.
- Booth, J.; Moseley, G.L.; Schiltenwolf, M.; Cashin, A.; Davies, M.; Hübscher, M. Exercise for chronic musculoskeletal pain: A biopsychosocial approach. Musculoskelet. Care 2017, 15, 413–421.
- Cimmino, M.A.; Ferrone, C.; Cutolo, M. Epidemiology of chronic musculoskeletal pain. Best Pr. Res. Clin. Rheumatol. 2011, 25, 173–183.
- Sims, A.B.; Demerjian, G.G. Temporomandibular Joint Dysfunction, Trigeminal Nerve Inflammation, and Biomechanical Dental Treatments for the Suppression of Neurological and Neuropsychiatric Symptoms. In Temporomandibular Joint and Airway Disorders; Springer: Berlin/Heidelberg, Germany, 2018; pp. 95–123.
- Manfredini, D.; Guarda-Nardini, L.; Winocur, E.; Piccotti, F.; Ahlberg, J.; Lobbezoo, F. Research diagnostic criteria for temporomandibular disorders: A systematic review of axis I epidemiologic findings. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2011, 112, 453–462.
- Armijo-Olivo, S.L.; Gadotti, I.C. Temporomandibular Disorders. In Pathology and Intervention in Musculoskeletal Rehabilitation; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015; Volume 3, pp. 119–156.
- National Institute of Dental and Craniofacial Research. Facial Pain; National Institute of Dental and Craniofacial Research: Bethesda, MD, USA, 2018.
- Alshaban, K.K.; Waheed, Z.G.A. Prevalence of TMJ Disorders among the Patients Attending the Dental Clinic of Ajman University of Science and Technology–Fujairah Campus, UAE. Int. J. Dent. 2018, 2018, 1–6.
- Van Bellinghen, X.; Idoux-Gillet, Y.; Pugliano, M.; Strub, M.; Bornert, F.; Clauss, F.; Schwinté, P.; Keller, L.; Benkirane-Jessel, N.; Kuchler-Bopp, S.; et al. Temporomandibular Joint Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 446.
- Han, M.; Markiewicz, M.; Miloro, M.; Wolford, L.; Pogrel, M.A. Surgery of the Temporomandibular Joint: Discectomy and Arthroplasty. In Contemporary Management of Temporomandibular Disorders; Springer: Berlin/Heidelberg, Germany, 2019; pp. 107–127.
- Aryaei, A.; Vapniarsky, N.; Hu, J.C.; Athanasiou, K.A. Recent Tissue Engineering Advances for the Treatment of Temporomandibular Joint Disorders. Curr. Osteoporos. Rep. 2016, 14, 269–279.
- Ingawalé, S.; Goswami, T. Temporomandibular Joint: Disorders, Treatments, and Biomechanics. Ann. Biomed. Eng. 2009, 37, 976–996.
- Murphy, M.K.; MacBarb, R.F.; Wong, M.E.; Athanasiou, K.A. Temporomandibular Joint Disorders: A Review of Etiology, Clinical Management, and Tissue Engineering Strategies. Int. J. Oral Maxillofac. Implant. 2013, 28, e393.
- Ângelo, D.F. A Letter to the Editor on “Root of helix inter tragus notch incision (RHITNI) for temporomandibular open surgery. ” Int. J. Surg. 2020, 83, 233–234.
- Zhu, H.; Yang, Z.; He, D.; Hu, N.; Cheng, Z. The effect of TMJ disk repositioning by suturing through open incision on adolescent mandibular asymmetry with and without a functional orthodontic appliance. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 405–414.
- Vapniarsky, N.; Huwe, L.W.; Arzi, B.; Houghton, M.K.; Wong, M.E.; Wilson, J.W.; Hatcher, D.C.; Hu, J.C.; Athanasiou, K.A. Tissue engineering toward temporomandibular joint disc regeneration. Sci. Transl. Med. 2018, 10, eaaq1802.
- Dimitroulis, G. A critical review of interpositional grafts following temporomandibular joint discectomy with an overview of the dermis-fat graft. Int. J. Oral Maxillofac. Surg. 2011, 40, 561–568.
- Wolford, L.; Pitta, M.; Reiche-Fischel, O.; Franco, P. TMJ Concepts/Techmedica custom-made TMJ total joint prosthesis: 5-year follow-up study. Int. J. Oral Maxillofac. Surg. 2003, 32, 268–274.
- Lin, Y.; Lin, H.; Ramamoorthi, M.; Wu, D.; Zhang, Z.; Tran, S.D. Scaffolds for temporomandibular joint disc engineering. In Handbook of Tissue Engineering Scaffolds; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 437–455.
- Gupta, S.K.; Mishra, N.C.; Dhasmana, A. Decellularization Methods for Scaffold Fabrication. In Breast Cancer; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–10.
- Schwarz, S.; Koerber, L.; Elsaesser, A.F.; Goldberg-Bockhorn, E.; Seitz, A.M.; Dürselen, L.; Ignatius, A.; Walther, P.; Breiter, R.; Rotter, N. Decellularized Cartilage Matrix as a Novel Biomatrix for Cartilage Tissue-Engineering Applications. Tissue Eng. Part A 2012, 18, 2195–2209.
- Benders, K.E.; van Weeren, P.R.; Badylak, S.F.; Saris, D.B.; Dhert, W.; Malda, J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013, 31, 169–176.
- Lumpkins, S.B.; Pierre, N.; McFetridge, P.S. A mechanical evaluation of three decellularization methods in the design of a xenogeneic scaffold for tissue engineering the temporomandibular joint disc. Acta Biomater. 2008, 4, 808–816.
- Kim, Y.S.; Majid, M.; Melchiorri, A.J.; Mikos, A.G. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng. Transl. Med. 2019, 4, 83–95.
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479.
- Crapo, P.M.; Gilbert, T.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243.
More
Information
Subjects:
Orthopedics
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revision:
1 time
(View History)
Update Date:
12 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




