
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrzej Bajguz | + 2989 word(s) | 2989 | 2021-07-08 04:59:35 | | | |
| 2 | Bruce Ren | Meta information modification | 2989 | 2021-07-12 12:03:33 | | | | |
| 3 | Bruce Ren | Meta information modification | 2989 | 2021-07-14 09:44:08 | | |
Video Upload Options
Phytocannabinoids are a structurally diverse class of bioactive naturally occurring compounds found in angiosperms, fungi, and liverworts and produced in several plant organs such as the flower and glandular trichrome of Cannabis sativa, the scales in Rhododendron, and oil bodies of liverworts such as Radula species; they show a diverse role in humans and plants. Moreover, phytocannabinoids are prenylated polyketides, i.e., terpenophenolics, which are derived from isoprenoid and fatty acid precursors. Additionally, targeted productions of active phytocannabinoids have beneficial properties via the genes involved and their expression in a heterologous host. Bioactive compounds show a remarkable non-hallucinogenic biological property that is determined by the variable nature of the side chain and prenyl group defined by the enzymes involved in their biosynthesis. Phytocannabinoids possess therapeutic, antibacterial, and antimicrobial properties; thus, they are used in treating several human diseases.
1. Introduction
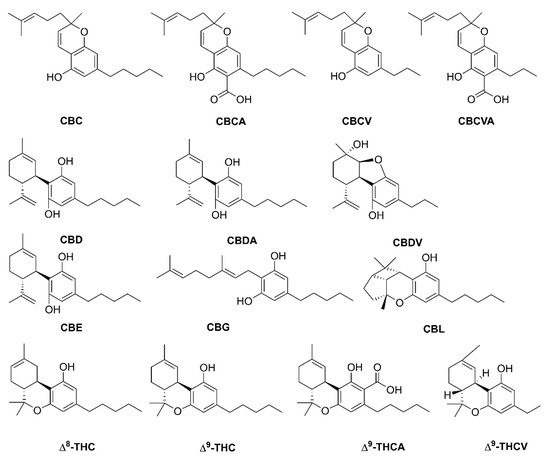
2. Phytocannabinoid Biosynthesis Sites
3. Biosynthesis of Phytocannabinoids
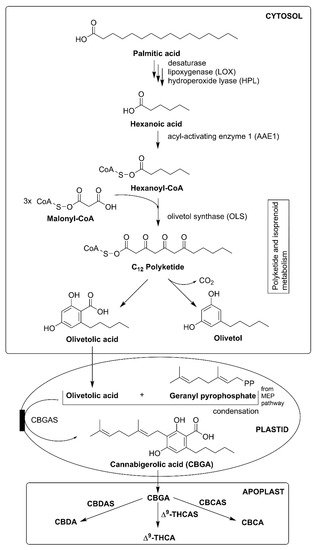
3.1. Cannabis sativa
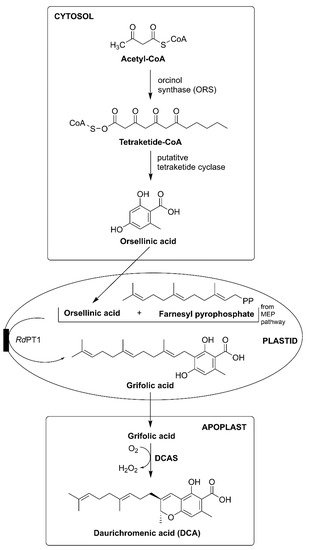
3.2. Rhododendron
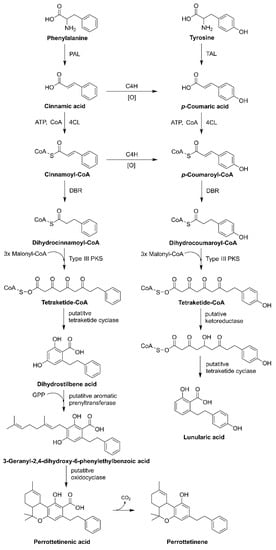
3.3. Liverworts
3.4. Application of Biotechnological Approaches to Phytocannabinoids Production in Heterologous Hosts
3.5. Production of Phytocannabinoids through Synthetic Approaches
References
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and biosynthesis. Trends Plant Sci. 2020, 25, 985–1004.
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392.
- ElSohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548.
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 14280.
- Pollastro, F.; Caprioglio, D.; Del Prete, D.; Rogati, F.; Minassi, A.; Taglialatela-Scafati, O.; Munoz, E.; Appendino, G. Cannabichromene. Nat. Prod. Commun. 2018, 13, 1189–1194.
- Happyana, N.; Kayser, O. Monitoring metabolite profiles of Cannabis sativa L. trichomes during flowering period using 1H NMR-based metabolomics and real-time PCR. Planta Med. 2016, 82, 1217–1223.
- Chanda, D.; Neumann, D.; Glatz, J.F.C. The endocannabinoid system: Overview of an emerging multi-faceted therapeutic target. Prostaglandins Leukot. Essent. Fatty Acids 2019, 140, 51–56.
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary metabolism in Cannabis. Phytochem. Rev. 2008, 7, 615–639.
- Dei Cas, M.; Casagni, E.; Casiraghi, A.; Minghetti, P.; Fornasari, D.M.M.; Ferri, F.; Arnoldi, S.; Gambaro, V.; Roda, G. Phytocannabinoids profile in medicinal cannabis oils: The impact of plant varieties and preparation methods. Front. Pharmacol. 2020, 11, 570616.
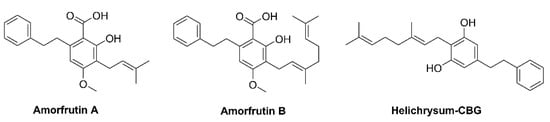
- Pollastro, F.; De Petrocellis, L.; Schiano-Moriello, A.; Chianese, G.; Heyman, H.; Appendino, G.; Taglialatela-Scafati, O. Amorfrutin-type phytocannabinoids from Helichrysum umbraculigerum. Fitoterapia 2017, 123, 13–17.
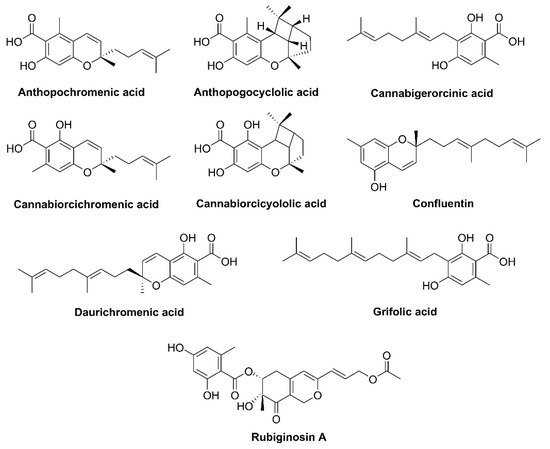
- Taura, F.; Iijima, M.; Kurosaki, F. Daurichromenic acid and grifolic acid: Phytotoxic meroterpenoids that induce cell death in cell culture of their producer Rhododendron dauricum. Plant Signal. Behav. 2018, 13, e1422463.
- Taura, F.; Iijima, M.; Lee, J.-B.; Hashimoto, T.; Asakawa, Y.; Kurosaki, F. Daurichromenic acid-producing oxidocyclase in the young leaves of Rhododendron dauricum. Nat. Prod. Commun. 2014, 9, 1329–1332.
- Kashiwada, Y.; Yamazaki, K.; Ikeshiro, Y.; Yamagishi, T.; Fujioka, T.; Mihashi, K.; Mizuki, K.; Cosentino, L.M.; Fowke, K.; Morris-Natschke, S.L.; et al. Isolation of rhododaurichromanic acid B and the anti-HIV principles rhododaurichromanic acid A and rhododaurichromenic acid from Rhododendron dauricum. Tetrahedron 2001, 57, 1559–1563.
- Rogachev, A.; Komarova, N.; Korchagina, D.; Dolgikh, M.; Sorokina, I.; Baev, D.; Tolstikova, T.; Fomenko, V.; Salakhutdinov, N. Some prenylated phenols of Rhododendron adamsii: Isolation, modification and pharmacological tests. Chem. Sustain. Dev. 2009, 17, 185–193.
- Yang, Y.-X.; Wang, J.-X.; Wang, Q.; Li, H.-L.; Tao, M.; Luo, Q.; Liu, H. New chromane and chromene meroterpenoids from flowers of Rhododendron rubiginosum Franch. var. rubiginosum. Fitoterapia 2018, 127, 396–401.
- Iwata, N.; Kitanaka, S. New cannabinoid-like chromane and chromene derivatives from Rhododendron anthopogonoides. Chem. Pharm. Bull. 2011, 59, 1409–1412.
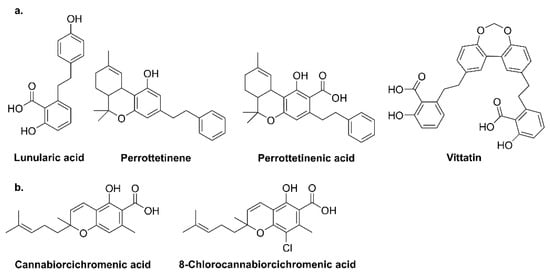
- Chicca, A.; Schafroth, M.A.; Reynoso-Moreno, I.; Erni, R.; Petrucci, V.; Carreira, E.M.; Gertsch, J. Uncovering the psychoactivity of a cannabinoid from liverworts associated with a legal high. Sci. Adv. 2018, 4, eaat2166.
- Hussain, T.; Espley, R.V.; Gertsch, J.; Whare, T.; Stehle, F.; Kayser, O. Demystifying the liverwort Radula marginata, a critical review on its taxonomy, genetics, cannabinoid phytochemistry and pharmacology. Phytochem. Rev. 2019, 18, 953–965.
- Métoyer, B.; Lebouvier, N.; Hnawia, E.; Herbette, G.; Thouvenot, L.; Asakawa, Y.; Nour, M.; Raharivelomanana, P. Chemotypes and biomarkers of seven species of new Caledonian liverworts from the Bazzanioideae subfamily. Molecules 2018, 23, 1353.
- Pryce, R.J. Lunularic acid, a common endogenous growth inhibitor of liverworts. Planta 1971, 97, 354–357.
- Quaghebeur, K.; Coosemans, J.; Toppet, S.; Compernolle, F. Cannabiorci- and 8-chlorocannabiorcichromenic acid as fungal antagonists from Cylindrocarpon olidum. Phytochemistry 1994, 37, 159–161.
- Elhendawy, M.A.; Wanas, A.S.; Radwan, M.M.; Azzaz, N.A.; Toson, E.S.; ElSohly, M.A. Chemical and biological studies of Cannabis sativa roots. Med. Cannabis Cannabinoids 2019, 1, 104–111.
- Happyana, N.; Agnolet, S.; Muntendam, R.; Van Dam, A.; Schneider, B.; Kayser, O. Analysis of cannabinoids in laser-microdissected trichomes of medicinal Cannabis sativa using LCMS and cryogenic NMR. Phytochemistry 2013, 87, 51–59.
- Livingston, S.J.; Quilichini, T.D.; Booth, J.K.; Wong, D.C.J.; Rensing, K.H.; Laflamme-Yonkman, J.; Castellarin, S.D.; Bohlmann, J.; Page, J.E.; Samuels, A.L. Cannabis glandular trichomes alter morphology and metabolite content during flower maturation. Plant J. 2019, 101, 37–56.
- Mahlberg, P.G.; Kim, E.-S. Cuticle development on glandular trichomes of Cannabis sativa (Cannabaceae). Am. J. Bot. 1991, 78, 1113–1122.
- Eichhorn Bilodeau, S.; Wu, B.-S.; Rufyikiri, A.-S.; MacPherson, S.; Lefsrud, M. An update on plant photobiology and implications for cannabis production. Front. Plant Sci. 2019, 10, 296.
- Iijima, M.; Munakata, R.; Takahashi, H.; Kenmoku, H.; Nakagawa, R.; Kodama, T.; Asakawa, Y.; Abe, I.; Yazaki, K.; Kurosaki, F.; et al. Identification and characterization of daurichromenic acid synthase active in anti-HIV biosynthesis. Plant Physiol. 2017, 174, 2213–2230.
- Asakawa, Y.; Ludwiczuk, A. Chemical constituents of bryophytes: Structures and biological activity. J. Nat. Prod. 2017, 81, 641–660.
- He, X.; Sun, Y.; Zhu, R.-L. The oil bodies of liverworts: Unique and important organelles in land plants. Crit. Rev. Plant Sci. 2013, 32, 293–302.
- Geissler, M.; Volk, J.; Stehle, F.; Kayser, O.; Warzecha, H. Subcellular localization defines modification and production of Δ9-tetrahydrocannabinolic acid synthase in transiently transformed Nicotiana benthamiana. Biotechnol. Lett. 2018, 40, 981–987.
- Luo, X.; Reiter, M.A.; d’Espaux, L.; Wong, J.; Denby, C.M.; Lechner, A.; Zhang, Y.; Grzybowski, A.T.; Harth, S.; Lin, W.; et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 2019, 567, 123–126.
- Rodziewicz, P.; Loroch, S.; Marczak, Ł.; Sickmann, A.; Kayser, O. Cannabinoid synthases and osmoprotective metabolites accumulate in the exudates of Cannabis sativa L. glandular trichomes. Plant Sci. 2019, 284, 108–116.
- Gagne, S.J.; Stout, J.M.; Liu, E.; Boubakir, Z.; Clark, S.M.; Page, J.E. Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. Proc. Natl. Acad. Sci. USA 2012, 109, 12811–12816.
- Raharjo, T.J.; Chang, W.-T.; Choi, Y.H.; Peltenburg-Looman, A.M.G.; Verpoorte, R. Olivetol as product of a polyketide synthase in Cannabis sativa L. Plant Sci. 2004, 166, 381–385.
- Laverty, K.U.; Stout, J.M.; Sullivan, M.J.; Shah, H.; Gill, N.; Holbrook, L.; Deikus, G.; Sebra, R.; Hughes, T.R.; Page, J.E.; et al. A physical and genetic map of Cannabis sativa identifies extensive rearrangements at the THC/CBD acid synthase loci. Genome Res. 2019, 29, 146–156.
- Saeki, H.; Hara, R.; Takahashi, H.; Iijima, M.; Munakata, R.; Kenmoku, H.; Fuku, K.; Sekihara, A.; Yasuno, Y.; Shinada, T.; et al. An aromatic farnesyltransferase functions in biosynthesis of the anti-HIV meroterpenoid daurichromenic acid. Plant Physiol. 2018, 178, 535–551.
- Taura, F.; Iijima, M.; Yamanaka, E.; Takahashi, H.; Kenmoku, H.; Saeki, H.; Morimoto, S.; Asakawa, Y.; Kurosaki, F.; Morita, H. A novel class of plant type III polyketide synthase involved in orsellinic acid biosynthesis from Rhododendron dauricum. Front. Plant Sci. 2016, 7, 1452.
- Katsuyama, Y.; Kita, T.; Funa, N.; Horinouchi, S. Curcuminoid biosynthesis by two type III polyketide synthases in the herb Curcuma longa. J. Biol. Chem. 2009, 284, 11160–11170.
- Hussain, T.; Plunkett, B.; Ejaz, M.; Espley, R.V.; Kayser, O. Identification of putative precursor genes for the biosynthesis of cannabinoid-like compound in Radula marginata. Front. Plant Sci. 2018, 9, 537.
- Akiyama, T.; Shibuya, M.; Liu, H.-M.; Ebizuka, Y. p-Coumaroyltriacetic acid synthase, a new homologue of chalcone synthase, from Hydrangea macrophylla var. thunbergii. Eur. J. Biochem. 1999, 263, 834–839.
- Austin, M.B.; Noel, J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 2002, 20, 79–110.
- Imoto, S.A.; Ohta, Y. Intracellular localization of lunularic acid and prelunularic acid in suspension cultured cells of Marchantia polymorpha. Plant Physiol. 1985, 79, 751–755.
- Toyota, M.; Shimamura, T.; Ishii, H.; Renner, M.; Braggins, J.; Asakawa, Y. New bibenzyl cannabinoid from the New Zealand liverwort Radula marginata. Chem. Pharm. Bull. 2002, 50, 1390–1392.
- Yang, X.; Matsui, T.; Kodama, T.; Mori, T.; Zhou, X.; Taura, F.; Noguchi, H.; Abe, I.; Morita, H. Structural basis for olivetolic acid formation by a polyketide cyclase from Cannabis sativa. FEBS J. 2016, 283, 1088–1106.
- Toyota, M.; Kinugawa, T.; Asakawa, Y. Bibenzyl cannabinoid and bisbibenzyl derivative from the liverwort Radula perrottetii. Phytochemistry 1994, 37, 859–862.
- Fuentes, P.; Armarego-Marriott, T.; Bock, R. Plastid transformation and its application in metabolic engineering. Curr. Opin. Biotechnol. 2018, 49, 10–15.
- Kodym, A.; Leeb, C.J. Back to the roots: Protocol for the photoautotrophic micropropagation of medicinal Cannabis. Plant Cell Tissue Organ Cult. 2019, 138, 399–402.
- Lange, K.; Schmid, A.; Julsing, M.K. Δ9-Tetrahydrocannabinolic acid synthase: The application of a plant secondary metabolite enzyme in biocatalytic chemical synthesis. J. Biotechnol. 2016, 233, 42–48.
- Lata, H.; Chandra, S.; Khan, I.A.; ElSohly, M.A. Micropropagation of Cannabis sativa L. -An update. In Cannabis sativa L. Botany and Biotechnology; Chandra, S., Lata, H., ElSohly, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 285–297.
- Wróbel, T.; Dreger, M.; Wielgus, K.; Słomski, R. The application of plant in vitro cultures in cannabinoid production. Biotechnol. Lett. 2017, 40, 445–454.
- Andersen-Ranberg, J.; Kongstad, K.T.; Nielsen, M.T.; Jensen, N.B.; Pateraki, I.; Bach, S.S.; Hamberger, B.; Zerbe, P.; Staerk, D.; Bohlmann, J.; et al. Expanding the landscape of diterpene structural diversity through stereochemically controlled combinatorial biosynthesis. Angew. Chem. Int. Ed. 2016, 55, 2142–2146.
- Chen, R.; Gao, B.; Liu, X.; Ruan, F.; Zhang, Y.; Lou, J.; Feng, K.; Wunsch, C.; Li, S.-M.; Dai, J.; et al. Molecular insights into the enzyme promiscuity of an aromatic prenyltransferase. Nat. Chem. Biol. 2017, 13, 226–234.
- Kannangara, R.; Siukstaite, L.; Borch-Jensen, J.; Madsen, B.; Kongstad, K.T.; Staerk, D.; Bennedsen, M.; Okkels, F.T.; Rasmussen, S.A.; Larsen, T.O.; et al. Characterization of a membrane-bound C-glucosyltransferase responsible for carminic acid biosynthesis in Dactylopius coccus Costa. Nat. Commun. 2017, 8, 1987.
- Yazaki, K.; Sasaki, K.; Tsurumaru, Y. Prenylation of aromatic compounds, a key diversification of plant secondary metabolites. Phytochemistry 2009, 70, 1739–1745.
- Ban, Z.; Qin, H.; Mitchell, A.J.; Liu, B.; Zhang, F.; Weng, J.-K.; Dixon, R.A.; Wang, G. Noncatalytic chalcone isomerase-fold proteins in Humulus lupulus are auxiliary components in prenylated flavonoid biosynthesis. Proc. Natl. Acad. Sci. USA 2018, 115, E5223–E5232.
- Gribble, G.W. Biological activity of recently discovered halogenated marine natural products. Mar. Drugs 2015, 13, 4044–4136.
- Hansen, E.H.; Osmani, S.A.; Kristensen, C.; Møller, B.L.; Hansen, J. Substrate specificities of family 1 UGTs gained by domain swapping. Phytochemistry 2009, 70, 473–482.
- Khan, B.A.; Warner, P.; Wang, H. Antibacterial properties of hemp and other natural fibre plants: A review. BioResources 2014, 9, 3642–3659.




