Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chaojie Zhu | + 1318 word(s) | 1318 | 2021-07-02 11:21:00 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhu, C. Nanotechnology-facilitated Bacterial Therapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/11880 (accessed on 07 February 2026).
Zhu C. Nanotechnology-facilitated Bacterial Therapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/11880. Accessed February 07, 2026.
Zhu, Chaojie. "Nanotechnology-facilitated Bacterial Therapy" Encyclopedia, https://encyclopedia.pub/entry/11880 (accessed February 07, 2026).
Zhu, C. (2021, July 09). Nanotechnology-facilitated Bacterial Therapy. In Encyclopedia. https://encyclopedia.pub/entry/11880
Zhu, Chaojie. "Nanotechnology-facilitated Bacterial Therapy." Encyclopedia. Web. 09 July, 2021.
Copy Citation
In recent years, advancing nanotechnology has extended bacterial therapies to a higher level through tailoring bacteria on a nanoscale, such as bacteria-derived nanovesicles and bacterial membrane-coated nanoparticles, or endowing bacteria with abilities to serve as drug carriers, photosensitizers, and sonosensitizers.
bacterial therapy
nanotechnology
drug and gene
delivery system
combinational therapy
cancer treatment
1. Introduction
Malignant tumors are the second most common threat to human life and health [1]. Countless efforts have been dedicated to countering tumor growth and rapidly progressing associated diseases. Currently, conventional clinical interventions like chemotherapy still face problems such as off-target toxicity, limited therapeutic agent enrichment in target lesions, and drug resistance despite being the first-line clinical treatment against cancer [2]. Certain bacteria exhibit promising properties in handling these defects. In 1813, Vautier found that those suffering from cancer had their condition improved after the development of gas gangrene. The underlying therapeutic efficacy is mainly attributed to the ability to localize a hypoxic environment, toxin release, and immune activation using pathogenic bacteria [3]. In recent years, advancing nanotechnology has extended bacterial therapies to a higher level through tailoring bacteria on a nanoscale, such as bacteria-derived nanovesicles and bacterial membrane-coated nanoparticles, or endowing bacteria with abilities to serve as drug carriers, photosensitizers, and sonosensitizers (Figure 1). In this review, cancer hallmarks, current management regimens and their deficiencies are first introduced. Then, we elaborate on the history and therapeutic mechanisms of conventional bacterial therapy. Most importantly, we highlight recent advances in nanotechnology-facilitated bacterial therapy. The superiority of such hybrid nanoformulations, either performing as drug and gene delivery vectors or active pharmaceuticals themselves, is described in detail. Overall, rapidly advancing nanotechnology has facilitated bacterial therapy, unlocking a new stage in cancer treatment.
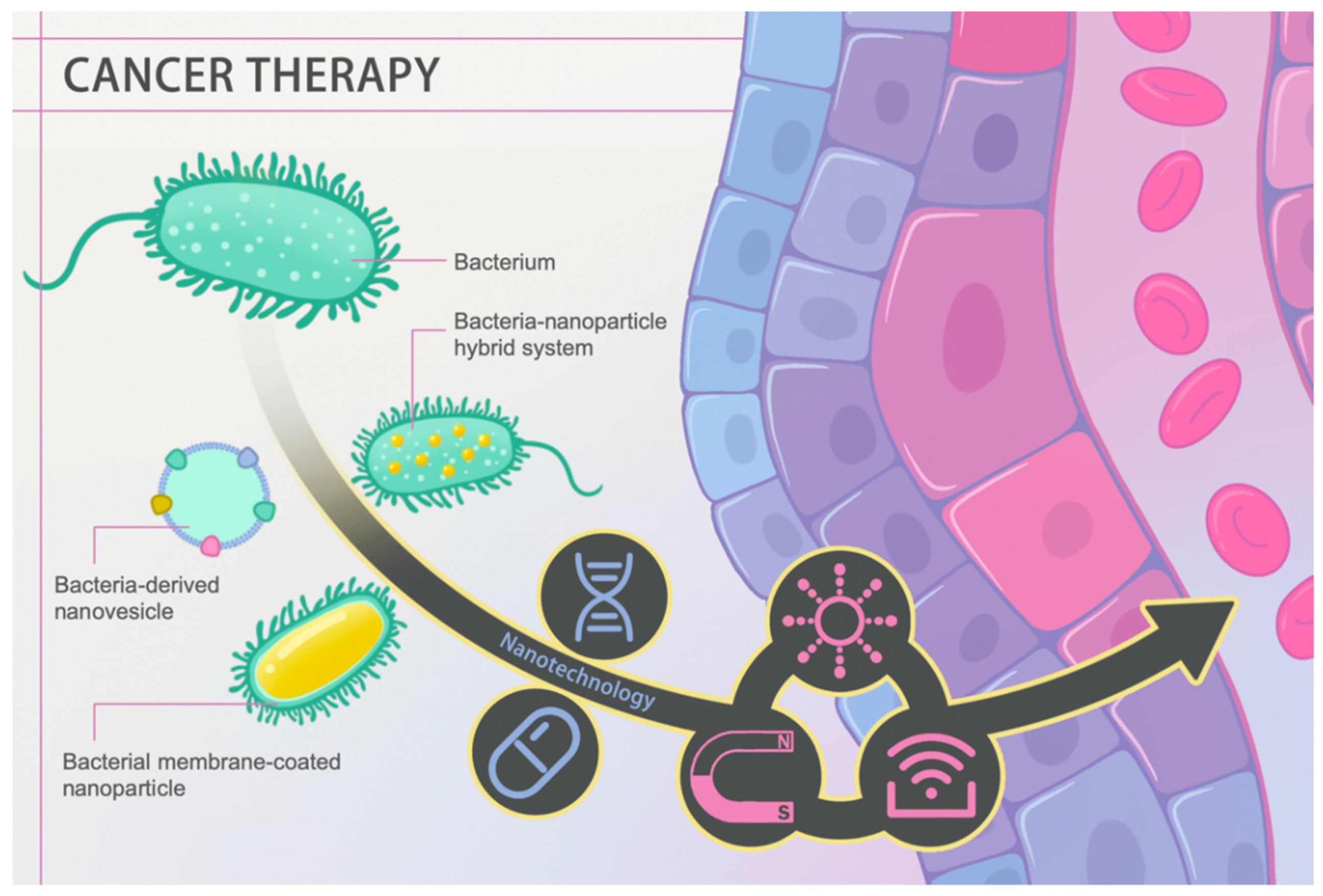 Figure 1. Schematic diagram of nanotechnology-facilitated bacteria-based cancer therapy. Bacteria-derived nanovesicles, bacterial membrane-coated nanoparticles, and bacteria–nanoparticle hybrid systems represent the three main representative delivery platforms used to date. Such platforms not only facilitate drug/gene loading and delivery, but they can also perform diverse functions in response to external stimuli, such as light, magnetism, and ultrasound, achieving better therapeutic efficacy.
Figure 1. Schematic diagram of nanotechnology-facilitated bacteria-based cancer therapy. Bacteria-derived nanovesicles, bacterial membrane-coated nanoparticles, and bacteria–nanoparticle hybrid systems represent the three main representative delivery platforms used to date. Such platforms not only facilitate drug/gene loading and delivery, but they can also perform diverse functions in response to external stimuli, such as light, magnetism, and ultrasound, achieving better therapeutic efficacy.2. Bacteria, an Old Player against Cancer
Bacteria are some of the most notorious killers in human history. In the 14th century, the Black Death claimed millions of human lives, which was caused by the bacterium Yersinia pestis [4]. However, bacteria also contain promising antitumor properties beneath their ‘evil masks’. In this section, we elaborate on the history (Table 1) and therapeutic mechanisms of bacterial therapy. Bottlenecks confronted in clinical trials are also mentioned to acquire a comprehensive understanding of the current role of bacteria in cancer treatment at the same time.
Table 1. Timeline of several typical examples of bacteria use in cancer treatment.
| Year | Bacteria | Cancer Type | Brief Description | Ref. |
|---|---|---|---|---|
| 1868 | Streptococcus pyogenes | Sarcoma | First use of bacteria in cancer treatment | [5] |
| 1891 | Streptococcus pyogenes | Malignant sarcoma | Coley’s toxins | [6] |
| 1989 | Mycobacterium bovis | Bladder cancer | Bacillus Calmette–Guerin vaccine (BCG) approved by the FDA | [7] |
| 2000 | Salmonella typhimurium VNP20009 | Solid tumor | Deletion of the purI and msbB genes which reduce the virulence and the risk of septic shock | [8] |
| 2005 | Clostridium novyi-NT | HCT116 colorectal cancer | Combination of bacterial therapy and traditional drug therapy | [9] |
| 2006 | Escherichia coli | HeLa, HepG2, and U2OS cell lines | Characterization of invasin from Yersinia pseudotuberculosis as an output module | [10] |
| 2011 | Salmonella Typhimurium SL7207 | Colorectal carcinoma | Engineered to survive only in anaerobic conditions without otherwise affecting its functions | [11] |
2.1. Tumor-Targeting Mechanisms
Currently, two main mechanisms explain the tumor-targeting ability of bacteria, which are high hypoxia and immunosuppression in the tumor microenvironment. When some anaerobic bacteria, for example, Salmonella, were injected intravenously into mice, there was no significant difference in the amount of bacteria between the tumor and the liver at the beginning. Subsequently, the bacteria localized near the tumor proliferated due to a suitable hypoxic environment and immunosuppressed conditions [12]. In addition, those situated at normal tissues or in the body’s circulation were rapidly eliminated due to natural immune clearance [13].
In addition, interstitial fluid pressure (IFP) has been reported to be higher in tumor tissues due to blood vessel leakiness and poor lymphoid fluid drainage [14]. As a result, such increased tumor IFP hinders conventional therapeutic agents from entering deep tumor tissues, thus impacting their uptake by cancer cells. The flagellum of bacteria can well handle this predicament through active migration toward tumor tissues and even deeper into their necrotic core [15]. Other factors like the entrapment of bacteria in chaotic tumor vasculatures and chemotaxis toward compounds that derive from cancer tissues also contribute to their tumor-targeting ability [16][17][18].
2.2. Therapeutic Mechanisms
The therapeutic mechanisms of bacteria can be classified into three groups: (1) swelling and apoptosis of tumor cells induced by bacterial invasion, (2) secretion of bacterial toxins, and (3) antitumor immune activation. First, bacteria can kill tumor cells by initiating autophagy or inducing cell apoptosis through infection and intracellular multiplications [19]. In addition, bacteria can secrete toxins which can activate downstream apoptotic pathways. For example, cytolysin A (ClyA) can trigger caspase-mediated cell death and form gaps in cell membranes [20]. Escherichia coli K-12 can secrete ClyA and inhibit tumor growth. Besides, nitric oxide (NO) correlates with tumor progression. A high concentration of NO has been reported to mediate cancer cell apoptosis and tumor regression [21]. However, under normal conditions, NO is converted to its nontoxic form NO3−. In this case, the NO generation enzyme produced by E. coli reoxidizes NO3− into NO to block cancer progression [22].
Apart from them, therapeutic effects rely on the antitumor immune responses to a large extent. Bacteria exhibit outstanding immune activation capability. For example, Salmonella can colonize macrophages and dendritic cells to induce the production of interleukin-1β (IL-1β) [23]. Salmonella infection can also lead to the upregulation of connexin 43 (Cx43) and the formation of functional gap junctions between dendritic cells and tumor cells [24]. Such junctions assist tumor-associated antigens being presented to T cells from dendritic cells, resulting in significant antitumor immune responses. In addition, pathogen-associated molecular patterns (PAMPs) show the capability to activate inflammatory responses and facilitate proinflammatory cytokine release which can contribute to cancer immunotherapy [25]. For example, lipopolysaccharide (LPS) can induce toll-like receptor 4 (TLR4) signal transduction and promote macrophage secretion of IL-1β [26]. Flagellin is also a potential stimulator of natural killer cells that can induce the production of interferon-γ [27][28].
3. Conclusions and Prospects
We outlined the therapeutic roles of nanotechnology-facilitated bacteria-based drug and gene delivery systems. With the help of nanotechnology, such hybrid systems exhibit strong capabilities of delivering drugs and genetic information to targeted tumor sites at high specificity for precise subcellular locations. In addition, nanomaterials can serve as active pharmaceutic compounds by themselves or when hybridized with bacteria and bring additional therapeutic potential to bacterial therapy, including proven techniques such as photothermal or catalytic combinational treatment. As a result, such hybridization exhibits no noticeable impact on bacterial targeting of tumor tissues and exerts great synergistic therapeutic efficacy against cancer. On the other hand, the appropriate selection of bacteria is critical for improving drug-targeting ability. For example, Felfoul et al. conjugated drug-loaded liposomes to Magnetococcus marinus strain MC-1 [29]. As a result, up to 55% of the MC-1 cells penetrated hypoxic regions of HCT116 colorectal xenografts when injected near the tumor with the aid of external magnetic forces. Such hybrid drug delivery systems can significantly improve the therapeutic index of various small-molecule drugs in the tumor’s hypoxic regions. Therefore, the rational design of bacteria–nanoparticle hybrid systems is essential to achieve their full potential performance for treating cancer.
Despite great achievements and promising outlooks in this area, several critical issues must be solved before their possible translation into clinical use. Firstly, latent inflammation and toxicity induced by bacterial membrane components need to be well-managed to avoid severe systemic inflammation [30]. Secondly, therapeutic efficacy and replicability need to be verified carefully in trials. Many variables, such as the amounts of bacteria, nanoparticles, drugs, and genetic information, and the method of nanoparticle hybridization with bacteria must be carefully considered during the construction of such hybrid systems. Overall, nanotechnology has unlocked a new era for bacteria-based cancer therapy and will bring benefits to clinical cancer treatment in new, innovative ways.
References
- Nagai, H.; Kim, Y.H. Cancer Prevention from the Perspective of Global Cancer Burden Patterns. J. Thorac. Dis. 2017, 9, 448–451.
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorganic Chem. 2019, 88, 102925.
- Minton, N.P. Clostridia in Cancer Therapy. Nat. Rev. Microbiol. 2003, 1, 237–242.
- Raoult, D.; Mouffok, N.; Bitam, I.; Piarroux, R.; Drancourt, M. Plague: History and Contemporary Analysis. J. Infect. 2013, 66, 18–26.
- Pawelek, J.M.; Low, K.B.; Bermudes, D. Bacteria as Tumour-Targeting Vectors. Lancet Oncol. 2003, 4, 548–556.
- Nauts, H.C.; Swift, W.E. The Treatment of Malignant Tumors by Bacterial Toxins as Developed by the Late William I3. Coley, M.D., Reviewed in the Light of Modern Research. Cancer Res. 1946, 6, 205–216.
- Guallar-Garrido, S.; Julián, E. Bacillus Calmette-Guérin (BCG) Therapy for Bladder Cancer: An Update. ITT 2020, 9, 1–11.
- Clairmont, C.; Lee, K.C.; Pike, J.; Ittensohn, M.; Low, K.B.; Pawelek, J.; Bermudes, D.; Brecher, S.M.; Margitich, D.; Turnier, J.; et al. Biodistribution and Genetic Stability of the Novel Antitumor Agent VNP20009, a Genetically Modified Strain of Salmonella typhimurium. J. Infect Dis. 2000, 181, 1996–2002.
- Smith, A.B.; Freeze, B.S.; LaMarche, M.J.; Sager, J.; Kinzler, K.W.; Vogelstein, B. Discodermolide Analogues as the Chemical Component of Combination Bacteriolytic Therapy. Bioorg. Med. Chem. Lett. 2005, 15, 3623–3626.
- Anderson, J.C.; Clarke, E.J.; Arkin, A.P.; Voigt, C.A. Environmentally Controlled Invasion of Cancer Cells by Engineered Bacteria. J. Mol. Biol. 2006, 355, 619–627.
- Yu, B.; Yang, M.; Shi, L.; Yao, Y.; Jiang, Q.; Li, X.; Tang, L.-H.; Zheng, B.-J.; Yuen, K.-Y.; Smith, D.K.; et al. Explicit Hypoxia Targeting with Tumor Suppression by Creating an “Obligate” Anaerobic Salmonella Typhimurium Strain. Sci. Rep. 2012, 2, 436.
- Pawelek, J.M.; Low, K.B.; Bermudes, D. Tumor-Targeted Salmonella as a Novel Anticancer Vector. Cancer Res. 1997, 57, 4537–4544.
- Chandra, D.; Jahangir, A.; Quispe-Tintaya, W.; Einstein, M.H.; Gravekamp, C. Myeloid-Derived Suppressor Cells Have a Central Role in Attenuated Listeria Monocytogenes-Based Immunotherapy against Metastatic Breast Cancer in Young and Old Mice. Br. J. Cancer 2013, 108, 2281–2290.
- Heldin, C.-H.; Rubin, K.; Pietras, K.; Östman, A. High Interstitial Fluid Pressure—An Obstacle in Cancer Therapy. Nat. Rev. Cancer 2004, 4, 806–813.
- Duong, M.T.-Q.; Qin, Y.; You, S.-H.; Min, J.-J. Bacteria-Cancer Interactions: Bacteria-Based Cancer Therapy. Exp. Mol. Med. 2019, 51, 1–15.
- Kasinskas, R.W.; Forbes, N.S. Salmonella Typhimurium Specifically Chemotax and Proliferate in Heterogeneous Tumor Tissue in Vitro. Biotechnol. Bioeng. 2006, 94, 710–721.
- Kasinskas, R.W.; Forbes, N.S. Salmonella Typhimurium Lacking Ribose Chemoreceptors Localize in Tumor Quiescence and Induce Apoptosis. Cancer Res. 2007, 67, 3201–3209.
- Forbes, N.S.; Munn, L.L.; Fukumura, D.; Jain, R.K. Sparse Initial Entrapment of Systemically Injected Salmonella Typhimurium Leads to Heterogeneous Accumulation within Tumors. Cancer Res. 2003, 63, 5188–5193.
- Uchugonova, A.; Zhang, Y.; Salz, R.; Liu, F.; Suetsugu, A.; Zhang, L.; Koenig, K.; Hoffman, R.M.; Zhao, M. Imaging the Different Mechanisms of Prostate Cancer Cell- Killing by Tumor-Targeting Salmonella Typhimurium A1-R. Anticancer. Res. 2015, 5, 5225–5229.
- Lai, X.-H.; Arencibia, I.; Johansson, A.; Wai, S.N.; Oscarsson, J.; Kalfas, S.; Sundqvist, K.-G.; Mizunoe, Y.; Sjöstedt, A.; Uhlin, B.E. Cytocidal and Apoptotic Effects of the ClyA Protein from Escherichia Coli on Primary and Cultured Monocytes and Macrophages. Infect. Immun. 2000, 68, 4363–4367.
- Vannini, F.; Kashfi, K.; Nath, N. The Dual Role of INOS in Cancer. Redox Biol. 2015, 6, 334–343.
- Charles, G. The Role of Nitric Oxide in Cancer. Cell Res. 2002, 12, 311–320.
- Kim, J.-E.; Phan, T.X.; Nguyen, V.H.; Dinh-Vu, H.-V.; Zheng, J.H.; Yun, M.; Park, S.-G.; Hong, Y.; Choy, H.E.; Szardenings, M.; et al. Salmonella Typhimurium Suppresses Tumor Growth via the Pro-Inflammatory Cytokine Interleukin-1β. Theranostics 2015, 5, 1328–1342.
- Saccheri, F.; Pozzi, C.; Avogadri, F.; Barozzi, S.; Faretta, M.; Fusi, P.; Rescigno, M. Bacteria-Induced Gap Junctions in Tumors Favor Antigen Cross-Presentation and Antitumor Immunity. Sci. Transl. Med. 2010, 2, 44–57.
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in Clinical Cancer Immunotherapy. Br. J. Cancer 2019, 120, 6–15.
- Phan, T.X.; Nguyen, V.H.; Duong, M.T.-Q.; Hong, Y.; Choy, H.E.; Min, J.-J. Activation of Inflammasome by Attenuated Salmonella Typhimurium in Bacteria-Mediated Cancer Therapy: Bacteriotherapy and Inflammasome. Microbiol. Immunol. 2015, 59, 664–675.
- Tsujimoto, H.; Uchida, T.; Efron, P.A.; Scumpia, P.O.; Verma, A.; Matsumoto, T.; Tschoeke, S.K.; Ungaro, R.F.; Ono, S.; Seki, S.; et al. Flagellin Enhances NK Cell Proliferation and Activation Directly and through Dendritic Cell-NK Cell Interactions. J. Leukoc. Biol. 2005, 78, 888–897.
- Kupz, A.; Curtiss, R.; Bedoui, S.; Strugnell, R.A. In Vivo IFN-γ Secretion by NK Cells in Response to Salmonella Typhimurium Requires NLRC4 Inflammasomes. PLoS ONE 2014, 9, e97418.
- Felfoul, O.; Mohammadi, M.; Taherkhani, S.; de Lanauze, D.; Zhong Xu, Y.; Loghin, D.; Essa, S.; Jancik, S.; Houle, D.; Lafleur, M.; et al. Magneto-Aerotactic Bacteria Deliver Drug-Containing Nanoliposomes to Tumour Hypoxic Regions. Nat. Nanotech. 2016, 11, 941–947.
- Nedeva, C.; Menassa, J.; Puthalakath, H. Sepsis: Inflammation Is a Necessary Evil. Front. Cell Dev. Biol. 2019, 7, 108.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Entry Collection:
Biopharmaceuticals Technology
Revision:
1 time
(View History)
Update Date:
09 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




